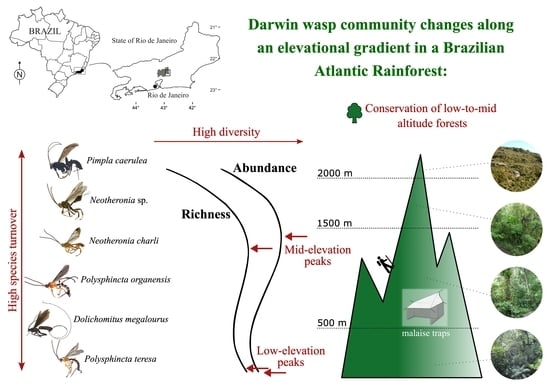
Variation in a Darwin Wasp (Hymenoptera: Ichneumonidae) Community along an Elevation Gradient in a Tropical Biodiversity Hotspot: Implications for Ecology and Conservation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
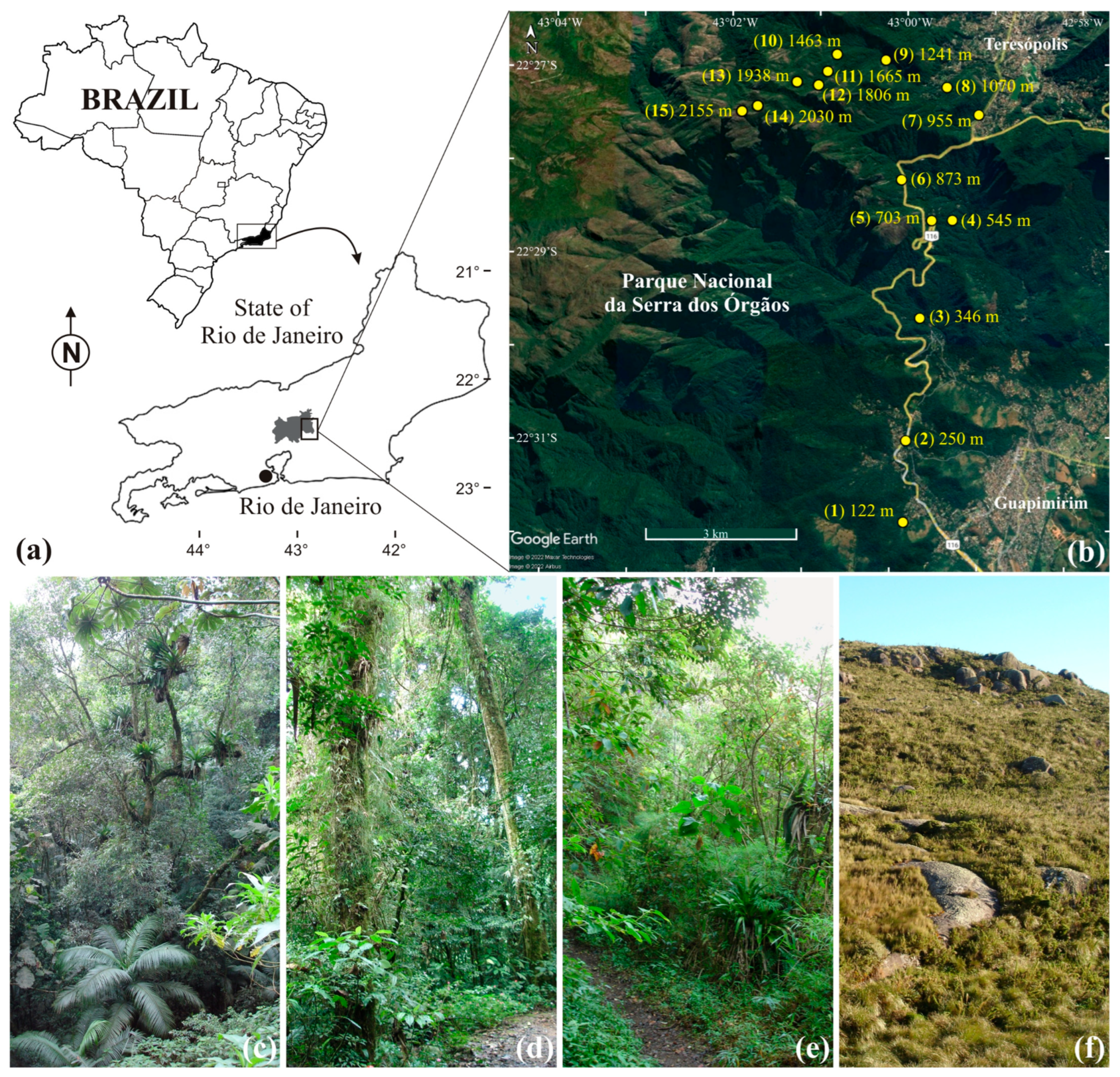
2.1. Study Site
2.2. Study Species
2.3. Pimplinae Collections
2.4. Environmental Variables
2.5. Analysis
3. Results
3.1. Species Richness
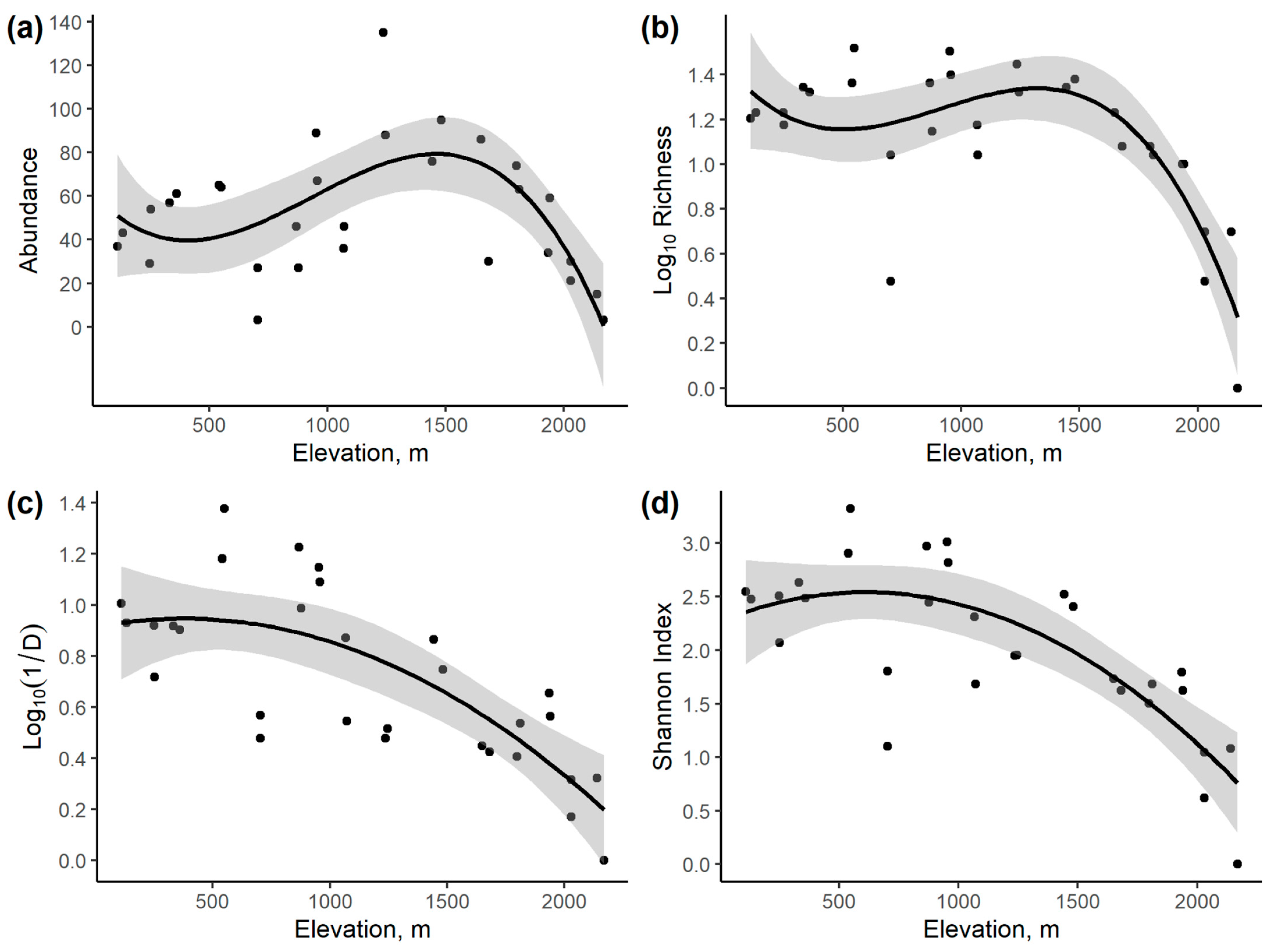
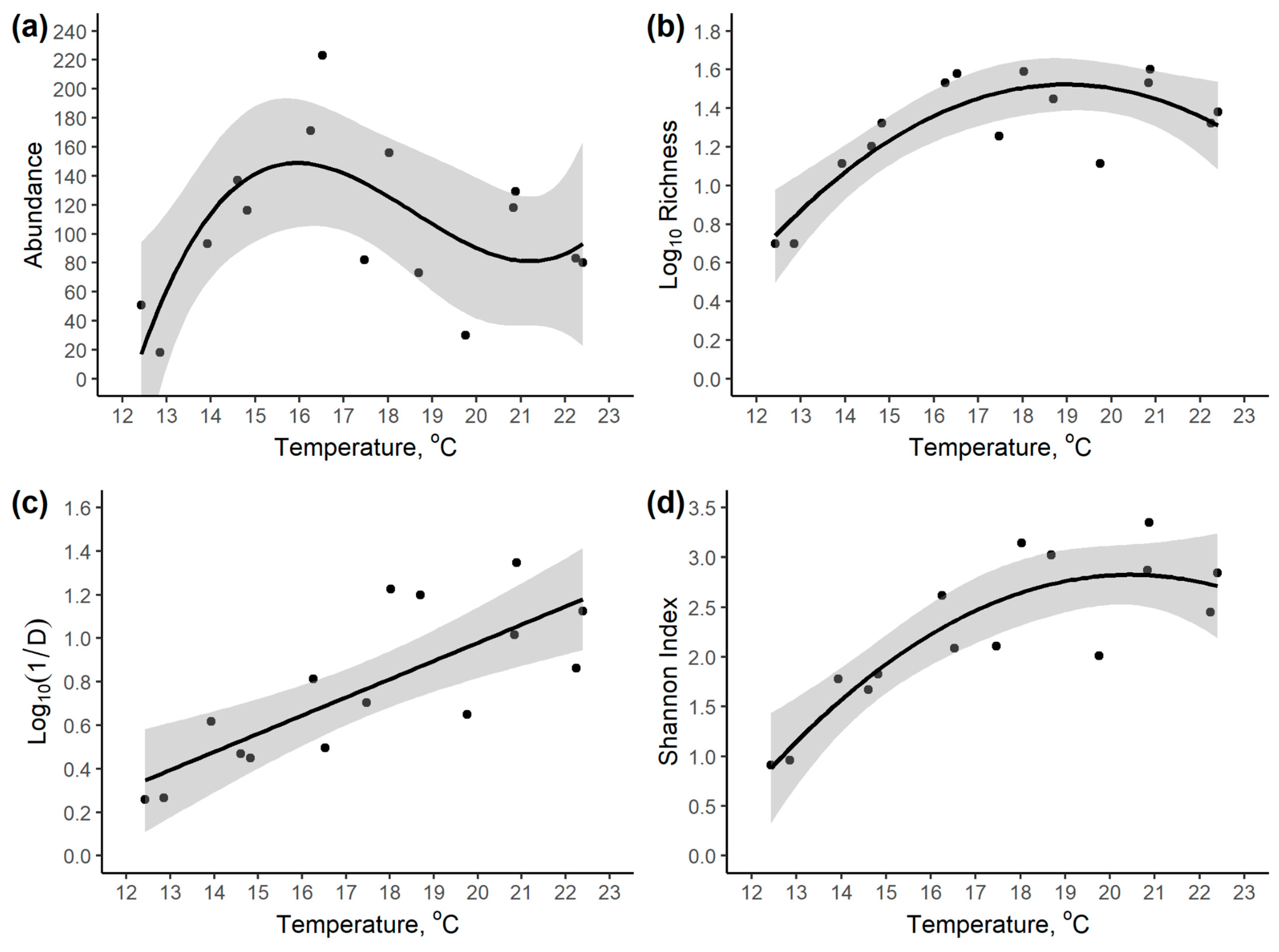
3.2. Community Variation with Elevation and Temperature
3.3. Associations between Pimpline Community and Other Habitat Properties
3.4. Vegetation Predictors of the Pimpline Community
4. Discussion
4.1. Effect of Latitude
4.2. Effect of Elevation and Temperature
4.3. Effects of Vegetation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.; Chan, K.M.; et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T.; Hudson, L.N.; Arnell, A.P.; Contu, S.; De Palma, A.; Ferrier, S.; Hill, S.L.; Hoskins, A.J.; Lysenko, I.; Phillips, H.R.; et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 2016, 353, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Tittensor, D.P.; Walpole, M.; Hill, S.L.; Boyce, D.G.; Britten, G.L.; Burgess, N.D.; Butchart, S.H.; Leadley, P.W.; Regan, E.C.; Alkemade, R.; et al. A mid-term analysis of progress toward international biodiversity targets. Science 2014, 346, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Brondizio, E.S.; Settele, J.; Díaz, S.; Ngo, H.T. (Eds.) Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; 1148p. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Freckleton, R.P.; Godfray, H.C.J.; Beissinger, S.R.; Benton, T.; Cameron, D.D.; Carmel, Y.; Coomes, D.A.; Coulson, T.; Emmerson, M.C.; et al. Identification of 100 fundamental ecological questions. J. Ecol. 2013, 101, 58–67. [Google Scholar] [CrossRef]
- Gaston, K. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Tittensor, D.P.; Mora, C.; Jetz, W.; Lotze, H.K.; Ricard, D.; Berghe, E.V.; Worm, B. Global patterns and predictors of marine biodiversity across taxa. Nature 2010, 466, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Worm, B.; Tittensor, D.P. A Theory of Global Biodiversity; Princeton University Press: Princeton, NJ, USA, 2018; 232p. [Google Scholar]
- Boakes, E.H.; McGowan, P.J.; Fuller, R.A.; Chang-qing, D.; Clark, N.E.; O’Connor, K.; Mace, G.M. Distorted views of biodiversity: Spatial and temporal bias in species occurrence data. PLoS Biol. 2010, 8, e1000385. [Google Scholar] [CrossRef]
- Clark, J.A.; May, R.M. Taxonomic bias in conservation research. Science 2002, 297, 191–192. [Google Scholar] [CrossRef]
- McRae, L.; Deinet, S.; Freeman, R. The diversity-weighted living planet index: Controlling for taxonomic bias in a global biodiversity indicator. PLoS ONE 2017, 12, e0169156. [Google Scholar] [CrossRef]
- Amano, T.; Lamming, J.D.L.; Sutherland, W.J. Spatial gaps in global biodiversity information and the role of citizen science. BioScience 2016, 66, 393–400. [Google Scholar] [CrossRef]
- Troudet, J.; Grandcolas, P.; Blin, A.; Vignes-Lebbe, R.; Legendre, F. Taxonomic bias in biodiversity data and societal preferences. Sci. Rep. 2017, 7, 9132. [Google Scholar] [CrossRef]
- Klopfstein, S.; Santos, B.F.; Shaw, M.R.; Alvarado, M.; Bennett, A.M.; Dal Pos, D.; Giannotta, M.; Herrera Florez, A.F.; Karlsson, D.; Khalaim, A.I.; et al. Darwin wasps: A new name heralds renewed efforts to unravel the evolutionary history of Ichneumonidae. Entomol. Commun. 2019, 1, ec01006. [Google Scholar] [CrossRef]
- Butchart, S.H.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.; Almond, R.E.; Baillie, J.E.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity, indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T.; Bentley, L.F.; Hill, S.L.L.; Edgar, M.J.; Horton, M.; Su, G.; Şekercioğlu, Ç.H.; Collen, B.; Purvis, A. Global effects of land use on biodiversity differ among functional groups. Funct. Ecol. 2020, 34, 684–693. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Essl, F.; Lenzner, B.; Bacher, S.; Bailey, S.; Capinha, C.; Daehler, C.; Dullinger, S.; Genovesi, P.; Hui, C.; Hulme, P.E.; et al. Drivers of future alien species impacts: An expert-based assessment. Glob. Chang. Biol. 2020, 26, 4880–4893. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.; Watson, J.E.M. Biodiversity: The ravages of guns, nets and bulldozers. Nature 2016, 436, 143–145. [Google Scholar] [CrossRef]
- Beier, P.; Sutcliffe, P.; Hjort, J.; Faith, D.P.; Pressey, R.L.; Albuquerque, F. A review of selection-based tests of abiotic surrogates for species representation. Conserv. Biol. 2015, 29, 668–679. [Google Scholar] [CrossRef]
- Hughes, A.C.; Orr, M.C.; Yang, Q.; Qiao, H. Effectively and accurately mapping global biodiversity patterns for different regions and taxa. Glob. Ecol. Biogeogr. 2021, 30, 1375–1388. [Google Scholar] [CrossRef]
- Wiens, J.A.; Hayward, G.D.; Holthausen, R.S.; Wisdom, M.J. Using surrogate species and groups for conservation planning and management. BioScience 2008, 58, 241–252. [Google Scholar] [CrossRef]
- Kukkala, A.S.; Moilanen, A. Core concepts of spatial prioritisation in systematic conservation planning. Biol. Rev. 2013, 88, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Sumner, S. Endless Forms: The Secret World of Wasps; Harper Collins: London, UK, 2022; 400p. [Google Scholar]
- Bánki, O.; Roskov, Y.; Döring, M.; Ower, G.; Vandepitte, L.; Hobern, D.; Remsen, D.; Schalk, P.; DeWalt, R.E.; Keping, M.; et al. Catalogue of Life Checklist; Version 2022-12-19; Catalogue of Life: Leiden, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Forbes, A.A.; Bagley, R.K.; Beer, M.A.; Hippie, A.C.; Widmayer, H.A. Quantifying the unquantifiable: Why Hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecol. 2018, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J. Parasitoids, Behavioural and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; 488p. [Google Scholar]
- Quicke, D.L.J. Parasitic Wasps; Chapman and Hall: London, UK, 1997; 470p. [Google Scholar]
- Pinto, C.P.G. Parasitoid wasps as biological control agents: A review. J. Entomol. Zool. 2020, 8, 967–971. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Shi, M.; Huang, J.; Chen, X. Parasitoid wasps as effective biological control agents. J. Integr. Agric. 2019, 18, 705–715. [Google Scholar] [CrossRef]
- Hochberg, M.E. What, conserve parasitoids? In Parasitoid Population Biology; Hochberg, M.E., Ives, A.R., Eds.; Princeton University Press: Princeton, NJ, USA, 2000; pp. 266–277. [Google Scholar]
- Shaw, M.R.; Hochberg, M.E. The neglect of parasitic Hymenoptera in insect conservation strategies: The British fauna as a prime example. J. Insect Conserv. 2001, 5, 253–263. [Google Scholar] [CrossRef]
- Kruess, A.; Tscharntke, T. Habitat fragmentation, species loss, and biological control. Science 1994, 264, 1581–1584. [Google Scholar] [CrossRef]
- Stireman, J.O., III; Dyer, L.A.; Janzen, D.H.; Singer, M.S.; Lill, J.T.; Marquis, R.J.; Ricklefs, R.E.; Gentry, G.L.; Hallwachs, W.; Coley, P.D.; et al. Climatic unpredictability and parasitism of caterpillars: Implications of global warming. Proc. Natl. Acad. Sci. USA 2005, 102, 17384–17387. [Google Scholar] [CrossRef]
- Favreau, J.; Drew, C.; Hess, G.; Rubino, M.; Koch, F.; Eschelbach, K.A. Recommendations for assessing the effectiveness of surrogate species approaches. Biodivers. Conserv. 2006, 15, 3949–3969. [Google Scholar] [CrossRef]
- Rodriques, T.M.; Brooks, T.M. Shortcuts for biodiversity conservation planning: The effectiveness of surrogates. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 13–37. [Google Scholar] [CrossRef]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef] [PubMed]
- Quicke, D.L.J. We know too little about parasitoid wasp distributions to draw any conclusions about latitudinal trends in species richness, body size and biology. PLoS ONE 2012, 7, e32101. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.H. The peak in North American ichneumonid species richness lies between 38 degrees and 42 degrees N. Ecology 1981, 62, 532–537. [Google Scholar] [CrossRef]
- Timms, L.L.; Schwarzfeld, M.; Sääksjärvi, I.E. Extending understanding of latitudinal patterns in parasitoid wasp diversity. Insect Conserv. Divers. 2016, 9, 74–86. [Google Scholar] [CrossRef]
- Santos, A.M.C.; Quicke, D.L.J. Large-scale diversity patterns of parasitoid insects: Parasitoid diversity patterns. Entomol. Sci. 2011, 14, 371–382. [Google Scholar] [CrossRef]
- Sääksjärvi, I.E.; Haataja, S.; Neuvonen, S.; Gauld, I.D.; Jussila, R.; Salo, J.; Marmol Burgos, A. High local species richness of parasitic wasps (Hymenoptera: Ichneumonidae; Pimplinae and Rhyssinae) from the lowland rainforests of Peruvian Amazonia. Ecol. Entomol. 2004, 29, 735–743. [Google Scholar] [CrossRef]
- Goméz, I.C.; Saaksjarvi, I.E.; Mayhew, P.J.; Pollet, M.; Rey del Castillo, C.; Nieves-Aldrey, J.; Broad, G.R.; Roininen, H.; Tuomisto, H. Variation in the species richness of parasitoid wasps (Ichneumonidae: Pimplinae and Rhyssinae) across sites on different continents. Insect Conserv. Divers. 2018, 11, 305–316. [Google Scholar] [CrossRef]
- Hopkins, T.; Roininen, H.; van Noort, S.; Broad, G.R.; Kaunisto, K.; Sääksjärvi, I.E. Extensive sampling and thorough taxonomic assessment of Afrotropical Rhyssinae (Hymenoptera, Ichneumonidae) reveals two new species and demonstrates the limitations of previous sampling efforts. ZooKeys 2019, 878, 33–71. [Google Scholar] [CrossRef]
- Smith, M.A.; Rodriguez, J.J.; Whitfield, J.B.; Deans, A.R.; Janzen, D.H.; Hallwachs, W.; Hebert, P.D.N. Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proc. Natl. Acad. Sci. USA 2008, 105, 12359–12364. [Google Scholar] [CrossRef]
- Veijalainen, A.; Wahlberg, N.; Broad, G.R.; Erwin, T.L.; Longino, J.T.; Sääksjärvi, I.E. Unprecedented ichneumonid parasitoid wasp diversity in tropical forests. Proc. R. Soc. B 2012, 279, 4694–4698. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Blaschke, M.; Bässler, C. Altitudinal gradients in biodiversity research: The state of the art and future perspectives under climate change aspects. Wald. Landschaftsforschung Naturschutz 2011, 8, 35–47. [Google Scholar]
- McCain, C.M.; Grytnes, J.-A. Elevational Gradients in Species Richness. In Encyclopedia of Life Sciences (ELS); John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 1–10. [Google Scholar] [CrossRef]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns: Scale and species-richness patterns. Ecol. Lett. 2004, 8, 224–239. [Google Scholar] [CrossRef]
- Noyes, J.S. The diversity of Hymenoptera in the tropics with special reference to Parasitica in Sulawesi. Ecol. Entomol. 1989, 14, 197–207. [Google Scholar] [CrossRef]
- Van Noort, S. Ichneumonid (Hymenoptera: Ichneumonoidea) diversity across an elevational gradient on Monts Doudou in southwestern Gabon. Mem. Calif. Acad. Sci. 2004, 28, 187–216. [Google Scholar]
- Veijalainen, A.; Sääksjärvi, I.E.; Tuomisto, H.; Broad, G.R.; Bordera, S.; Jussila, R. Altitudinal trends in species richness and diversity of Mesoamerican parasitoid wasps (Hymenoptera: Ichneumonidae). Insect Conserv. Divers. 2014, 7, 496–507. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Pucci, T.M.; Hanula, J.L. The importance of forest type, tree species and wood posture to saproxylic wasp (Hymenoptera) communities in the southeastern United States. J. Insect Conserv. 2011, 15, 539–546. [Google Scholar] [CrossRef]
- Pak, D.; Iverson, A.L.; Ennis, K.K.; Gonthier, D.J.; Vandermeer, J.H. Parasitoid wasps benefit from shade tree size and landscape complexity in Mexican coffee agroecosystems. Agric. Ecosyst. Environ. 2015, 206, 21–32. [Google Scholar] [CrossRef]
- Peck, R.W.; Banko, P.C.; Schwarzfeld, M.; Euaparadorn, M.; Brinck, K.W. Alien dominance of the parasitoid wasp community along an elevation gradient on Hawai’i Island. Biol. Invasions 2008, 10, 1441–1455. [Google Scholar] [CrossRef]
- Mumladze, L.; Ulrich, W.; Asanidze, Z.; Japoshvili, G. An inverse elevational species richness gradient of Caucasian vascular plants and Encyrtidae (Hymenoptera, Chalcidoidea). Ecoscience 2017, 24, 75–79. [Google Scholar] [CrossRef]
- Aguirre, H.; Shaw, S.R.; Rodriguez-Jimenez, A. Contrasting patterns of altitudinal distribution between parasitoid wasps of the subfamilies Braconinae and Doryctinae (Hymenoptera: Braconidae). Insect Conserv. Divers. 2018, 11, 219–229. [Google Scholar] [CrossRef]
- Hall, C.R.; Burwell, C.J.; Nakamura, A.; Kitching, R.L. Altitudinal variation of parasitic Hymenoptera assemblages in Australian subtropical rainforest. Austral Entomol. 2014, 54, 246–258. [Google Scholar] [CrossRef]
- Macedo, M.V.; Monteiro, R.F.; Flinte, V.; Almeida-Neto, M.; Khattar, G.; da Silveira, L.F.L.; Araújo, C.d.O.; Araújo, R.d.O.; Colares, C.; Gomes, C.V.S.; et al. Insect elevational specialization in a tropical biodiversity hotspot. Insect Conserv. Divers. 2018, 11, 240–254. [Google Scholar] [CrossRef]
- Higa, P.T.; Penteado-Dias, A.M. Altitudinal effects of on diversity of Pimplinae (Hymenoptera: Ichneumonidae) from Southeast Brazil and description of new species. Braz.J. Biol. 2020, 80, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Arnan, X.; Bosch, J.; Comas, L.; Gracia, M.; Retana, J. Habitat determinants of abundance, structure and composition of flying Hymenoptera communities in mountain old-growth forests. Insect Conserv. Divers. 2011, 4, 200–211. [Google Scholar] [CrossRef]
- Fraser, S.E.M.; Dytham, C.; Mayhew, P.J. Determinants of parasitoid abundance and diversity in woodland habitats. J. Appl. Ecol. 2007, 44, 352–361. [Google Scholar] [CrossRef]
- Kendall, L.K.; Ward, D.F. Habitat determinants of the taxonomic and functional diversity of parasitoid wasps. Biodivers. Conserv. 2016, 25, 1955–1972. [Google Scholar] [CrossRef]
- Mazon, M.; Bordera, S. Diversity of Ichneumonidae (Insecta: Hymenoptera) in a protected area of Central Spain: What are we protecting? Insect Conserv. Divers. 2014, 7, 432–452. [Google Scholar] [CrossRef]
- Lassau, S.A.; Hochuli, D.F. Wasp community responses to habitat complexity in Sydney sandstone forests. Austral Ecol. 2005, 30, 179–187. [Google Scholar] [CrossRef]
- Lassau, S.A.; Hochuli, D.F. Associations between wasp communities and forest structure: Do strong local patterns hold across landscapes? Austral Ecol. 2007, 32, 656–662. [Google Scholar] [CrossRef]
- Szczepko, K.; Kruk, A.; Bartos, M.; Wisniowski, B. Factors influencing the diversity of cuckoo wasps (Hymenoptera: Chrysididae) in the post-agriculture area of the Kampinos National Park, Poland. Insect Conserv. Divers. 2013, 6, 339–353. [Google Scholar] [CrossRef]
- Piekarska-Boniecka, H.; Mazur, R.; Wagner, A.; Trzcinski, P. Selected elements of cultural landscape structure in Wielkopolska region of Poland as habitats for the parasitoid hymenoptera Pimplinae (Hymenoptera, Ichneumonidae). Insect Conserv. Divers. 2015, 8, 54–70. [Google Scholar] [CrossRef]
- Sperber, C.F.; Nakayama, K.; Valverde, M.J.; Neves, F.S. Tree species richness and density affect parasitoid diversity in cacao agroforestry. Basic Appl. Ecol. 2004, 3, 241–251. [Google Scholar] [CrossRef]
- Loyola, R.D.; Martins, R.P. Habitat structure components are effective predictors of trap-nesting Hymenoptera diversity. Basic Appl. Ecol. 2008, 9, 735–742. [Google Scholar] [CrossRef]
- Fabian, Y.; Sandau, N.; Bruggisser, O.T.; Aebi, A.; Kehrli, P.; Rohr, R.P.; Naisbit, R.E.; Bersier, L.-F. Plant diversity in a nutshell: Testing for small-scale effects on trap nesting wild bees and wasps. Ecosphere 2014, 5, 18. [Google Scholar] [CrossRef]
- Piekarska-Boniecka, H.; Zyprych-Walczak, J.; Rzanska-Wieczorek, M.; Siatkowski, I. The number and abundance of Ichneumonidae (Hymenoptera, Apocrita) subfamilies occurring in apple orchards and on their edges. Acta Sci. Pol. Hortorum Cultus 2018, 17, 93–103. [Google Scholar] [CrossRef]
- Sääksjärvi, I.E.; Ruokolainen, K.; Tuomisto, H.; Haataja, S.; Fine, P.V.A.; Cárdenas, G.; Mesones, I.; Vargas, V. Comparing composition and diversity of parasitoid wasps and plants in an Amazonian rain-forest mosaic. J. Trop. Ecol. 2006, 22, 167–176. [Google Scholar] [CrossRef]
- Fraser, S.E.M.; Beresford, A.E.; Peters, J.; Redhead, J.W.; Welch, A.J.; Mayhew, P.J.; Dytham, C. Effectiveness of vegetation surrogates for parasitoid wasps in reserve selection. Conserv. Biol. 2009, 23, 142–150. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots; Zachos, F., Habel, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Safford, H.D. Brazilian Páramos, I. An introduction to the physical environment and vegetation of the Campos de altitude. J. Biogeogr. 1999, 26, 693–712. [Google Scholar] [CrossRef]
- Rezende, C.L.; Scarano, F.R.; Assad, E.D.; Joly, C.A.; Metzger, J.P.; Strassburg, B.B.N.; Tabarelli, M.; Fonseca, G.A.; Mittermeier, R.A. From hotspot to hopespot: An opportunity for the Brazilian Atlantic forest. Perspect. Ecol. Conserv. 2018, 16, 208–214. [Google Scholar] [CrossRef]
- Scarano, F.R.; Ceotto, P. Brazilian Atlantic forest: Impact, vulnerability, and adaptation to climate change. Biodivers. Conserv. 2015, 24, 2319–2331. [Google Scholar] [CrossRef]
- Silveira, L.F.L.; Khattar, G.; Vaz, S.; Wilson, V.A.; Souto, P.M.; Mermudes, J.R.M.; Stanger-Hall, K.F.; Macedo, M.V.; Monteiro, R.F. Natural history of the fireflies of the Serra dos Órgãos mountain range (Brazil: Rio de Janeiro)—One of the ‘hottest’ firefly spots on Earth, with a key to genera (Coleoptera: Lampyridae). J. Nat. Hist. 2020, 54, 275–308. [Google Scholar] [CrossRef]
- Castro, E.B.V. Plano de Manejo do Parque Nacional da Serra dos Órgão; ICMBio: Brasilia, Brazil, 2008; 371p. [CrossRef]
- Rizzini, C.T. Flora Organensis: Lista preliminar das Cormophyta da Serra dos Órgãos. Arch. Jard. Bot. 1954, 13, 115–259. [Google Scholar]
- Veloso, H.P.; Rangel Filho, A.L.R.; Lima, J.C.A. Classificação da Vegetação Brasileira, Adaptada a um Sistema Universal; Fundação Instituto Brasileiro de Geografia e Estatística, Departamento de Recursos Naturais e Estudos Ambientais: Rio de Janeiro, Brazil, 1991; 124p. [Google Scholar]
- Colares, C.; Roza, A.S.; Mermudes, J.R.M.; Silveira, L.F.L.; Khattar, G.; Mayhew, P.J.; Monteiro, R.F.; Nunes, M.F.S.Q.C.; Macedo, M.V. Elevational specialization and the monitoring of the effects of climate change in insects: Beetles in a Brazilian rainforest mountain. Ecol. Indic. 2021, 120, 106888. [Google Scholar] [CrossRef]
- Gauld, I.D. The Ichneumonidae of Costa Rica, 1. Mem. Am. Entomol. Inst. 1991, 47, 1–589. [Google Scholar]
- Aguiar, A.; Deans, A.R.; Engel, M.S.; Forshage, M.; Huber, J.T.; Jennings, J.T.; Johnson, N.F.; Lelej, A.S.; Longino, J.T.; Lohrmann, V.; et al. Hymenoptera. In: Animal Biodiversity: An outline of higher-level classification and survey of taxonomic richness (Addenda 2013); Z.-Q. Zhang Ed. Zootaxa 2013, 3703, 51–62. [Google Scholar] [CrossRef]
- Quicke, D.L.J. The Braconid and Ichneumonid Parasitoid Wasps: Biology, Systematics, Evolution and Ecology; John Wiley & Sons: Chichester, UK, 2015; 675p. [Google Scholar]
- Broad, G.R.; Shaw, M.R.; Fitton, M.G. Ichneumonid Wasps (Hymenoptera: Ichneumonoidea): Their Classification and Biology; RES Handbooks for the Identification of British Insects; Field Studies Council: Shrewsbury, UK, 2018; Volume 7, 418p. [Google Scholar]
- Gauld, I.D.; Dubois, J. Phylogeny of the Polysphincta group of genera (Hymenoptera: Ichneumonidae; Pimplinae): A taxonomic revision of spider ectoparasitoids. Syst. Entomol. 2006, 31, 529–564. [Google Scholar] [CrossRef]
- Fraser, S.E.M.; Dytham, C.; Mayhew, P.J. The effectiveness and optimal use of Malaise traps for monitoring parasitoid wasps. Insect Conserv. Divers. 2008, 1, 22–31. [Google Scholar] [CrossRef]
- Oliver, I.; Beattie, A.J. Invertebrate morphospecies as surrogates for species: A case study. Conserv. Biol. 1996, 10, 99–109. [Google Scholar] [CrossRef]
- Derraik, J.G.; Closs, J.G.P.; Dickinson, K.J.; Sirvid, P.; Barratt, B.I.; Patrick, B.H. Arthropod morphospecies versus taxonomic species: A case study with Araneae, Coleoptera, and Lepidoptera. Conserv. Biol. 2002, 16, 1015–1023. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-2. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 April 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2002; Available online: https://www.R-project.org/ (accessed on 1 April 2022).
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; 256p. [Google Scholar]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, R.B. Species richness estimators: How many species can dance on the head of a pin? J. Anim. Ecol. 2005, 74, 375–386. [Google Scholar] [CrossRef]
- Chiu, C.H.; Wang, Y.T.; Walther, B.A.; Chao, A. Improved nonparametric lower bound of species richness via a modified Good-Turing frequency formula. Biometrics 2014, 70, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference a Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; 488p. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.46.0. 2022. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 1 April 2022).
- Tanque, R.L.; Frieiro-Costa, F.A. Pimplinae (Hymenoptera, Ichneumonidae) em um fragmento de Cerrado na Reserva Biológica Unilavras/Boqueirão, Ingaí, Minas Gerais, Brasil. Biota Neotrop. 2011, 11, 169–171. [Google Scholar] [CrossRef]
- Gaston, K.J.; Gauld, I.D. How many species of Pimplines (Hymenoptera: Ichneumonidae) are there in Costa Rica? J. Trop. Ecol. 1993, 9, 491–499. [Google Scholar] [CrossRef]
- Idris, A.B.; Hainidah, J. Diversity of ichneumonid wasps in the logged over forest of Langat Basin in Selangor, Malaysia. J. Biol. Sci. 2003, 3, 259–270. [Google Scholar] [CrossRef]
- Eagalle, T.; Smith, M.A. Diversity of parasitoid and parasitic wasps across a latitudinal gradient: Using public DNA records to work within a taxonomic impediment. FACETS 2017, 2, 937–954. [Google Scholar] [CrossRef]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Broad, G. Checklist of British and Irish Hymenoptera—Ichneumonidae. Biodivers. Data J. 2016, 4, e9042. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.R.; Purvis, A.; Baumgart, E.; Quicke, D.L.J. Using taxonomic revision data to estimate the geographic and taxonomic distribution of undescribed species richness in the Braconidae (Hymenoptera: Ichneumonoidea). Insect Conserv. Divers. 2009, 2, 204–212. [Google Scholar] [CrossRef]
- Idris, A.B.; Nor Zaneedarwaty, N.; Gonzaga, A.D.; Zaidi, M.I.; Azman, S.; Salmah, Y. A study on four methods of sampling Ichneumonidae and Braconidae at two different habitats of Fraser’s Hill, Malaysia. Pak. J. Biol. Sci. 2001, 4, 1515–1517. [Google Scholar] [CrossRef][Green Version]
- Li, T.; Sheng, M.-L.; Sun, S.-P.; Chen, G.-F.; Guo, Z.-H. Effect of the trap color on the capture of ichneumonids wasps (Hymenoptera). Rev. Colom. Entomol. 2012, 38, 338–342. [Google Scholar] [CrossRef]
- Mazón, M.; Bordera, S. Effectiveness of two sampling methods used for collecting Ichneumonidae (Hymenoptera) in the Cabañeros National Park (Spain). Eur. J. Entomol. 2008, 105, 879–888. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M. Pattern and Process in Macroecology; Blackwell Scientific Publications: Oxford, UK, 2000; 392p. [Google Scholar] [CrossRef]
- Sanders, N.J. Elevational gradients in ant species rich-ness: Area, geometry, and Rapoport’s rule. Ecography 2002, 25, 25–32. [Google Scholar] [CrossRef]
- Trigas, P.; Panitsa, M.; Tsiftsis, S. Elevational gradient of vascular plant species richness and endemism in Crete—The effect of post-isolation mountain uplift on a continental island system. PLoS ONE 2013, 8, e59425. [Google Scholar] [CrossRef]
- McCain, C.M. The mid-domain effect applied to elevational gradients: Species richness of small mammals in Costa Rica. J. Biogeogr. 2004, 31, 19–31. [Google Scholar] [CrossRef]
- Khattar, G.; Vaz, S.; Braga, P.H.P.; Macedo, M.; Silveira, L.F.L. Life history traits modulate the influence of environmental stressors on biodiversity: The case of fireflies, climate and artificial light at night. Divers. Distrib. 2022, 28, 1820–1831. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Hawkins, B.A. Pattern and Process in Host-Parasitoid Communities; Cambridge University Press: Cambridge, UK, 1994; 190p. [Google Scholar] [CrossRef]
- Korenko, S.; Sýkora, J.; Černecká, Ľ.; Gajdoš, P.; Purgat, P.; Černecký, J.; Holý, K.; Heneberg, P.; Agnarsson, I. Elevation gradient affects the distribution and host utilisation of Zatypota anomala (Hymenoptera, Ichneumonidae) associated with mesh web weaving spiders (Araneae, Dictynidae). J. Hymenopt. Res. 2022, 93, 89–100. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
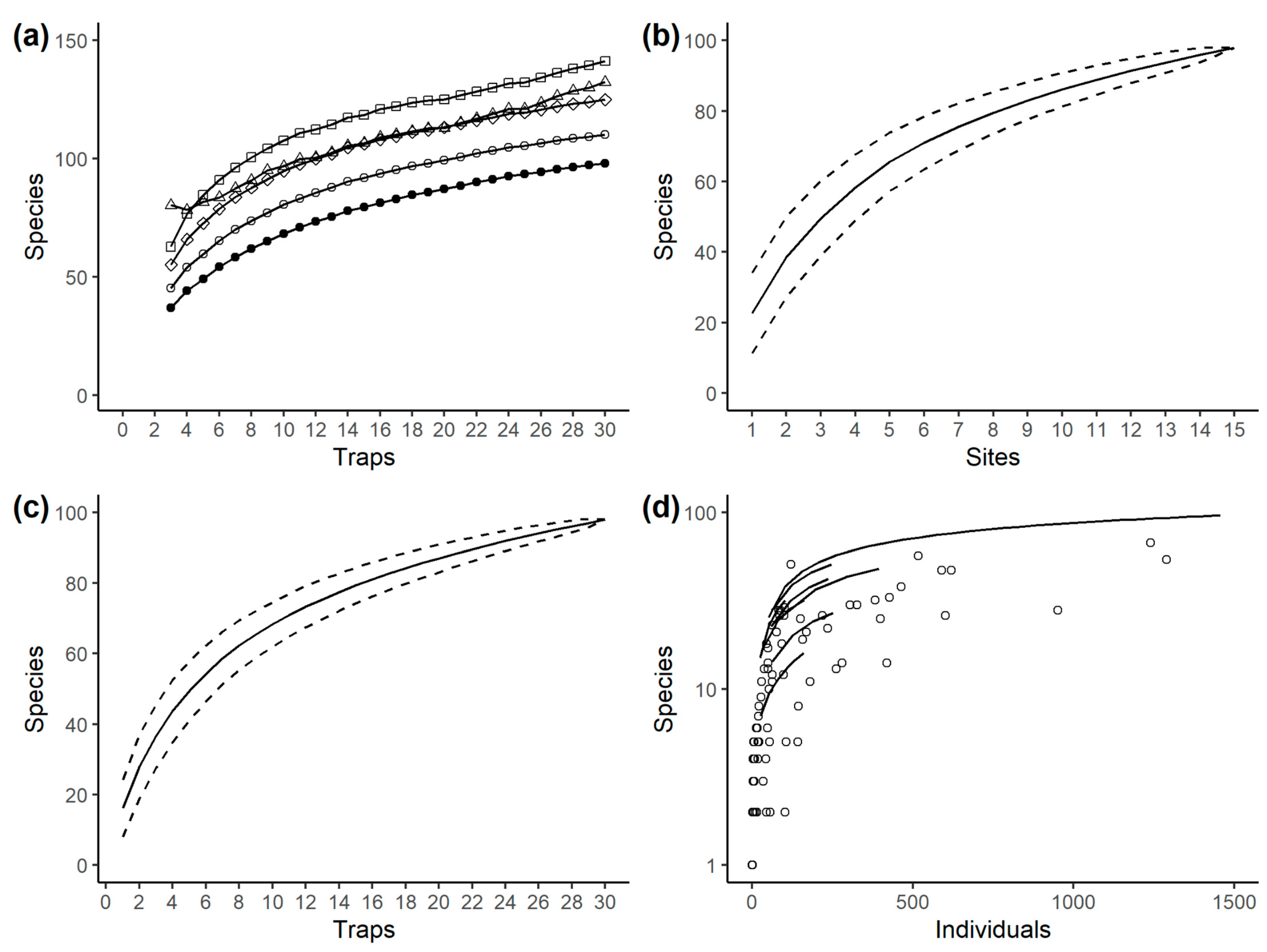



| Method | Trap Level Data (n = 30) | Site Level Data (n = 15) |
|---|---|---|
| Observed richness | 98 | 98 |
| Chao | 132 ± 18 | 132 ± 17 |
| First-order jackknife | 125 ± 8 | 127 ± 11 |
| Second-order jackknife | 141 | 143 |
| Bootstrap | 110 ± 5 | 111 ± 6 |
| Bias-corrected Chao | 123 ± 13 | |
| ACE | 122 ± 6 | |
| Response Variable | Intercept | Elevation | Elevation2 | Elevation3 | Elevation4 | Model r2 | AICc |
|---|---|---|---|---|---|---|---|
| Abundance | 54.90 * | −0.00254 | - | - | - | 0.003 | 294.05 |
| 19.60 | 0.0882 * | −3.946 × 10−5 * | - | - | 0.238 | 288.66 | |
| 62.10 * | −0.1217 | −1.912 × 10−4 * | −6.835 × 10−8 * | - | 0.424 | 283.20 | |
| 55.75 | −7.606 × 10−2 | 1.073 × 10−4 | −1.270 × 10−8 | −1.211 × 10−11 | 0.425 | 286.26 | |
| Log10 Species Richness | 1.423 * | −2.723 × 10−4 * | 0.255 | 16.79 | |||
| 1.032 * | 7.323 × 10−4 * | –4.369 × 10−7 * | 0.495 | 8.835 | |||
| 1.456 * | −1.362 × 10−3 | 1.866 × 10−6 * | −6.822 × 10−10 * | 0.633 | 2.196 | ||
| 1.228 | 2.745 × 10−4 | −1.145 × 10−6 | 1.315 × 10−9 | −4.344 × 10−13 | 0.649 | 3.691 | |
| Log10 Inverse Simpson’s Index (1/D) | 1.122 * | −3.599 × 10−4 * | 0.512 | 3.85 | |||
| 0.9136 * | 1.765 × 10−4 | −2.333 × 10−7 | 0.576 | 2.31 | |||
| 0.8901 * | 2.925 × 10−4 | −3.608 × 10−7 | 3.778 × 10−11 | 0.576 | 5.18 | ||
| 0.7449 * | 1.3 × 10−3 | −2.213 × 10−6 | 1.267 × 10−9 | −2.673 × 10−13 | 0.583 | 7.87 | |
| Shannon Index | 2.925 * | −0.000790 * | 0.479 | 54.93 | |||
| 2.268 * | 8.973 × 10−4 | −7.339 × 10−7 * | 0.602 | 49.53 | |||
| 2.643 * | −9.526 × 10−4 | 1.3 × 10−6 | −6.025 × 10−10 | 0.624 | 50.74 | ||
| 2.266 * | 1.756 × 10−3 | −3.863 × 10−6 | 2.702 × 10−9 | −7.19 × 10−13 | 0.633 | 53.15 |
| Response Variable | Intercept | Temperature | Temperature2 | Temperature3 | Model R2 |
|---|---|---|---|---|---|
| Abundance | −5497.70 | +945.02 | −51.91 | +0.9314 | 0.458 |
| Log10 Richness | 1.221 * | 7.036 × 10−4 * | −4.225 × 10−7 * | - | 0.670 |
| Log10 (1/D) | −0.6927 | +0.0836 * | - | - | 0.614 |
| Shannon Index | −9.803 * | +1.234 * | −0.0302 * | - | 0.769 |
| Variable | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Elevation | −0.326 | −0.138 | −0.090 | −0.117 |
| Mean temperature | 0.328 | 0.103 | 0.133 | 0.152 |
| Max. temperature | 0.303 | 0.153 | 0.224 | 0.182 |
| Min. temperature | 0.333 | 0.084 | 0.107 | 0.139 |
| Temperature amplitude | −0.258 | 0.159 | 0.290 | 0.050 |
| Humidity | 0.082 | 0.298 | −0.337 | −0.362 |
| Dry litter mass | 0.091 | −0.541 | −0.020 | −0.012 |
| Litter moisture | −0.298 | 0.071 | −0.207 | −0.078 |
| Large tree density | 0.028 | −0.413 | −0.456 | 0.152 |
| Small tree density | −0.087 | 0.133 | −0.385 | 0.420 |
| Tree fern density | 0.113 | 0.179 | −0.047 | −0.657 |
| Bamboo ground cover | −0.326 | −0.078 | 0.041 | 0.050 |
| Fern ground cover | −0.290 | 0.197 | −0.080 | −0.020 |
| Herb ground cover | −0.174 | 0.430 | 0.058 | 0.289 |
| Epiphyte density | −0.317 | 0.085 | 0.011 | 0.073 |
| Liana density | 0.189 | 0.216 | −0.445 | 0.200 |
| Palm density | 0.189 | 0.156 | −0.324 | −0.088 |
| Initial eigenvalue | 8.354 | 2.657 | 1.804 | 1.476 |
| % variance | 49.14 | 15.63 | 10.61 | 8.69 |
| % cumulative variance | 49.14 | 64.78 | 75.39 | 84.07 |
| Response Variable | Intercept | Predictor Variables | Model R2 | |
|---|---|---|---|---|
| Abundance | 145.75 * | −6.80 × PC1 | −5.35 × PC12 * | 0.467 |
| Log10 richness | 1.444 * | +0.040 × PC1 | −0.019 × PC12 * | 0.745 |
| Log10(1/D) | 0.766 * | +0.104 × PC1 * | 0.719 | |
| Shannon Index | 2.244 * | +0.227 × PC1 * | 0.772 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flinte, V.; Pádua, D.G.; Durand, E.M.; Hodgin, C.; Khattar, G.; da Silveira, L.F.L.; Fernandes, D.R.R.; Sääksjärvi, I.E.; Monteiro, R.F.; Macedo, M.V.; et al. Variation in a Darwin Wasp (Hymenoptera: Ichneumonidae) Community along an Elevation Gradient in a Tropical Biodiversity Hotspot: Implications for Ecology and Conservation. Insects 2023, 14, 861. https://doi.org/10.3390/insects14110861
Flinte V, Pádua DG, Durand EM, Hodgin C, Khattar G, da Silveira LFL, Fernandes DRR, Sääksjärvi IE, Monteiro RF, Macedo MV, et al. Variation in a Darwin Wasp (Hymenoptera: Ichneumonidae) Community along an Elevation Gradient in a Tropical Biodiversity Hotspot: Implications for Ecology and Conservation. Insects. 2023; 14(11):861. https://doi.org/10.3390/insects14110861
Chicago/Turabian StyleFlinte, Vivian, Diego G. Pádua, Emily M. Durand, Caitlin Hodgin, Gabriel Khattar, Luiz Felipe L. da Silveira, Daniell R. R. Fernandes, Ilari E. Sääksjärvi, Ricardo F. Monteiro, Margarete V. Macedo, and et al. 2023. "Variation in a Darwin Wasp (Hymenoptera: Ichneumonidae) Community along an Elevation Gradient in a Tropical Biodiversity Hotspot: Implications for Ecology and Conservation" Insects 14, no. 11: 861. https://doi.org/10.3390/insects14110861
APA StyleFlinte, V., Pádua, D. G., Durand, E. M., Hodgin, C., Khattar, G., da Silveira, L. F. L., Fernandes, D. R. R., Sääksjärvi, I. E., Monteiro, R. F., Macedo, M. V., & Mayhew, P. J. (2023). Variation in a Darwin Wasp (Hymenoptera: Ichneumonidae) Community along an Elevation Gradient in a Tropical Biodiversity Hotspot: Implications for Ecology and Conservation. Insects, 14(11), 861. https://doi.org/10.3390/insects14110861