Electrophysiological and Behavioral Responses of Batocera horsfieldi Hope to Volatiles from Pistacia chinensis Bunge
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. Host Plant Choice Tests
2.3. Collection and Identification of Volatile Compounds
2.4. Electroantennographic (EAG) Assays
2.5. Y-Tube Behavioral Experiments
2.6. Effect of Plant Volatiles on Feeding
2.7. Statistical Analysis
3. Results
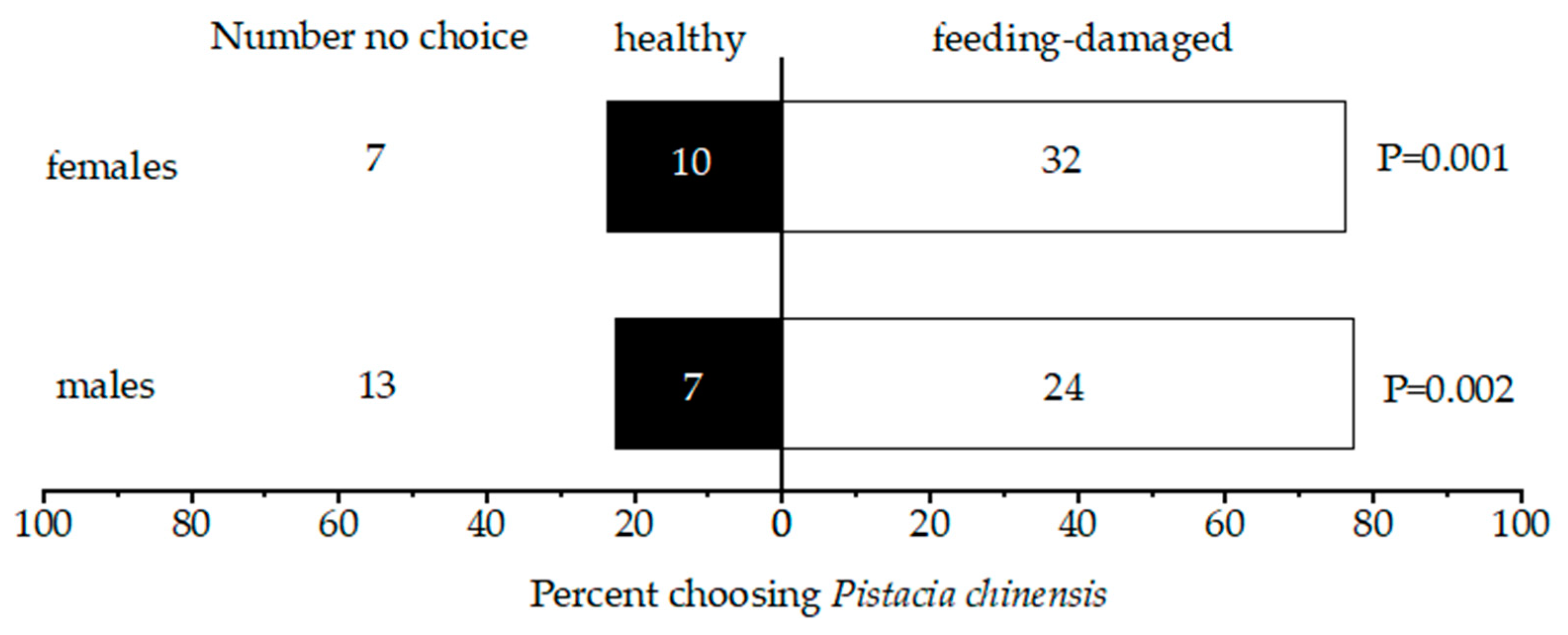
3.1. Host Plant Choice Tests
3.2. Collection and Identification of Volatile Compounds
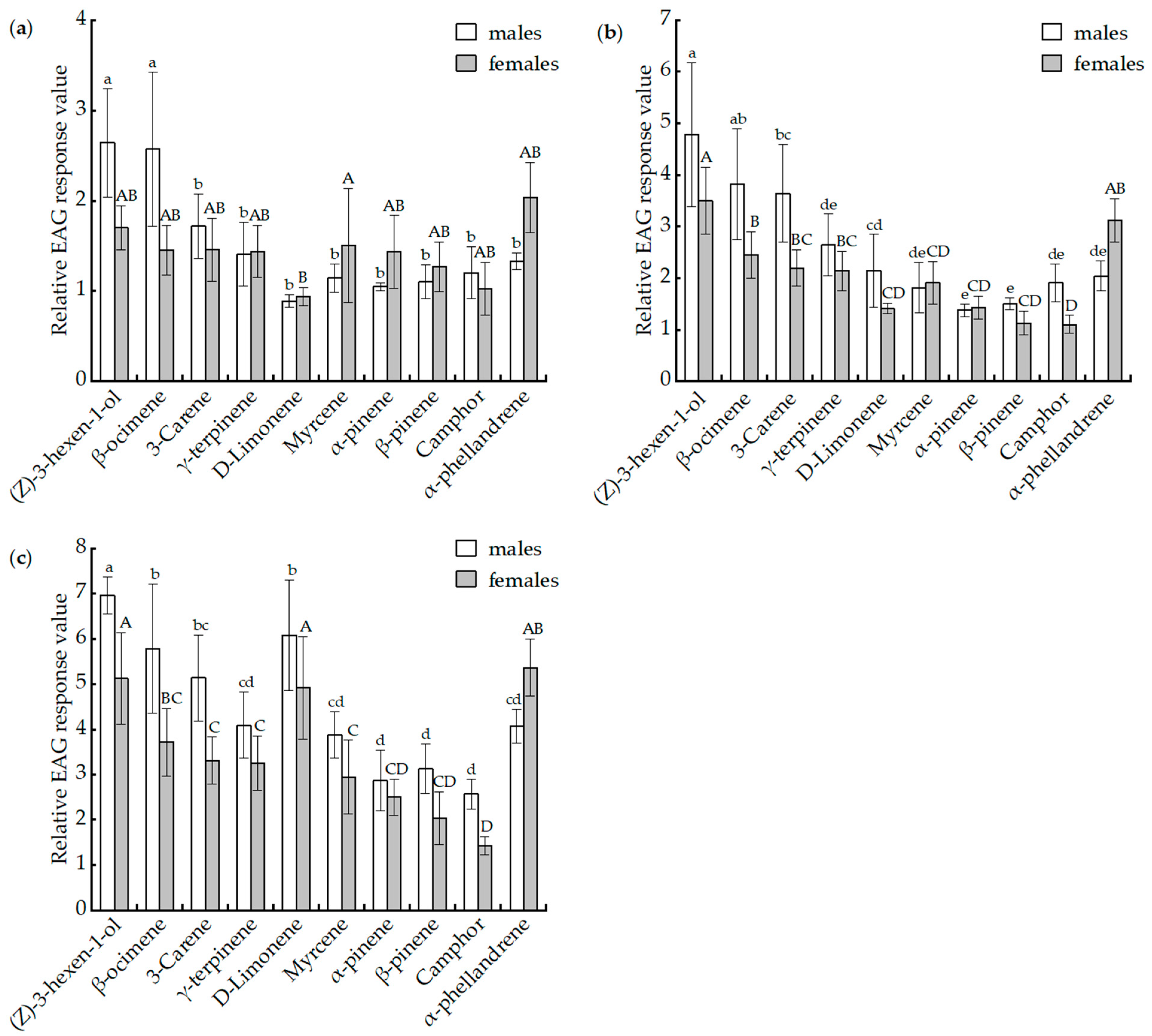
3.3. Electroantennographic (EAG) Assays
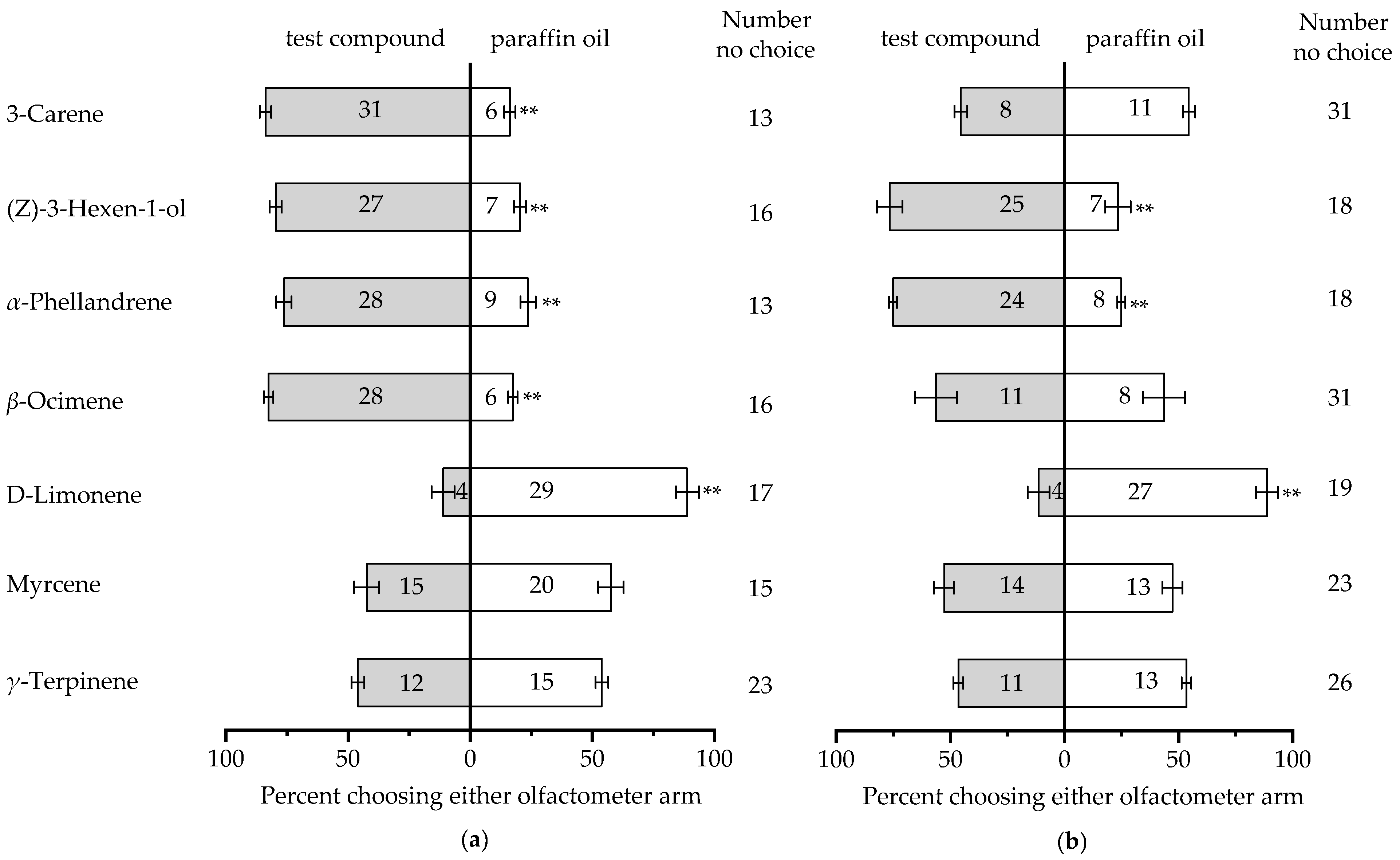
3.4. Y-Tube Behavioral Experiments
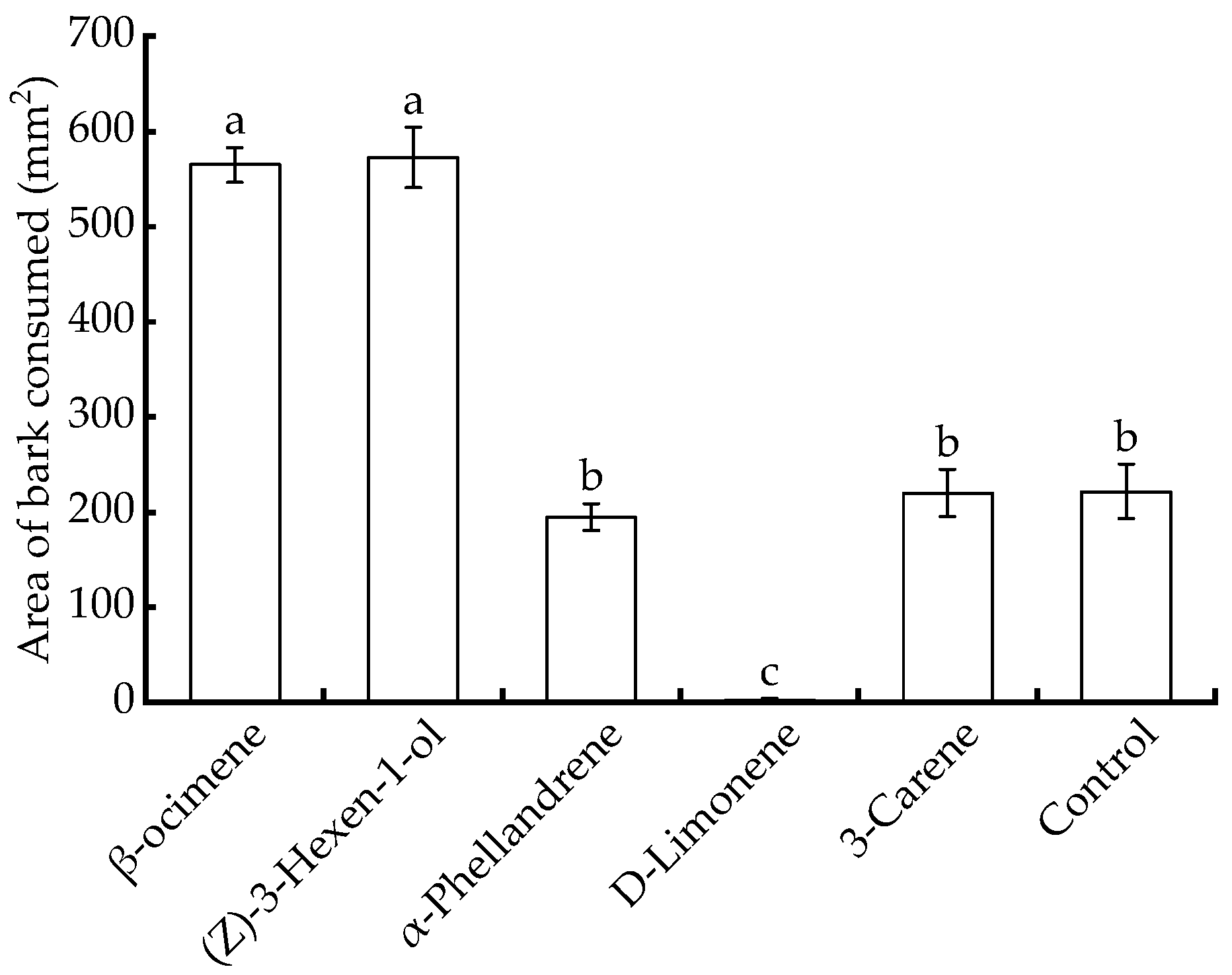
3.5. Effect of Plant Volatiles on Feeding
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Q.Y.; Zhao, Z.C. A preliminary study on Batocera horsfieldi. J. Jiangsu For. Sci. Technol. 1991, 2, 22–25. [Google Scholar]
- Gao, R.T.; Wang, H.Q.; Wang, X.Q.; Xu, B.X.; Zheng, S.K.; Gong, Y.H. Study on the habit of absorbing replenishing nutrition of Batocera horsfieldi and its relation with the host trees. For. Res. 1995, 8, 619–623. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.Q.; Zhang, Z.C.; Chen, J.Y.; Zhang, G.A. Behavioral response of Batocera horsfieldi adults to plant volatiles. Sci. Silvae Sin. 2008, 44, 168–170. [Google Scholar]
- Liang, X.Y.; Yang, W.; Yang, Y.L.; Yang, C.P.; Yang, Y. Preference of Batocera horsfieldi for adults to feeding plants. Chin. Bull. Entomol. 2008, 45, 78–82. [Google Scholar]
- Li, J.Q.; Yang, Z.Q.; Mei, Z.X.; Zhang, Y.L. Pest risk analysis and control countermeasure of Batocera horsfieldi. For. Res. 2009, 22, 148–153. [Google Scholar] [CrossRef]
- Yang, H.; Yang, W.; Yang, C.P.; Zhu, T.H.; Huang, Q.; Han, S.; Xiao, J.J. Electrophysiological and behavioral responses of the whitestriped longhorned beetle, Batocera lineolata, to the diurnal rhythm of host plant volatiles of holly, Viburnum awabuki. J. Insect Sci. 2013, 13, 85. [Google Scholar] [CrossRef]
- Zheng, Z.C.; Li, D.Z.; Zhou, A.M.; Yi, S.C.; Liu, H.; Wang, M.Q. Predicted structure of a Minus-C OBP from Batocera horsfieldi (Hope) suggests an intermediate structure in evolution of OBPs. Sci. Rep. 2016, 6, 33981. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cai, Y.; Zhuo, Z.H.; Yang, W.; Yang, C.P.; Zhang, J.; Yang, Y.; Wang, B.X.; Guan, F.R. Correction: Transcriptome analysis in different developmental stages of Batocera horsfieldi (Coleoptera: Cerambycidae) and comparison of candidate olfactory genes. PLoS ONE 2019, 14, e0214472. [Google Scholar] [CrossRef]
- Zhuge, P.P.; Luo, S.L.; Wang, M.Q.; Zhang, G. Electrophysiological responses of Batocera horsfieldi (Hope) adults to plant volatiles. J. Appl. Entomol. 2010, 134, 600–607. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Wang, M.Q. Chemosensory protein genes of Batocera horsfieldi (Hope): Identification and expression pattern. J. Appl. Entomol. 2012, 136, 781–792. [Google Scholar] [CrossRef]
- Li, H.; Zhang, A.J.; Chen, L.Z.; Zhang, G.A.; Wang, M.Q. Construction and analysis of cDNA libraries from the antennae of Batocera horsfieldi and expression pattern of putative odorant binding proteins. J. Insect Sci. 2014, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, X.Y.; Chen, L.Z.; Zhang, A.; Wang, D.X.; Wang, M.Q. Expression profile and ligand-binding characterization of odorant-binding protein 2 from Batocera horsfieldi (Hope). J. Appl. Entomol. 2015, 139, 361–369. [Google Scholar] [CrossRef]
- Takken, W.; Knols, B.G. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu. Rev. Entomol. 1999, 44, 131–157. [Google Scholar] [CrossRef] [PubMed]
- van der Goes van Naters, W.; Carlson, J.R. Insects as chemosensors of humans and crops. Nature 2006, 444, 302–307. [Google Scholar] [CrossRef]
- Wang, N.; Wang, N.X.; Niu, L.M.; Bian, S.N.; Xiao, J.H.; Huang, D.W. Odorant-binding protein (OBP) genes affect host specificity in a fig-pollinator mutualistic system. Insect Mol. Biol. 2014, 23, 621–631. [Google Scholar] [CrossRef]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in Insect olfaction: To smell or not to smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef]
- Morewood, W.D.; Neiner, P.R.; McNeil, J.R.; Sellmer, J.C.; Hooveret, K. Oviposition preference and larval performance of Anoplophora glabripennis (Coleoptera: Cerambycidae) in four eastern North American hardwood tree species. Environ. Entomol. 2003, 32, 1028–1034. [Google Scholar] [CrossRef]
- Mohammed, K.; Agarwal, M.; Li, B.; Newman, J.; Liu, T.; Ren, Y. Evaluation of R-(+)-limonene and β-Ocimene as Attractants of Aphytis melinus (Hymenoptera: Aphelinidae), a Parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp. Insects 2020, 11, 44. [Google Scholar] [CrossRef]
- Wang, J.J.; Gao, Q.; Li, C.Z.; Lou, Y.G. Molecular interactions between plants and phytophagous insects: Fundamentals and applications. Chin. J. Environ. Entomol. 2021, 43, 901–908. [Google Scholar] [CrossRef]
- Chénier, J.V.; Philogène, B.J. Field responses of certain forest Coleoptera to conifer monoterpenes and ethanol. J. Chem. Ecol. 1989, 15, 1729–1745. [Google Scholar] [CrossRef]
- Wang, T. Studies on the Biological Characters of Semanotus bifasciatus and Attractive Activity from Platycladus orientalis. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2005. [Google Scholar]
- Nehme, M.E.; Keena, M.A.; Zhang, A.; Baker, T.C.; Xu, Z.; Hoover, K. Evaluating the use of male-produced pheromone components and plant volatiles in two trap designs to monitor Anoplophora glabripennis. Environ. Entomol. 2010, 39, 169–176. [Google Scholar] [CrossRef]
- Zheng, K.W.; Wu, S.X.; Zhang, D.Y.; Wu, J.H.; Du, Y.B.; Fan, J.T. Differences in feeding and oviposition behavior of different populations of Batocera horsfieldi. J. Zhejiang. A&F Univ. 2022, 39, 159–165. [Google Scholar]
- Turlings, T.; Erb, M. Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Ning, T.; Fan, J.T.; Fang, Y.L.; Sun, J.H. Changes in contents of host volatile terpenes under different damaged states and electroantennogram response of Monochamus alternatus (Hope) to these volatiles. Acta Entomol. Sin. 2006, 49, 179–188. [Google Scholar] [CrossRef]
- Fatzinger, C.W.; Merkel, E.P. Oviposition and feeding preferences of the southern pine coneworm (Lepidoptera: Pyralidae) for different host-plant materials and observations on monoterpenes as an oviposition stimulant. J. Chem. Ecol. 1985, 11, 689–699. [Google Scholar] [CrossRef]
- Li, J.G.; Jin, Y.J.; Luo, Y.Q.; Xu, Z.C. Leaf volatiles for host tree Acer negundo: Diurnal rhythm and behavior responses of Anoplophora glabripennis to volatiles in field. Acta Bot Sin. 2003, 45, 177–182. [Google Scholar]
- Liu, F.H.; Wang, X.L.; Liu, T.; Wang, H.L. Biological characters and control of Batocera horsfieldi (Hope). J. Anhui Agric. Sci. 2014, 42, 7814–7816. [Google Scholar]
- Gregg, P.C.; del Socorro, A.P.; Henderson, G.S. Development of a synthetic plant volatiole-based attracticide for female noctuid moths. II. Bioassays of synthetic plant volatiles as attractants for the adults of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 21–30. [Google Scholar] [CrossRef]
- Moghbeli Gharaei, A.; Ziaaddini, M.; Frérot, B.; Nejad Ebrahimi, S.; Jalali, M.A.; Reddy, G.V.P. Identification and evaluation of four cucurbitaceous host plant volatiles attractive to Diaphania indica (Saunders) (Lep.: Pyralidae). Chemoecology 2020, 30, 173–182. [Google Scholar] [CrossRef]
- Mei, H.Z.; Xia, D.G.; Zhao, Q.L.; Zhang, G.Z.; Qiu, Z.Y.; Qian, P.; Lu, C. Molecular cloning, expression, purification and characterization of a novel cellulase gene (Bh-EGaseI) in the beetle Batocera horsfieldi. Gene 2016, 576 Pt 1, 45–51. [Google Scholar] [CrossRef]
- Zhong, J.P.; Xiao, X.Y.; Jin, M.X.; Tu, Y.G.; Xie, G.A.; Wang, W.H. A Bibliometric Analysis of Researches of Batocera horsfieldi. Biol. Disaster Sci. 2022, 45, 194–198. [Google Scholar]
- Wu, J.H.; Wu, L.P.; Zheng, K.W.; Gu, Y.T.; Fan, J.T. Electrophysiological and behavioral responses of Anoplophora chinensis (Coleoptera: Cerambycidae) to the volatiles from Salix ohsidare. Acta Entomol. Sin. 2022, 65, 1477–1487. [Google Scholar] [CrossRef]
- Hu, X.G.; Zhou, Q.X. Novel hydrated graphene ribbon unexpectedly promotes aged seed germination and root differentiation. Sci. Rep. 2014, 4(1), 3782. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, L.; Arman, D.; Duan, X.; Zhang, J.P.; Chen, J. Electrophysiological responses of the Monolept ahieroglyphica (motschulsky) to 13 volatile compounds. Xinjiang Agric. Sci. 2021, 58, 1282–1290. [Google Scholar]
- Anastasaki, E.; Drizou, F.; Milonas, P.G. Electrophysiological and Oviposition Responses of Tuta absoluta Females to Herbivore-Induced Volatiles in Tomato Plants. J. Chem. Ecol. 2018, 44, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejek, J.; Patykowski, J.; Wala, M. Effect of light, gibberellic acid and nitrogen source on germination of eight taxa from dissapearing European temperate forest, Potentillo albae—Quercetum. Sci. Rep. 2017, 7, 13924. [Google Scholar] [CrossRef] [PubMed]
- Skoczek, A.; Piesik, D.; Wenda-Piesik, A.; Buszewski, B.; Bocianowski, J.; Wawrzyniak, M. Volatile organic compounds released by maize following herbivory or insect extract application and communication between plants. J. Appl. Entomol. 2017, 141, 630–643. [Google Scholar] [CrossRef]
- Nehme, M.E.; Keena, M.A.; Zhang, A.; Baker, T.C.; Hoover, K. Attraction of Anoplophora glabripennis to male-produced pheromone and plant volatiles. Environ. Entomol. 2009, 38, 1745–1755. [Google Scholar] [CrossRef]
- Sweeney, J.; de Groot, P.; MacDonald, L.; Smith, S.; Cocquempot, C.; Kenis, M.; Gutowski, J.M. Host volatile attractants and traps for detection of Tetropium fuscum (F.), Tetropium castaneum L., And Other Longhorned Beetles (Coleoptera: Cerambycidae). Environ. Entomol. 2004, 33, 844–854. [Google Scholar] [CrossRef]
- Sun, X.L.; Wang, G.C.; Cai, X.M.; Jin, S.; Gao, Y.; Chen, Z.M. The tea weevil, Myllocerinus aurolineatus, is attracted to volatiles induced by conspecifics. J. Chem. Ecol. 2010, 36, 388–395. [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, H.H.; Li, J.K.; Zhang, R.; Turlings, T.C.; Chen, L. Volatiles released by Chinese liquorice roots mediate host location behaviour by neonate Porphyrophora sophorae (Hemiptera: Margarodidae). Pest Manag. Sci. 2016, 72, 1959–1964. [Google Scholar] [CrossRef]
- Blassioli-Moraes, M.C.; Michereff, M.F.F.; Magalhães, D.M.; Morais, S.D.; Hassemer, M.J.; Laumann, R.A.; Meneghin, A.M.; Birkett, M.A.; Withall, D.M.; Medeiros, J.N.; et al. Influence of constitutive and induced volatiles from mature green coffee berries on the foraging behaviour of female coffee berry borers, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae: Scolytinae). Arthropod-Plant Interact. 2019, 13, 349–358. [Google Scholar] [CrossRef]
- Wu, Y.H.; Heng, S.; Zhou, F.C.; Zhang, H.B.; Han, D.B. Repellent effect of celery volatiles on Bemisia tabaci. Chin. J. Environ. Entomol. 2019, 41, 900–907. [Google Scholar]
- Li, Z.Y.; Han, Y.; Tang, L.D.; Wu, J.H.; Ali, S. Behavioral responses of Megalurothrips usitatus (Thysanoptera: Thripoidae) to host plant and volatile compounds. Chin. J. Environ. Entomol. 2021, 43, 1566–1580. [Google Scholar]
- Tang, X.A.; Li, X.L.; Chi, Z.C. Tendency response of Diaphorina citri Kuwayama to shoots and volatile components of twelve host plants. Chin. J. Environ. Entomol. 2021, 43, 485–491. [Google Scholar]
- Allison, J.D.; Borden, J.H.; Seybold, S.J. A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 2004, 14, 123–150. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef]
- Fan, J.T.; Zhang, D.Y.; Zhang, Z.P.; Meng, J.G.; Wang, Y.P. Feeding behavior of Monochamus alternatus and its relationship with the host volatiles. J. Zhejiang A&F Univ. 2014, 31, 78–82. [Google Scholar]
- Li, G.W.; Lu, Y.; Chen, X.L.; Shang, T.C. Extraction and identification of host-plant volatiles of Acer mono and EAG responses of Anoplophora nobilis to the primary compounds of A. mono volatiles. J. Henan Norm. Univ. (Nat. Sci. Ed.) 2017, 45, 53–59. [Google Scholar]
- Fan, J.T.; Sun, J.H.; Shi, J. Attraction of the Japanese pine sawyer, Monochamus alternatus, to volatiles from stressed host in China. Ann. For. Sci. 2007, 64, 67–71. [Google Scholar] [CrossRef]
- Phillips, T.W.; Wilkening, A.J.; Atkinson, T.H.; Nation, J.L.; Wilkinson, R.C.; Foltz, J.L. Synergism of turpentine and ethanol as attractants for certain pine-infesting beetles (Coleoptera). Environ. Entomol. 1988, 17, 456–462. [Google Scholar] [CrossRef]
- Schroeder, L.M.; Lindelöw, Å. Attraction of scolytids and associated beetles by different absolute amounts and proportions of α-pinene and ethanol. J. Chem. Ecol. 1989, 15, 807–817. [Google Scholar] [CrossRef]
- Loughrin, J.H.; Manukian, A.; Heach, R.R.; Turlings, T.C.; Tumlinson, J.H. Diurnal cycle of emission of induced volatile terpe-noids by herbivore-injured cotton plants. Proc. Natl. Acad. Sci. USA 1994, 91, 11836–11840. [Google Scholar] [CrossRef]
- Bolter, C.J.; Dicke, M.; van Loon, J.J.A.; Visser, J.H.; Posthumus, M.A. Attraction of colorado potato beetle to herbivore damaged plants during herbivory and after its termination. J. Chem. Ecol. 1997, 23, 1003–1023. [Google Scholar] [CrossRef]
- Bian, L.; Cai, X.M.; Luo, Z.X.; Li, Z.Q.; Xin, Z.J.; Chen, Z.M. Design of an Attractant for Empoasca onukii (Hemiptera: Cicadellidae) Based on the Volatile Components of Fresh Tea Leaves. J. Econ. Entomol. 2018, 2, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Zhang, F.; Zhang, Z.N. Electrophysiological Responses and Reproductive Behavior of Fall Webworm Moths (Hyphantria cunea Drury) are Influenced by Volatile Compounds from Its Mulberry Host (Morus alba L.). Insects 2016, 7, 19. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Westlind, D.J. Attraction of red turpentine beetle and other Scolytinae to ethanol, 3-carene or ethanol + 3-carene in an Oregon pine forest. Agric. For. Entomol. 2018, 20, 272–278. [Google Scholar] [CrossRef]
- Chaaban, A.; Richardi, V.S.; Carrer, A.R.; Brum, J.S.; Cipriano, R.R.; Martins, C.E.N.; Navarro-Silva, M.A.; Deschamps, C.; Molento, M.B. Cuticular damage of Lucilia cuprina larvae exposed to Curcuma longa leaves essential oil and its major compound α-phellandrene. Data Brief 2018, 21, 1776–1778. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Papi, R.M.; Paraskevopoulou, A.; Achilias, D.S. Synthesis of R-(+)-limonene Loaded Polymeric Nanoparticles with Enhanced Antimicrobial Properties for Potential Application in Food Packaging. Nanomaterials 2021, 11, 191. [Google Scholar] [CrossRef]
- Corrêa, A.N.R.; Weimer, P.; Rossi, R.C.; Hoffmann, J.F.; Koester, L.S.; Suyenaga, E.S.; Ferreira, C.D. Lime and orange essential oils and R-(+)-limonene as a potential COVID-19 inhibitor: Computational, in chemico, and cytotoxicity analysis. Food Biosci. 2023, 51, 102348. [Google Scholar] [CrossRef]
- Yang, H.; Yang, W.; Yang, M.F.; Yang, C.P.; Zhu, T.; Huang, Q. Effects of Plant Volatiles on the EAG and Behavioral Responses of Batocera horsfieldi Hope (Coleoptera: Cerambycidae). J. Agric. Urban Entomol. 2010, 27, 20–32. [Google Scholar] [CrossRef]
- Switzer, P.V.; Enstrom, P.C.; Schoenick, C.A. Behavioral explanations underlying the lack of trap effectiveness for small-scale management of Japanese beetles (Coleoptera: Scarabaeidae). J. Econ. Entomol. 2009, 102, 934–940. [Google Scholar] [CrossRef]
| Chemicals | Purity% | Compound Source |
|---|---|---|
| Ethanol | Analytically pure 95% | Macklin Biochemical Technology, Shanghai, China |
| Acetone | Analytically pure 99.5% | J&K Scientific, Shanghai, China |
| n-Hexane | Analytically pure 95% | J&K Scientific, Shanghai, China |
| Paraffin oil | 99% | Hushi Laboratorial Equipment Co., Ltd., Shanghai, China |
| α-Pinene | 99% | J&K Scientific, Shanghai, China |
| β-Pinene | 98% | J&K Scientific, Shanghai, China |
| γ-Terpinene | 95% | J&K Scientific, Shanghai, China |
| β-Ocimene | 98% | J&K Scientific, Shanghai, China |
| (Z)-3-Hexen-1-ol | 98% | J&K Scientific, Shanghai, China |
| Myrcene | 95% | J&K Scientific, Shanghai, China |
| n-Dodecane | 99% | J&K Scientific, Shanghai, China |
| n-Hexane | 99% | J&K Scientific, Shanghai, China |
| Camphor | 98% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| D-limonene | 95% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 3-Carene | 90% | Acros Organics, Morris, NJ, USA |
| α-Phellandrene | 85% | Sigma-Aldrich, St. Louis, MO, USA |
| No. | Compounds | CAS no. | Retention Time (min) | Extraction Solution Concentration (μg /L) | |
|---|---|---|---|---|---|
| Healthy | Feeding-Damaged | ||||
| 1 | (Z)-3-Hexen-1-ol | 928-96-1 | 7.398 | - | 6.74 ± 2.46 |
| 2 | Styrene | 100-42-5 | 7.630 | 38.51 ± 7.47 | 41.17 ± 11.56 |
| 3 | α-Pinene | 80-56-8 | 8.424 | 10.39 ± 1.49 | 20.83 ± 4.04 * |
| 4 | 5-Methyl-3-hexen-2-one | 5166-53-0 | 9.105 | 1.39 ± 0.30 | - |
| 5 | β-Pinene | 18172-67-3 | 9.402 | 7.43 ± 1.76 | 15.12 ± 1.56 * |
| 6 | Myrcene | 123-35-3 | 9.996 | - | 89.23 ± 19.20 |
| 7 | 3-Thujene | 2867-5-2 | 10.082 | - | 0.23 ± 0.21 |
| 8 | α-Phellandrene | 99-83-2 | 10.110 | 1.61 ± 1.02 | 5.85 ± 1.35 * |
| 9 | 2-Carene | 554-61-0 | 10.736 | 4.95 ± 1.34 | 5.28 ± 1.80 |
| 10 | γ-Terpinene | 99-85-4 | 10.801 | - | 20.36 ± 4.37 |
| 11 | 3-Carene | 13466-78-9 | 10.288 | 8.9 ± 2.32 | - |
| 12 | D-limonene | 5989-27-5 | 10.779 | 25.04 ± 5.80 | 9.14 ± 2.25 * |
| 13 | (1S,3R)-(Z)-4-Carene | 5208-49-1 | 10.882 | - | 6.33 ± 1.33 |
| 14 | β-Ocimene | 3779-61-1 | 10.968 | 133.07 ± 24.13 | 66.58 ± 12.36 * |
| 15 | Camphor | 464-49-3 | 13.902 | 6.30 ± 1.32 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Zheng, K.; Xie, P.; Dong, Y.; Gu, Y.; Wickham, J.D. Electrophysiological and Behavioral Responses of Batocera horsfieldi Hope to Volatiles from Pistacia chinensis Bunge. Insects 2023, 14, 911. https://doi.org/10.3390/insects14120911
Fan J, Zheng K, Xie P, Dong Y, Gu Y, Wickham JD. Electrophysiological and Behavioral Responses of Batocera horsfieldi Hope to Volatiles from Pistacia chinensis Bunge. Insects. 2023; 14(12):911. https://doi.org/10.3390/insects14120911
Chicago/Turabian StyleFan, Jianting, Kaiwen Zheng, Ping Xie, Yifan Dong, Yutong Gu, and Jacob D. Wickham. 2023. "Electrophysiological and Behavioral Responses of Batocera horsfieldi Hope to Volatiles from Pistacia chinensis Bunge" Insects 14, no. 12: 911. https://doi.org/10.3390/insects14120911
APA StyleFan, J., Zheng, K., Xie, P., Dong, Y., Gu, Y., & Wickham, J. D. (2023). Electrophysiological and Behavioral Responses of Batocera horsfieldi Hope to Volatiles from Pistacia chinensis Bunge. Insects, 14(12), 911. https://doi.org/10.3390/insects14120911






