Distribution and Organization of Descending Neurons in the Brain of Adult Helicoverpa armigera (Insecta)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Preparations and Backfill Labeling
2.3. Immunocytochemistry
2.4. Confocal Microscopy and Image Acquisition
2.5. Data Analysis
2.6. Nomenclature
3. Results
3.1. Distribution and Number of Somata of DNs in H. armigera
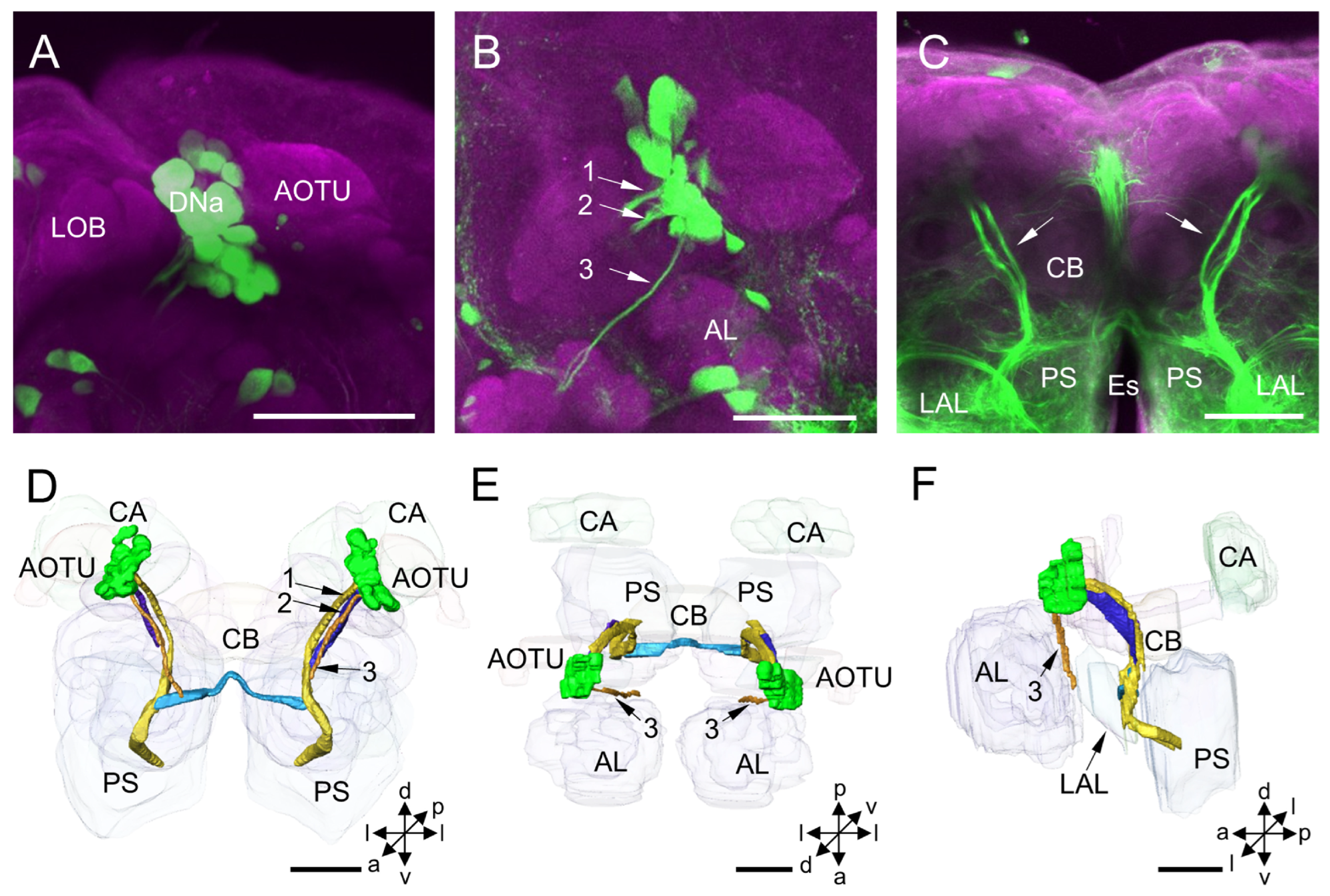
3.1.1. DNa Cluster
3.1.2. DNd Cluster
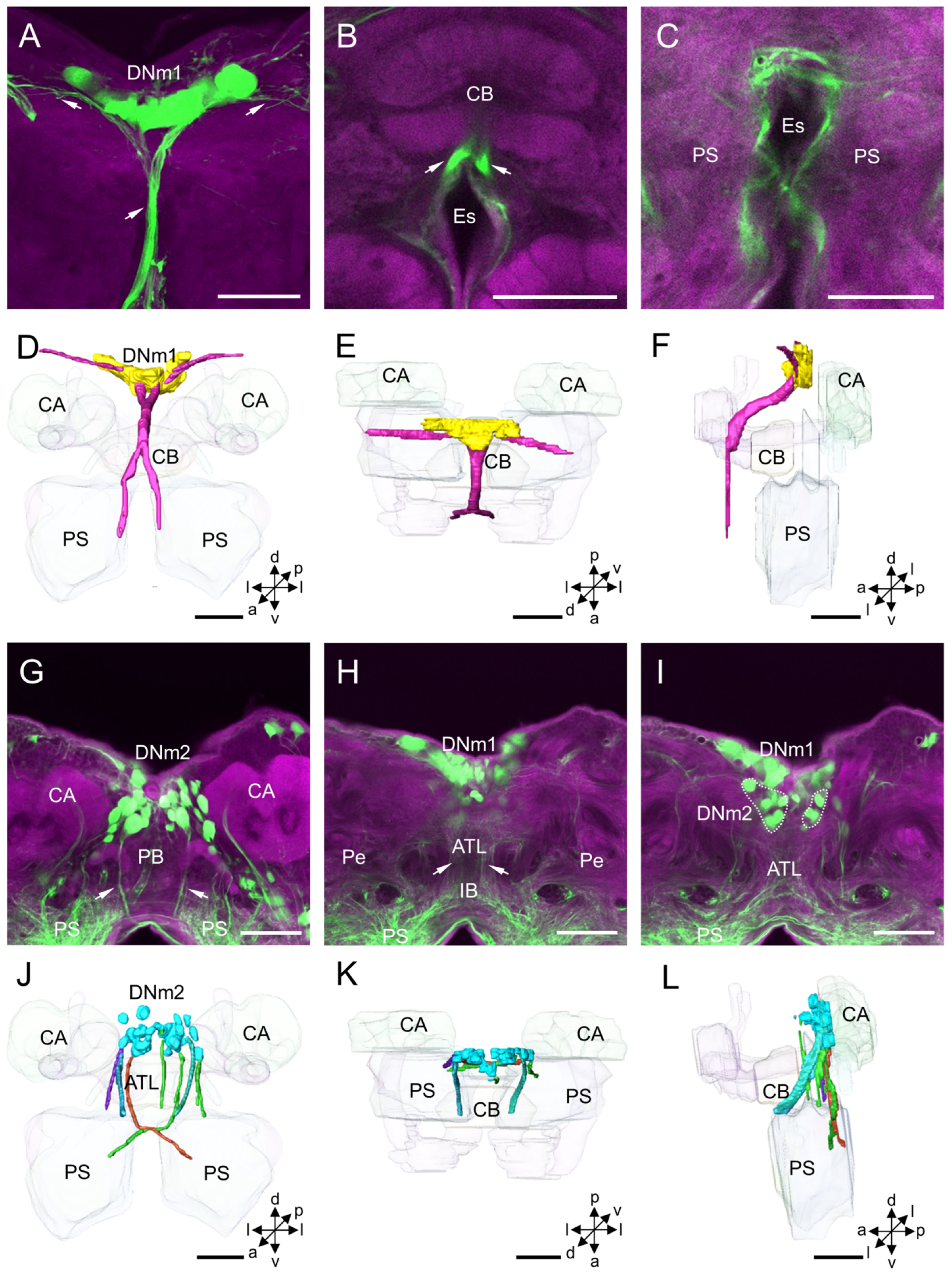
3.1.3. DNm Cluster
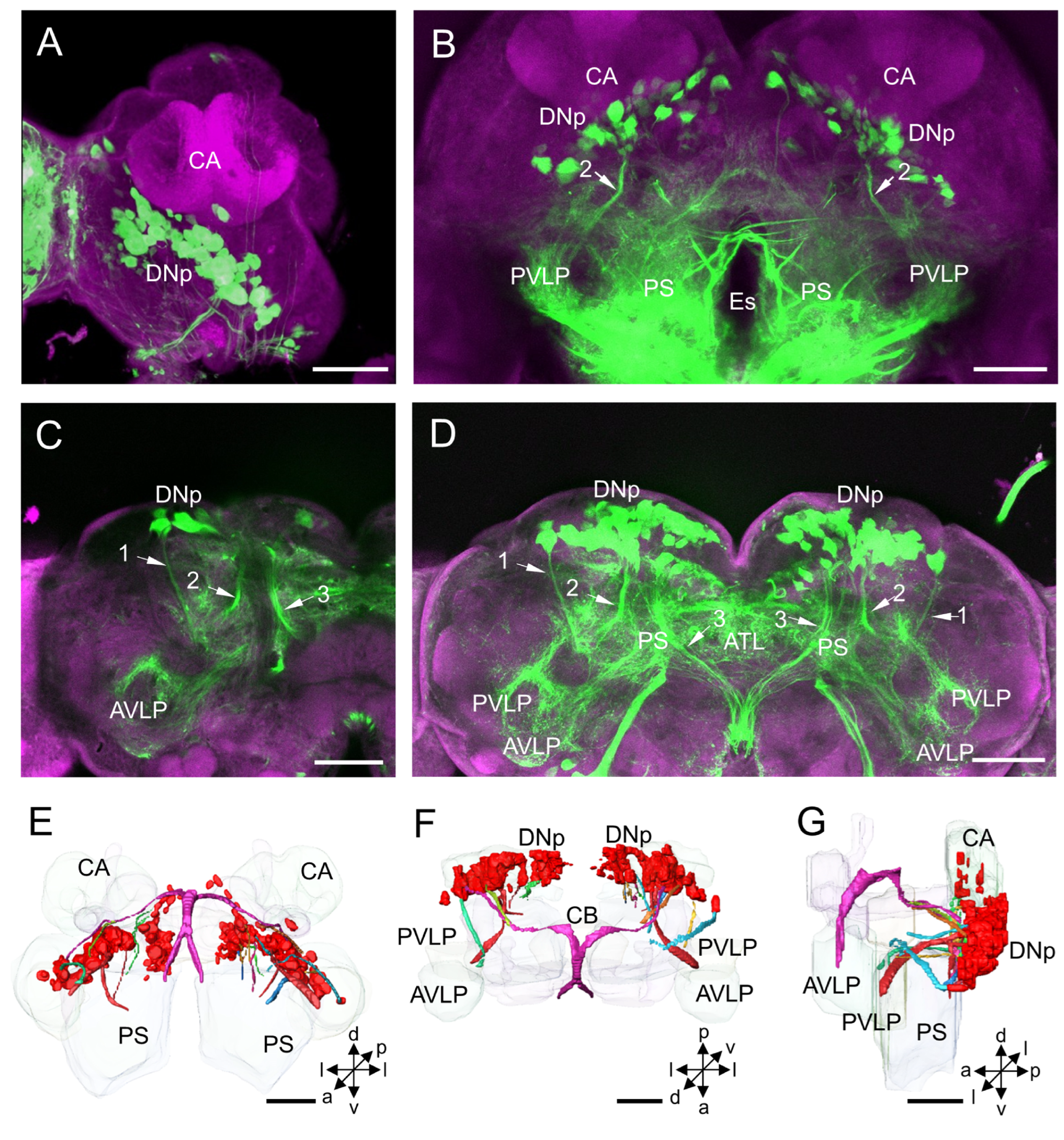
3.1.4. DNp Cluster
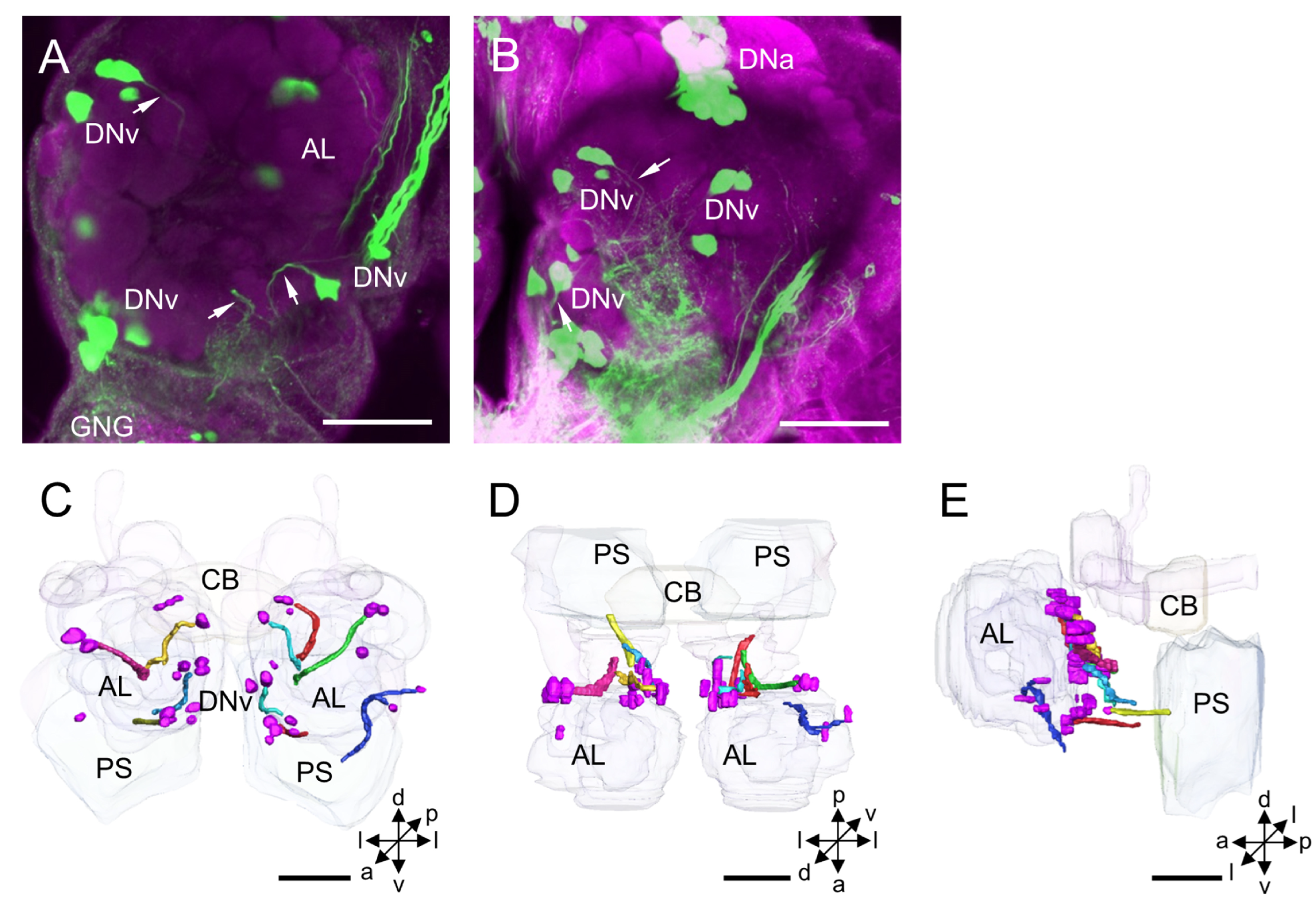
3.1.5. DNv Cluster
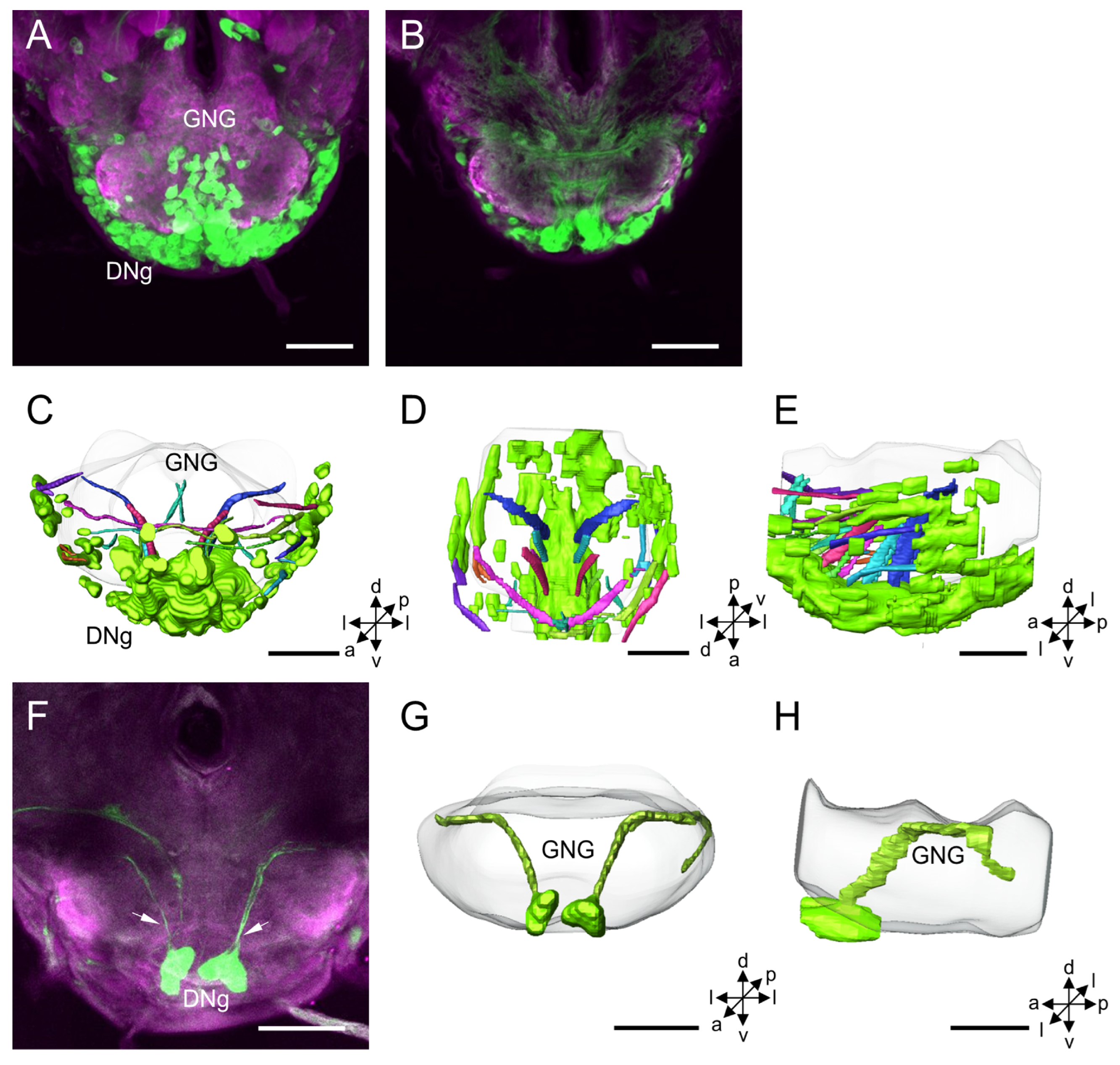
3.1.6. DNg Cluster
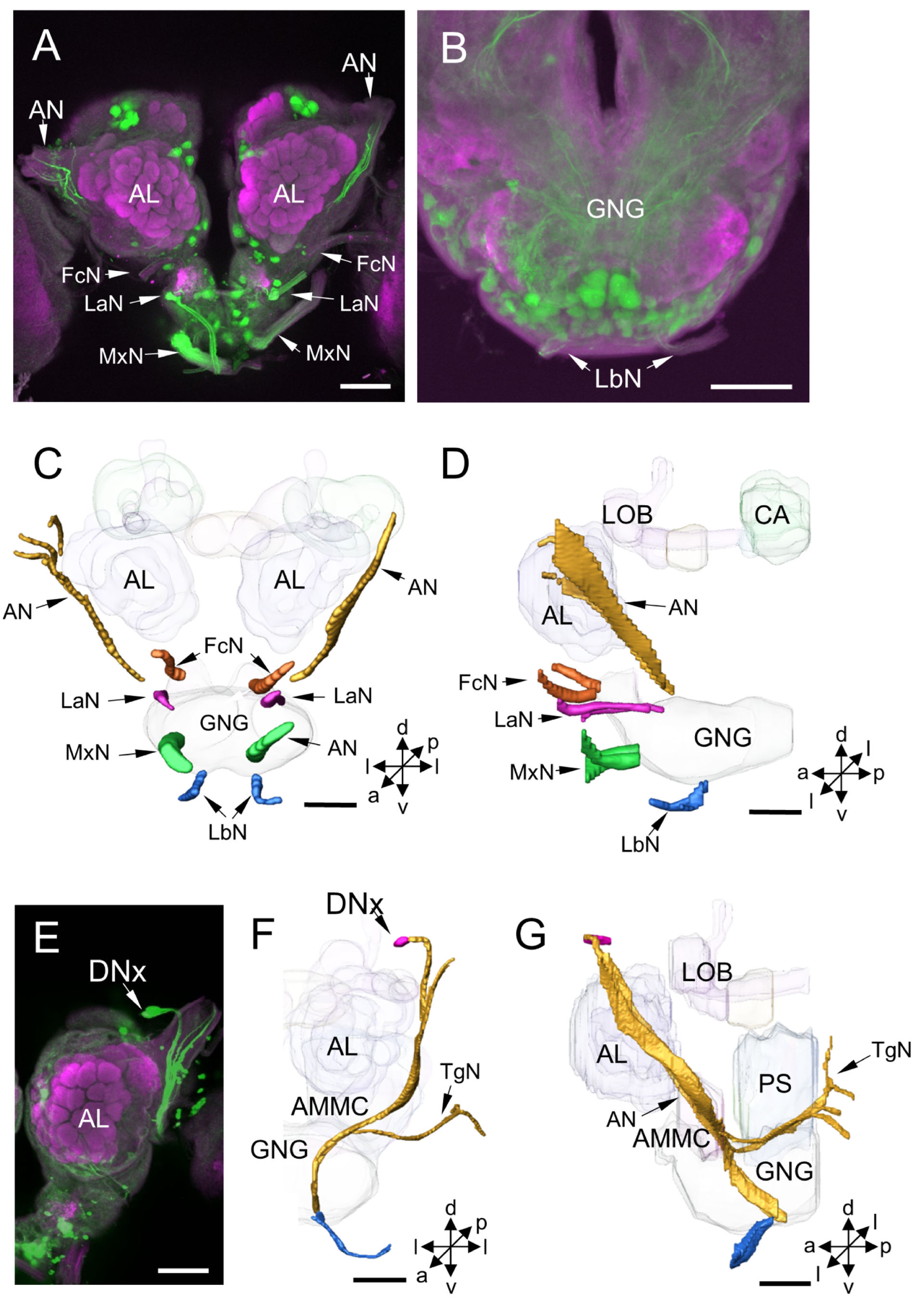
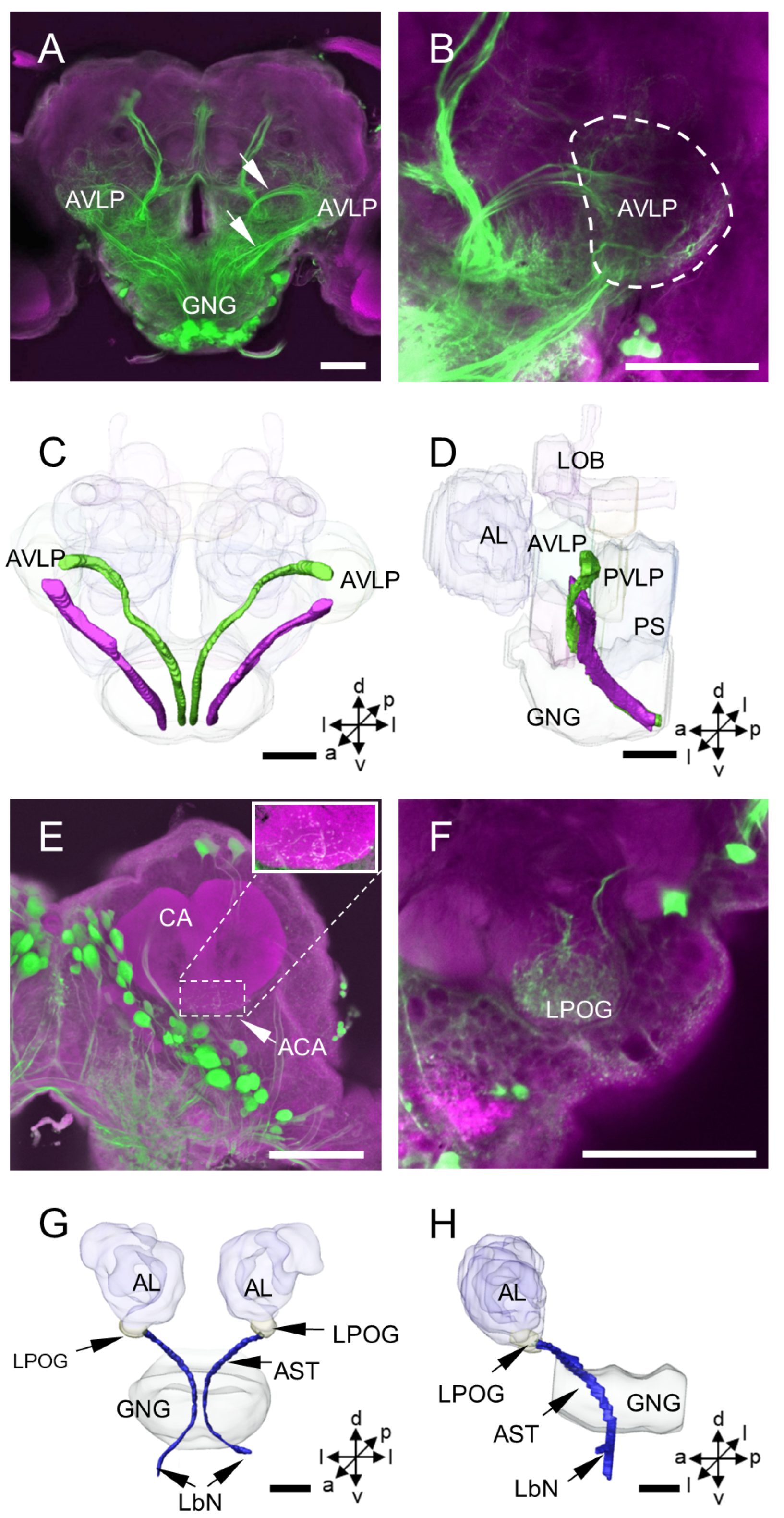
3.2. Other Stained Neurons and Innervation Patterns in the Brain
4. Discussion
4.1. DNa Cluster
4.2. DNd Cluster
4.3. DNm Cluster
4.4. DNp Cluster
4.5. DNv Cluster
4.6. DNg Cluster
4.7. Innervation Patterns of DNs Cross Insect Species
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowell, C.H.F. The orthopteran descending movement detector (DMD) neurons: A characterization and review. Z. Vergl. Physiol. 1971, 73, 167–194. [Google Scholar] [CrossRef]
- Gronenberg, W.; Strausfeld, N.J. Descending neurons supplying the neck and flight motor of Diptera: Physiological and anatomical characteristics. J. Comp. Neurol. 1990, 302, 973–991. [Google Scholar] [CrossRef] [PubMed]
- Mishima, T.; Kanzaki, R. Physiological and morphological characterization of olfactory descending interneurons of the male silkworm moth, Bombyx mori. J. Comp. Physiol. A 1999, 184, 143–160. [Google Scholar] [CrossRef]
- Burdohan, J.A.; Comer, C.M. Cellular organization of an antennal mechanosensory pathway in the cockroach, Periplaneta americana. J. Neurosci. 1996, 16, 5830–5843. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Comer, C.M. Correspondence of escape-turning behavior with activity of descending mechanosensory interneurons in the cockroach, Periplaneta americana. J. Neurosci. 1996, 16, 5844–5853. [Google Scholar] [CrossRef]
- Staudacher, E.; Schildberger, K. Gating of sensory responses of descending brain neurones during walking in crickets. J. Exp. Biol. 1998, 201, 559–572. [Google Scholar] [CrossRef]
- Griss, C.; Rowell, C.H.F. Three descending interneurons reporting deviation from course in the locust. I Anatomy. J. Comp. Physiol. A 1986, 158, 765–774. [Google Scholar] [CrossRef]
- Rowell, C.H.F.; Reichert, H. Three descending interneurons reporting deviation from course in the locust. II. Physiology. J. Comp. Physiol. A 1986, 158, 775–794. [Google Scholar] [CrossRef]
- Gronenberg, W.; Milde, J.J.; Strausfeld, N.J. Oculomotor control in calliphorid flies: Organization of descending neurons to neck motor neurons responding to visual stimuli. J. Comp. Neurol. 1995, 361, 267–284. [Google Scholar] [CrossRef]
- Kanzaki, R.; Ikeda, A.; Shibuya, T. Morphological and physiological properties of pheromone-triggered flipflopping descending interneurons of the male silkworm moth Bombyx mori. J. Comp. Physiol. A 1994, 175, 1–14. [Google Scholar] [CrossRef]
- Hampel, S.; Franconville, R.; Simpson, J.H.; Seeds, A.M. A neural command circuit for grooming movement control. eLife 2015, 4, e08758. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, N.; Simpson, J.H. Descending neurons coordinate anterior grooming behavior in Drosophila. Curr. Biol. 2022, 32, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Namiki, S.; Ros, I.G.; Morrow, C.; Rowell, W.J.; Card, G.M.; Korff, W.; Dickinson, M.H. A population of descending neurons that regulates the flight motor of Drosophila. Curr. Biol. 2022, 32, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Namiki, S.; Dickinson, M.H.; Wong, A.M.; Korff, W.; Card, G.M. The functional organization of descending sensory-motor pathways in Drosophila. eLife 2018, 7, e34272. [Google Scholar] [CrossRef]
- Staudacher, E. Distribution and morphology of descending brain neurons in the cricket Gryllus bimaculatus. Cell Tissue Res. 1998, 294, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Sakura, M.; Mizunami, M. Distribution of dendrites of descending neurons and its implications for the basic organization of the cockroach brain. J. Comp. Neurol. 2003, 458, 158–174. [Google Scholar] [CrossRef]
- Hsu, C.T.; Bhandawat, V. Organization of descending neurons in Drosophila melanogaster. Sci. Rep. 2016, 6, 20259. [Google Scholar] [CrossRef]
- Severina, I.Y.; Isavnina, I.L.; Knyazev, A.N. Topographic anatomy of ascending and descending neurons of the supraesophageal, meso- and metathoracic ganglia in paleo- and neopterous insects. J. Evol. Biochem. Physiol. 2016, 52, 397–406. [Google Scholar] [CrossRef]
- Fitt, G.P. The ecology of heliothis species in relation to agroecosystems. Ann. Rev. Entomol. 1989, 34, 17–53. [Google Scholar] [CrossRef]
- Wu, K.M.; Guo, Y.Y. The evolution of cotton pest management practices in China. Ann. Rev. Entomol. 2005, 50, 31–52. [Google Scholar] [CrossRef]
- Zhang, J.P.; Salcedo, C.; Fang, Y.L.; Zhang, R.J.; Zhang, Z.N. An overlooked component: (Z)-9-tetradecenal as a sex pheromone in Helicoverpa armigera. J. Insect Physiol. 2012, 58, 1209–1216. [Google Scholar] [CrossRef]
- Guo, M.B.; Du, L.X.; Chen, Q.Y.; Feng, Y.L.; Zhang, J.; Zhang, X.X.; Tian, K.; Cao, S.; Huang, T.Y.; Jacquin-Joly, E.; et al. Odorant receptors for detecting flowering plant cues are functionally conserved across moths and butterflies. Mol. Biol. Evol. 2021, 38, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Chu, X.; Sun, L.L.; Wang, Y.N.; Xie, G.Y.; Chen, W.B.; Liu, Y.; Berg, B.G.; An, S.H.; Wang, G.R.; et al. Functional map of the macroglomerular complex of male Helicoverpa armigera (Hübner). Insect Sci. 2022. accepted. [Google Scholar] [CrossRef]
- Liu, X.L.; Yin, X.M.; Wang, G.R.; Zhao, X.C. Research progress in the molecular and neural mechanisms of sex pheromone reception in male Helicoverpa armigera (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2020, 63, 1136–1144. [Google Scholar] [CrossRef]
- Yang, K.; Wang, C.Z. Review of pheromone receptors in heliothine species: Expression, function, and evolution. Entomol. Exp. Appl. 2020, 169, 156–171. [Google Scholar] [CrossRef]
- Wada, S.; Kanzaki, R. Neural control mechanisms of the pheromone-triggered programmed behavior in male silkmoths revealed by double-labeling of descending interneurons and a motor neuron. J. Comp. Neurol. 2005, 484, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Namiki, S.; Wada, S.; Kanzaki, R. Descending neurons from the lateral accessory lobe and posterior slope in the brain of the silkmoth Bombyx mori. Sci. Rep. 2018, 8, 9663. [Google Scholar] [CrossRef]
- Chu, X.; Heinze, S.; Ian, E.; Berg, B.G. A novel major output target for pheromone-sensitive projection neurons in male moths. Front. Cell. Neurosci. 2020, 14, 147. [Google Scholar] [CrossRef]
- Ito, K.; Shinomiya, K.; Ito, M.; Armstrong, J.D.; Boyan, G.; Hartenstein, V.; Harzsch, S.; Heisenberg, M.; Homberg, U.; Jenett, A.; et al. A systematic nomenclature for the insect brain. Neuron 2014, 81, 755–765. [Google Scholar] [CrossRef]
- Kien, J.; Fletcher, W.A.; Altman, J.S.; Ramirez, J.M.; Roth, U.R. Organisation of intersegmental interneurons in the suboesophageal ganglion of Schistocerca gregaria (Forksal) and Locusta migratoria migratorioides (Reiche & Fairmaire) (Acrididae, Orthoptera). Int. J. Insect Morphol. Embryol. 1990, 19, 35–60. [Google Scholar] [CrossRef]
- Knebel, D.; Rillich, J.; Nadler, L.; Pfluger, H.J.; Ayali, A. The functional connectivity between the locust leg pattern generators and the subesophageal ganglion higher motor center. Neurosci. Lett. 2019, 692, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.D. Anatomical studies of the insect central nervous system: A ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera). J. Zool. 1975, 176, 67–86. [Google Scholar] [CrossRef]
- Zorovic, M.; Hedwig, B. Descending brain neurons in the cricket Gryllus bimaculatus (de Geer): Auditory responses and impact on walking. J. Comp. Physiol. A 2013, 199, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gronenberg, W.; Strausfeld, N.J. Descending pathways connecting the male-specific visual system of flies to the neck and flight motor. J. Comp. Physiol. A 1991, 169, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Schnell, B.; Ros, I.G.; Dickinson, M.H. A descending neuron correlated with the rapid steering maneuvers of flying Drosophila. Curr. Biol. 2017, 27, 1200–1205. [Google Scholar] [CrossRef]
- Zhao, X.C.; Chen, Q.Y.; Guo, P.; Xie, G.Y.; Tang, Q.B.; Guo, X.R.; Berg, B.G. Glomerular identification in the antennal lobe of the male moth Helicoverpa armigera. J. Comp. Neurol. 2016, 524, 2993–3013. [Google Scholar] [CrossRef]
- Mizunami, M. Morphology of higher-order ocellar interneurons in the cockroach brain. J. Comp. Neurol. 1995, 362, 293–304. [Google Scholar] [CrossRef]
- Hensler, K. Neuronal co-processing of course deviation and head movements in locusts. I. Descending deviation detectors. J. Comp. Physiol. A 1992, 171, 257–271. [Google Scholar] [CrossRef]
- Träger, U.; Homberg, U. Polarization-sensitive descending neurons in the locust: Connecting the brain to thoracic ganglia. J. Neurosci. 2011, 31, 2238–2247. [Google Scholar] [CrossRef]
- Koto, M.; Tanouye, M.A.; Ferrus, A.; Thomas, J.B.; Wyman, R.J. The morphology of the cervical giant fiber neuron of Drosophila. Brain Res. 1981, 221, 213–217. [Google Scholar] [CrossRef]
- Bacon, J.P.; Strausfeld, N.J. The dipteran ‘Giant fibre’ pathway: Neurons and signals. J. Comp. Physiol. A 1986, 158, 529–548. [Google Scholar] [CrossRef]
- O’Shea, M.; Rowell, C.H.F.; Williams, J.L.D. The anatomy of a locust visual interneurone; the descending contralateral movement detector. J. Exp. Biol. 1974, 60, 1–12. [Google Scholar] [CrossRef]
- Olberg, R.M. Identified target-selective visual interneurons descending from the dragonfly brain. J. Comp. Physiol. A 1986, 159, 827–840. [Google Scholar] [CrossRef]
- Kohatsu, S.; Koganezawa, M.; Yamamoto, D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 2011, 69, 498–508. [Google Scholar] [CrossRef]
- von Philipsborn, A.C.; Liu, T.; Yu, J.Y.; Masser, C.; Bidaye, S.S.; Dickson, B.J. Neuronal control of Drosophila courtship song. Neuron 2011, 69, 509–522. [Google Scholar] [CrossRef]
- Bidaye, S.S.; Machacek, C.; Wu, Y.; Dickson, B.J. Neuronal control of Drosophila walking direction. Science 2014, 344, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Israel, S.; Rozenfeld, E.; Weber, D.; Huetteroth, W.; Parnas, M. Olfactory stimuli and moonwalker SEZ neurons can drive backward locomotion in Drosophila. Curr. Biol. 2022, 32, 1131–1149. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.; Tyrer, M. The tritocerebral commissure giant (TCG): A bimodal interneurone in the locust Schistocerca gregaria. J. Comp. Physiol. A 1978, 126, 317–325. [Google Scholar] [CrossRef]
- Bacon, J.; Möhl, B. The tritocerebral commissure giant (TCG) wind-sensitive interneurone in the locust. I. Its activity in straight flight. J. Comp. Physiol. A 1983, 150, 439–452. [Google Scholar] [CrossRef]
- Tyrer, N.M.; Pozza, M.F.; Humbel, U.; Peters, B.H.; Bacon, J.P. The tritocerebral commissure ‘dwarf’ (TCD): A major GABA-immunoreactive descending interneuron in the locust. J. Comp. Physiol. A 1988, 164, 141–150. [Google Scholar] [CrossRef]
- Kanzaki, R.; Arbas, E.A.; Hildebrand, J.G. Physiology and morphology of descending neurons in pheromone-processing olfactory pathways in the male moth Manduca sexta. J. Comp. Physiol. A 1991, 169, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bräunig, P.; Pflüger, H.J.; Hustert, R. The specificity of central nervous projections of locust mechanoreceptors. J. Comp. Neurol. 1983, 218, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, R.B.; Couton, L.; Lazzari, C.R.; Insausti, T.C.; Minoli, S.A.; Fresquet, N.; Rospars, J.P.; Anton, S. Antennal pathways in the central nervous system of a blood-sucking bug, Rhodnius prolixus. Arthropod Struct. Dev. 2009, 38, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.Y.; Zhao, X.C.; Ma, B.W.; Guo, P.; Li, G.P.; Feng, H.Q.; Wu, G.L. Central projection of antennal sensory neurons in the central nervous system of the mirid bug Apolygus lucorum (Meyer-Dür). PLoS ONE 2016, 11, e0160161. [Google Scholar] [CrossRef]
- Kamikouchi, A.; Shimada, T.; Ito, K. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J. Comp. Neurol. 2006, 499, 317–356. [Google Scholar] [CrossRef]
- Ma, B.W.; Zhao, X.C.; Berg, B.G.; Xie, G.Y.; Tang, Q.B.; Wang, G.R. Central projections of antennal and labial palp sensory neurons in the migratory armyworm Mythimna separata. Front. Cell. Neurosci. 2017, 11, 370. [Google Scholar] [CrossRef]


| This Study | Drosophila melanogaster (Female) [17] | Periplaneta americana (Male) ** [16] | Gryllus bimaculatus (Female) ** [15] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster | H. armigera (Male) | H. armigera (Female) | Cluster Name | Med | Max | Cluster Name | Med | Max | Cluster Name | Med | Max | ||||
| Mean ± SD | Med | Max | Mean ± SD | Med | Max | ||||||||||
| DNa * | 18.60 ± 2.07 | 19 | 21 | 19.00 ± 2.00 | 19 | 21 | AOTU * | 19 | 38 | i5, i5n | 23 | 35 | i5, i5n | 10 | 22 |
| DNd * | 12.20 ± 2.39 | 12 | 15 | - | - | - | - | - | - | - | - | - | - | - | - |
| DNm1 | 28.40 ± 7.44 | 28 | 40 | 26.33 ± 4.73 | 28 | 30 | PI | 26 | 48 | PI | 2 | 6 | PI | 5 | 17 |
| DNm2 | 31.40 ± 3.58 | 32 | 35 | 33.67 ± 1.53 | 34 | 35 | - | - | - | i4, c4 | 18 | 26 | i4, c4 | 12 | 17 |
| DNp | 196.00 ± 17.09 | 204 | 214 | 188.67 ± 15.14 | 182 | 206 | SMP | 280 | 325 | i1-i3, c1-c3 | 98 | 163 | i1-i3, c1-c3 | 111 | 154 |
| DNv * | 14.60 ± 1.82 | 15 | 17 | 13.67 ± 1.53 | 14 | 15 | PENP | 18 | 80 | i7(a,b), c7 | 11 | 18 | c5, c6, i6, i7(a,b) | 8 | 27 |
| DNg | 506.80 ± 43.13 | 489 | 582 | 477.33 ± 28.92 | 483 | 503 | GNG | 462 | 526 | - | - | - | - | - | - |
| Cluster | Diameter (µm) | Volume (×102 µm3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| DNa | 9.96–24.65 | 9.33–22.07 | 16.21 ± 3.65 | 15.88 ± 3.54 | 12.50–45.18 | 11.08–41.71 | 22.85 ± 8.50 | 22.04 ± 7.62 |
| DNd | 8.24–15.45 | - | 11.57 ± 1.77 | - | 7.38–19.73 | - | 12.16 ± 3.30 | - |
| DNm1 | 7.13–18.85 | 8.30–17.87 | 11.91 ± 2.73 | 11.27 ± 2.14 | 6.42–27.65 | 5.76–18.15 | 13.35 ± 4.54 | 10.66 ± 2.85 |
| DNm2 | 8.09–19.19 | 9.37–17.33 | 13.10 ± 2.96 | 12.65 ± 1.83 | 5.47–34.18 | 7.63–50.36 | 17.07 ± 8.95 | 15.48 ± 9.17 |
| DNp | 8.51–18.11 | 8.65–16.81 | 12.46 ± 1.98 | 11.94 ± 2.35 | 8.07–25.46 | 6.61–31.28 | 15.63 ± 4.90 | 14.08 ± 5.95 |
| DNv | 8.60–20.43 | 8.41–18.65 | 13.68 ± 3.33 | 13.00 ± 2.27 | 7.34–33.34 | 8.16–32.57 | 18.01 ± 6.55 | 16.98 ± 5.57 |
| DNg | 8.44–20.65 | 9.37–17.07 | 14.13 ± 3.27 | 13.14 ± 2.32 | 6.22–35.10 | 11.25–31.96 | 19.86 ± 8.13 | 17.65 ± 5.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yang, S.; Sun, L.; Xie, G.; Chen, W.; Liu, Y.; Wang, G.; Yin, X.; Zhao, X. Distribution and Organization of Descending Neurons in the Brain of Adult Helicoverpa armigera (Insecta). Insects 2023, 14, 63. https://doi.org/10.3390/insects14010063
Liu X, Yang S, Sun L, Xie G, Chen W, Liu Y, Wang G, Yin X, Zhao X. Distribution and Organization of Descending Neurons in the Brain of Adult Helicoverpa armigera (Insecta). Insects. 2023; 14(1):63. https://doi.org/10.3390/insects14010063
Chicago/Turabian StyleLiu, Xiaolan, Shufang Yang, Longlong Sun, Guiying Xie, Wenbo Chen, Yang Liu, Guirong Wang, Xinming Yin, and Xincheng Zhao. 2023. "Distribution and Organization of Descending Neurons in the Brain of Adult Helicoverpa armigera (Insecta)" Insects 14, no. 1: 63. https://doi.org/10.3390/insects14010063
APA StyleLiu, X., Yang, S., Sun, L., Xie, G., Chen, W., Liu, Y., Wang, G., Yin, X., & Zhao, X. (2023). Distribution and Organization of Descending Neurons in the Brain of Adult Helicoverpa armigera (Insecta). Insects, 14(1), 63. https://doi.org/10.3390/insects14010063






