Enhancing Airtight Storage with Germinating Cowpea Seeds: Impacts on Insect Mortality, Progeny and Grain Quality
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Preparation and Germination
2.2. Insect Rearing
2.3. Experimental Setup and Design
2.4. Data Collection
2.4.1. Oxygen, Temperature, and Relative Humidity
2.4.2. Adult Mortality
2.4.3. Egg Count
2.4.4. Progeny Development
2.4.5. Moisture Content
2.5. Statistical Analysis
- β0 = intercept
- β1 = slope of log(time)
- β2 = slope of Treatments
- β3 = slope of interaction term of log(time) and Treatments
- ε = Error term
3. Results
3.1. Oxygen Concentration
3.2. Adult Mortality
3.3. Egg Count
3.4. Progeny Development
3.5. Moisture Content
3.6. Temperature and Relative Humidity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swella, G.B.; Mushobozy, D.M.K. Comparative susceptibility of different legume seeds to infestation by cowpea bruchid Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae). Plant Prot. Sci. 2009, 45, 19–24. [Google Scholar] [CrossRef]
- Southgate, B.J. Biology of the Bruchidae. Annu. Rev. Entomol. 1979, 24, 449–473. [Google Scholar] [CrossRef]
- Beck, C.W.; Blumer, L.S. A Handbook on Bean Beetles Callosobruchus Maculatus; National Science Foundation: Alexandria, VA, USA, 2014; pp. 1–17.
- Oluwafemi, A.R. Comparative effects of three plant powders and pirimiphos-methyl against the infestation of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in cowpea. SOAJ Entomol. Stud. 2012, 1, 108–117. [Google Scholar]
- Mahama, S. Comparative Assessment of Some Storage Technologies Used for Cowpea Storage in the Nadowli District of the Upper West Region of Ghana. Master’s Thesis, Nkwame Nkrumah University of Science and Technology, Kumasi, Ghana, 2012. [Google Scholar]
- Baoua, I.B.; Amadou, L.; Margam, V.; Murdock, L.L. Comparative evaluation of six storage methods for postharvest preservation of cowpea grain. J. Stored Prod. Res. 2012, 49, 171–175. [Google Scholar] [CrossRef]
- Bett, C.; Nguyo, R. Post-harvest storage practices and techniques used by farmers in semi-arid eastern and central Kenya. In Proceedings of the African Crop Science Conference Proceedings, El-Minia, Egypt, 27–31 October 2007; Volume 8, pp. 1023–1227. [Google Scholar]
- Benhalima, H.; Chaudhry, M.Q.; Mills, K.A.; Price, N.R. Phosphine resistance in stored-product insects collected from various grain storage facilities in Morocco. J. Stored Prod. Res. 2004, 40, 241–249. [Google Scholar] [CrossRef]
- Fields, P.G.; White, N.D.G. Alternatives to methyl bromide treatments for stored-product and quarantine insects. Annu. Rev. Entomol. 2002, 47, 331–359. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.A.G.; Faroni, L.R.D.A.; Guedes, R.N.C.; Sousa, A.H.; Tótola, M.R. Phosphine resistance in Brazilian populations of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Stored Prod. Res. 2009, 45, 71–74. [Google Scholar] [CrossRef]
- Villers, P.; De Bruin, T.; Navarro, S. Development and applications of the hermetic storage technology. In Proceedings of the 9th International Working Conference on Stored Product Protection, Sao Paulo, Brazil, 15–18 October 2006; Lorini, B.I., Bacaltchuk, H., Beckel, D., Deckers, E., Sundfeld, J.P., dos Santos, J.D., Biagi, J.C., Celaro, L.R.D’.A., Faroni, L.d.O.F., Bortolini, M.R., et al., Eds.; Brazilian Post-Harvest Association—ABRAPOS: São Paulo, Brazil, 2006; pp. 719–729. [Google Scholar]
- Hoback, W.W.; Stanley, D.W. Insects in hypoxia. J. Insect Physiol. 2001, 47, 533–542. [Google Scholar] [CrossRef]
- Navarro, S. Commercial applications of oxygen depleted atmospheres for the preservation of food commodities. In Case Studies in Novel Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2010; pp. 321–350. [Google Scholar]
- Murdock, L.L.; Margam, V.; Baoua, I.; Balfe, S.; Shade, R.E. Death by desiccation: Effects of hermetic storage on cowpea bruchids. J. Stored Prod. Res. 2012, 49, 166–170. [Google Scholar] [CrossRef]
- Donga, T.K.; Baributsa, D. Effect of Temperature and Insect Infestation Levels on Oxygen Depletion in Hermetic Storage. Insects 2023, 14, 621. [Google Scholar] [CrossRef]
- Bechoff, A.; Chijioke, U.; Tomlins, K.I.; Govinden, P.; Ilona, P.; Westby, A.; Boy, E. Carotenoid stability during storage of yellow gari made from biofortified cassava or with palm oil. J. Food Compos. Anal. 2015, 44, 36–44. [Google Scholar] [CrossRef]
- Gannon, B.; Kaliwile, C.; Arscott, S.A.; Schmaelzle, S.; Chileshe, J.; Kalungwana, N.; Mosonda, M.; Pixley, K.; Masi, C.; Tanumihardjo, S.A. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: A community-based, randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Katola, A.A.; Stark, A.H.; Ndolo, V.U.; Tembo, D.T.; Katundu, M.C. Provitamin A retention and sensory acceptability of landrace orange maize (MW5021) food products among school-aged children living in rural Malawi. Food Prod. Process. Nutr. 2023, 5, 57. [Google Scholar] [CrossRef]
- Taleon, V.; Mugode, L.; Cabrera-Soto, L.; Palacios-Rojas, N. Carotenoid retention in biofortified maize using different post-harvest storage and packaging methods. Food Chem. 2017, 232, 60–66. [Google Scholar] [CrossRef]
- Mugode, L.; Ha, B.; Kaunda, A.; Sikombe, T.; Phiri, S.; Mutale, R.; Davis, C.; Tanumihardjo, S.; De Moura, F.F. Carotenoid Retention of Biofortified Provitamin A Maize (Zea mays L.) after Zambian Traditional Methods of Milling, Cooking and Storage. J. Agric. Food Chem. 2014, 62, 6317–6325. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Lei, J.; Ahn, J.E.; Liu, T.X.; Zhu-Salzman, K. Effects of decreased O2 and elevated CO2 on survival, development, and gene expression in cowpea bruchids. J. Insect Physiol. 2012, 58, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Kandel, P.; Scharf, M.E.; Mason, L.J.; Baributsa, D. Effect of hypoxia on the lethal mortality time of adult Sitophilus oryzae L. Insects 2021, 12, 952. [Google Scholar] [CrossRef]
- Souza, R.; Peruch, G.; Santos Pires, A.C. dos Oxygen Scavengers: An Approach on Food Preservation. In Structure and Function of Food Engineering; InTech: Rijeka, Croatia, 2012; pp. 21–42. [Google Scholar]
- Navarro, S. The use of modified and controlled atmospheres for the disinfestation of stored products. J. Pest Sci. 2012, 85, 301–322. [Google Scholar] [CrossRef]
- Njoroge, A.W.; Mankin, R.W.; Smith, B.; Baributsa, D. Effects of hypoxia on acoustic activity of two stored-product pests, adult emergence, and grain quality. J. Econ. Entomol. 2019, 112, 1989–1996. [Google Scholar] [CrossRef]
- Dey, A.; Neogi, S. Oxygen scavengers for food packaging applications: A review. Trends Food Sci. Technol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Cichello, S.A. Oxygen absorbers in food preservation: A review. J. Food Sci. Technol. 2015, 52, 1889–1895. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ortiz, D.; Baributsa, D.; Hamaker, B.; Rocheford, T.; Ferruzzi, M.G. Assessment of oxygen sequestration on effectiveness of Purdue Improved Crop Storage (PICS) bags in reducing carotenoid degradation during post-harvest storage of two biofortified orange maize genotypes. J. Cereal Sci. 2019, 87, 68–77. [Google Scholar] [CrossRef]
- Lamsal, G.; Volenec, J.; Ambrose, K.; Baributsa, D. Assessing Germinating Seeds of Legume and Cereal Crops to Enhance Oxygen Depletion: A Novel Approach in Hermetic Storage. Sustainability 2023, 15, 16403. [Google Scholar] [CrossRef]
- Garima, G.; Khan, R.; Seal, D. Cowpea Weevil. Available online: https://entnemdept.ufl.edu/Creatures/VEG/BEAN/Callosobruchus_maculatus.htm (accessed on 5 December 2023).
- van der Meer, J. The specification of metameric order in the insect Callosobruchus maculatus Fabr. (Coleoptera). J. Embryol. Exp. Morph. 1979, 51, 1–26. [Google Scholar] [PubMed]
- Dobie, P.; Haines, C.; Hodges, R.; Prevett, P.; Rees, D. Insects and Arachnids of Tropical Stored Products: Their Biology and Identification; Natural Resources Institute: Chatham Maritime, UK, 1991. [Google Scholar]
- Lenth, R.; Love, J.; Herve, M. Population marginal means in the linear model: An alternative to least squares means. Am. Stat. 2018, 34, 216–221. [Google Scholar] [CrossRef]
- Matthews, P.G.D.; White, C.R. Regulation of gas exchange and haemolymph pH in the cockroach Nauphoeta cinerea. J. Exp. Biol. 2011, 214, 3062–3073. [Google Scholar] [CrossRef]
- Taher, H.; Bartosik, R. Hermetic storage of dry soybean (Glycine max): Creating an effective modified atmosphere using soaked grain as O2 depletor. In Proceedings of the 12th International Working Conference on Stored Product Protection (IWCSPP), Berlin, Germany, 7–11 October 2018; Volume 2, pp. 666–671. [Google Scholar]
- Storey, C.L. Mortality of cowpea weevil in a low-oxygen atmosphere. J. Econ. Entomol. 1978, 7, 833–834. [Google Scholar] [CrossRef]
- Banks, H.J.; Annis, P.C. Comparative advantages of high CO2 and low O2 types of controlled atmospheres for grain storage. In Food Preservation by Modified Atmospheres; Calderon, M., Barkai-Golan, R., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 93–122. [Google Scholar]
- Fleurat-Lessard, F. Effect of modified atmospheres on insect and mites infesting stored products. In Food Preservation by Modified Atmospheres; Calderon, M., Barkai-Golan, R., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 21–38. [Google Scholar]
- Navarro, S. The effects of low oxygen tensions on three stored-product insect pests. Phytoparasitica 1978, 6, 51–58. [Google Scholar] [CrossRef]
- Adler, C.; Corinth, H.G.; Reichmuth, C. Modified atmospheres. In Alternatives to Pesticides in Stored-Product IPM; Subramanyam, B., Hagstrum, D., Eds.; Kluwer Academic Publishing: Norwell, MA, USA, 2000; pp. 105–146. ISBN 9788578110796. [Google Scholar]
- Yan, Y.; Williams, S.B.; Baributsa, D.; Murdock, L.L. Hypoxia treatment of Callosobruchus maculatus females and its effects on reproductive output and development of progeny following exposure. Insects 2016, 7, 26. [Google Scholar] [CrossRef]
- Azzam, Z.S.; Sharabi, K.; Guetta, J.; Bank, E.M.; Gruenbaum, Y. The physiological and molecular effects of elevated CO2 levels. Cell Cycle 2010, 9, 1528–1532. [Google Scholar] [CrossRef]
- De Carli, M.; Bresolin, B.; Noreña, C.P.Z.; Lorini, I.; Brandelli, A. Efficacy of modified atmosphere packaging to control Sitophilus spp. in organic maize grain. Brazilian Arch. Biol. Technol. 2010, 53, 1469–1476. [Google Scholar] [CrossRef]
- Hashem, M.Y.; Risha, E.S.M.; El-Sherif, S.I.; Ahmed, S.S. The effect of modified atmospheres, an alternative to methyl bromide, on the susceptibility of immature stages of angoumois grain moth Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae). J. Stored Prod. Res. 2012, 50, 57–61. [Google Scholar] [CrossRef]
- Kharel, K.; Mason, L.J.; Murdock, L.L.; Baributsa, D. Efficacy of hypoxia against Tribolium castaneum (Coleoptera: Tenebrionidae) throughout ontogeny. J. Econ. Entomol. 2019, 112, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, J.A.; Sandoval, A.J. Kinetics of moisture adsorption during simulated storage of whole dry cocoa beans at various relative humidities. J. Food Eng. 2020, 273, 109869. [Google Scholar] [CrossRef]
- Fleurat-Lessard, F. Integrated management of the risks of stored grain spoilage by seedborne fungi and contamination by storage mould mycotoxins—An update. J. Stored Prod. Res. 2017, 71, 22–40. [Google Scholar] [CrossRef]
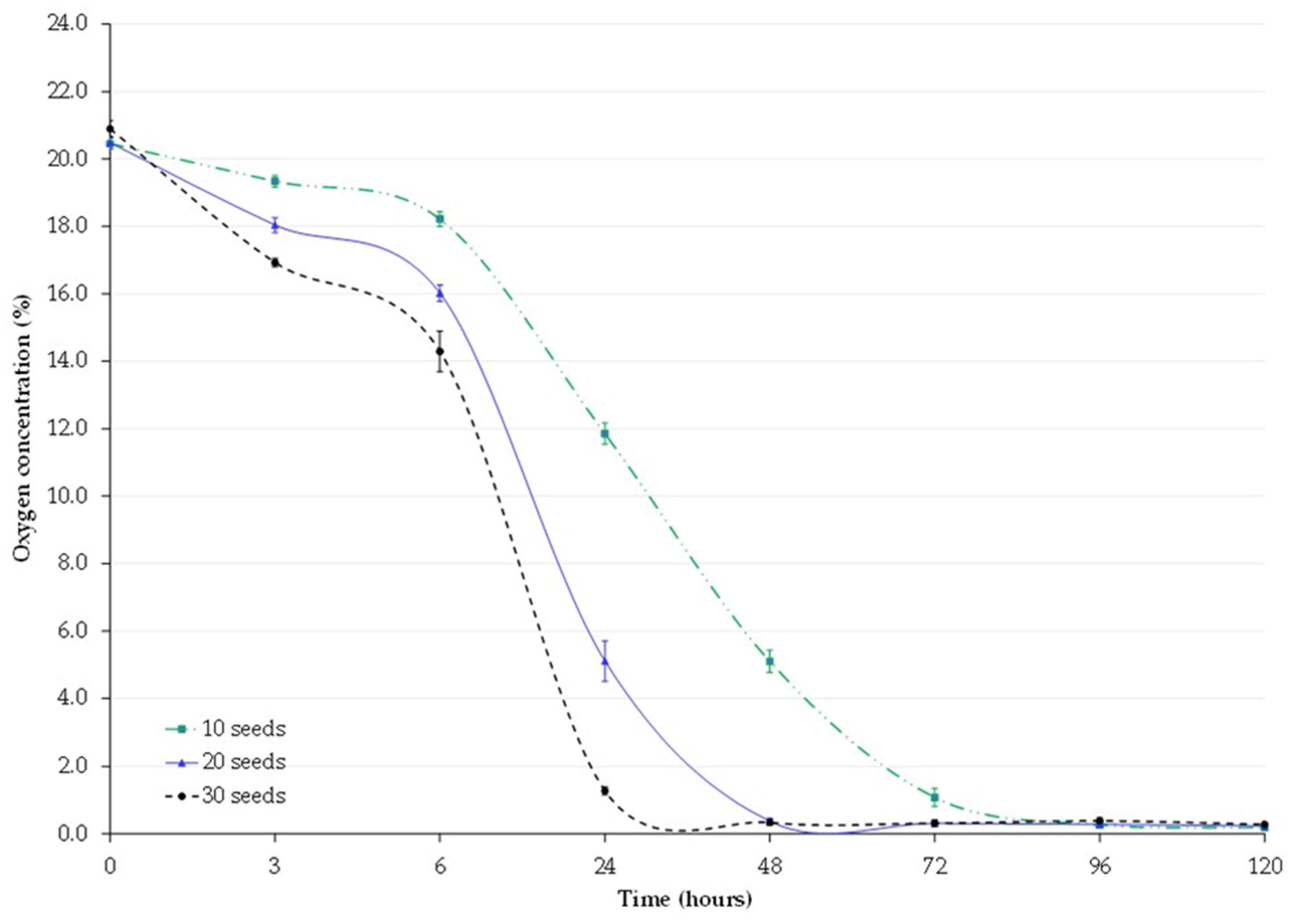
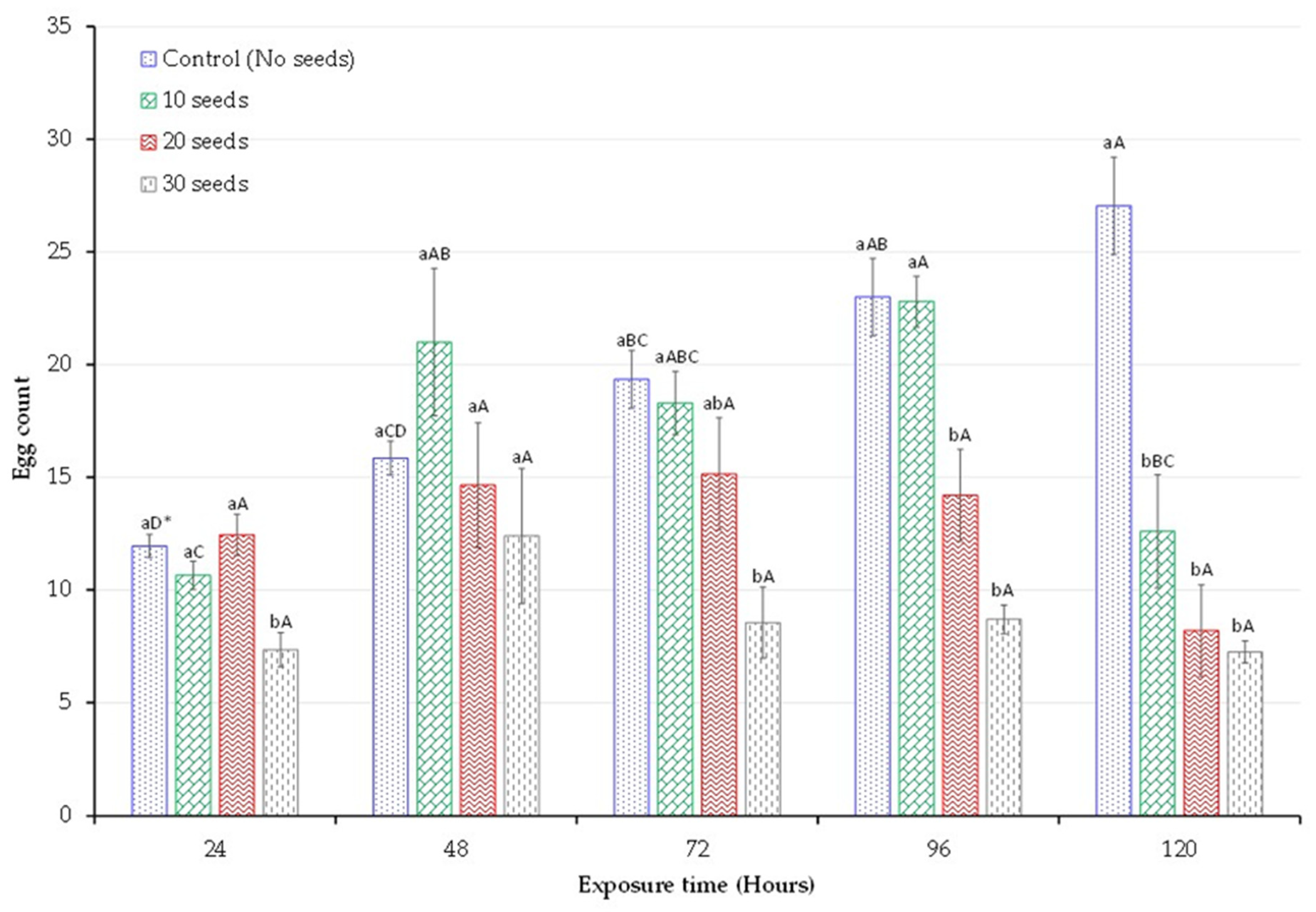
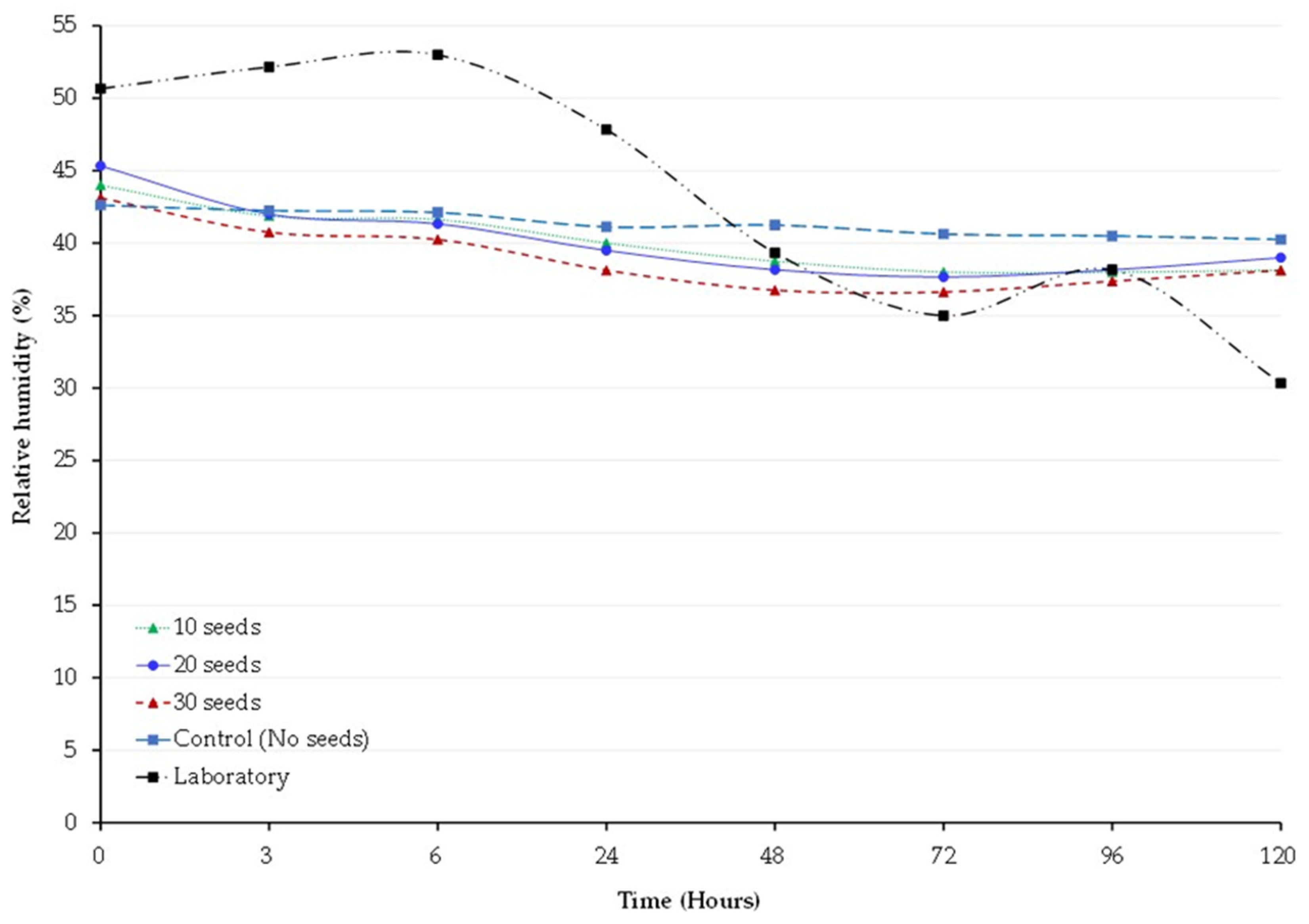
| Residual Oxygen Level (%, Mean ± SEM) | ||||||
|---|---|---|---|---|---|---|
| Time | ||||||
| Treatment | 6 h | 24 h | 48 h | 72 h | 96 h | 120 h |
| 10 Seeds | 18.22 ± 0.21 b * | 11.85 ± 0.32 b * | 5.10 ± 0.33 b | 1.08 ± 0.27 b | 0.27 ± 0.02 b | 0.19 ± 0.02 b |
| 20 Seeds | 16.02 ± 0.24 c | 5.11 ± 0.60 c | 0.38 ± 0.09 c | 0.31 ± 0.03 b | 0.29 ± 0.06 b | 0.24 ± 0.04 b |
| 30 Seeds | 14.39 ± 0.60 d | 1.28 ± 0.12 d | 0.34 ± 0.09 c | 0.32 ± 0.09 b | 0.39 ± 0.09 b | 0.28 ± 0.06 b |
| Control | 21.36 ± 0.90 a | 21.20 ± 0.93 a | 20.89 ± 0.96 a | 20.6 ± 0.86 a | 20.92 ± 0.94 a | 20.87 ± 0.85 a |
| Variables * | Estimate | Std. Error | t Value | Pr(>|t|) |
|---|---|---|---|---|
| (Intercept) (β0) | 21.37 | 0.42 | 50.30 | <0.001 |
| log_hr (β1) | −0.008 | 0.11 | −0.08 | 0.94 |
| 10 seeds (β2) | −3.62 | 0.60 | −6.02 | <0.001 |
| 20 seeds (β3) | −6.43 | 0.60 | −10.70 | <0.001 |
| 30 seeds (β4) | −8.42 | 0.60 | −14.01 | <0.001 |
| log_hr: 20 seeds (β5) | −0.55 | 0.15 | −3.63 | <0.001 |
| log_hr: 20 seeds (β6) | −1.03 | 0.15 | −6.75 | <0.001 |
| log_hr: 30 seeds (β7) | −1.39 | 0.15 | −9.11 | <0.001 |
| Adult Mortality (%, Mean ± SEM) | |||||
|---|---|---|---|---|---|
| Exposure Time | |||||
| Treatment | 24 h | 48 h | 72 h | 96 h | 120 h |
| 10 Seeds | 5 ± 2.89 abC * | 7.5 ± 2.5 cBC | 30 ± 10.8 bB | 97.5 ± 2.5 aA | 100 ± 0 aA |
| 20 Seeds | 10 ± 7.07 abC | 25 ± 6.45 bB | 100 ± 0 aA | 100 ± 0 aA | 100 ± 0 aA |
| 30 Seeds | 12.5 ± 4.79 aC | 85 ± 6.45 aB | 100 ± 0 aA | 100 ± 0 aA | 100 ± 0 aA |
| Control | 0 ± 0 bA | 5 ± 5 cA | 7.5 ± 4.79 cA | 7.5 ± 4.79 bA | 10 ± 4.08 bA |
| Adult Emergence (%, Mean ± SEM) | |||||
|---|---|---|---|---|---|
| Exposure Time | |||||
| Treatment | 24 h | 48 h | 72 h | 96 h | 120 h |
| 10 Seeds | 64.92 ± 2.99 bB * | 79 ± 1.38 aA | 30.89 ± 1.19 bC | 14.78 ± 1.57 bD | 0.29 ± 0.29 bE |
| 20 Seeds | 63.85 ± 1.54 bA | 40.66 ± 2.89 bB | 8.88 ± 1.72 cC | 2.54 ± 1.48 cD | 0 ± 0 bE |
| 30 Seeds | 50.09 ± 1.78 cA | 6.53 ± 1.40 cB | 0.40 ± 0.40 dC | 0 ± 0 cC | 0 ± 0 bC |
| Control | 83.24 ± 0.5 aABC | 86.13 ± 1.31 aAB | 82.92 ± 2.82 aBC | 76.03 ± 1.93 aC | 90.94 ± 1.51 aA |
| Treatment | Initial | 5 Days |
|---|---|---|
| 10 seeds | 8.90 ± 0.1 aA * | 9.13 ± 0.09 aA |
| 20 seeds | 8.90 ± 0.1 aA | 9.10 ± 0.06 aA |
| 30 seeds | 8.90 ± 0.1 aB | 9.31 ± 0.03 aA |
| Control | 8.90 ± 0.1 aB | 9.25 ± 0.10 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamsal, G.; Baributsa, D. Enhancing Airtight Storage with Germinating Cowpea Seeds: Impacts on Insect Mortality, Progeny and Grain Quality. Insects 2023, 14, 954. https://doi.org/10.3390/insects14120954
Lamsal G, Baributsa D. Enhancing Airtight Storage with Germinating Cowpea Seeds: Impacts on Insect Mortality, Progeny and Grain Quality. Insects. 2023; 14(12):954. https://doi.org/10.3390/insects14120954
Chicago/Turabian StyleLamsal, Gunakeshari, and Dieudonne Baributsa. 2023. "Enhancing Airtight Storage with Germinating Cowpea Seeds: Impacts on Insect Mortality, Progeny and Grain Quality" Insects 14, no. 12: 954. https://doi.org/10.3390/insects14120954
APA StyleLamsal, G., & Baributsa, D. (2023). Enhancing Airtight Storage with Germinating Cowpea Seeds: Impacts on Insect Mortality, Progeny and Grain Quality. Insects, 14(12), 954. https://doi.org/10.3390/insects14120954






