Assessment of Various Colors Combined with Insecticides in Devising Ovitraps as Attracting and Killing Tools for Mosquitoes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ovitraps
2.2. Laboratory Procedure for Color and Different Attractive Materials Experiments
2.2.1. Experiment 1
Attraction of Gravid Females to Various Colored Ovitraps in Laboratory
Coloured Ovitraps in the Field for Attracting Ae. aegypti Females
2.2.2. Experiment 2
Oviposition Preference of Gravid Females to Different Attractive Materials in the Laboratory
Ovitraps with Attractive Materials Installed in the Field
2.2.3. Experiment 3
Preparation of Lethal Ovitraps to Control Ae. aegypti in Laboratory
Lethal Ovitraps Used in the Field
2.2.4. Statistical Analysis
3. Results
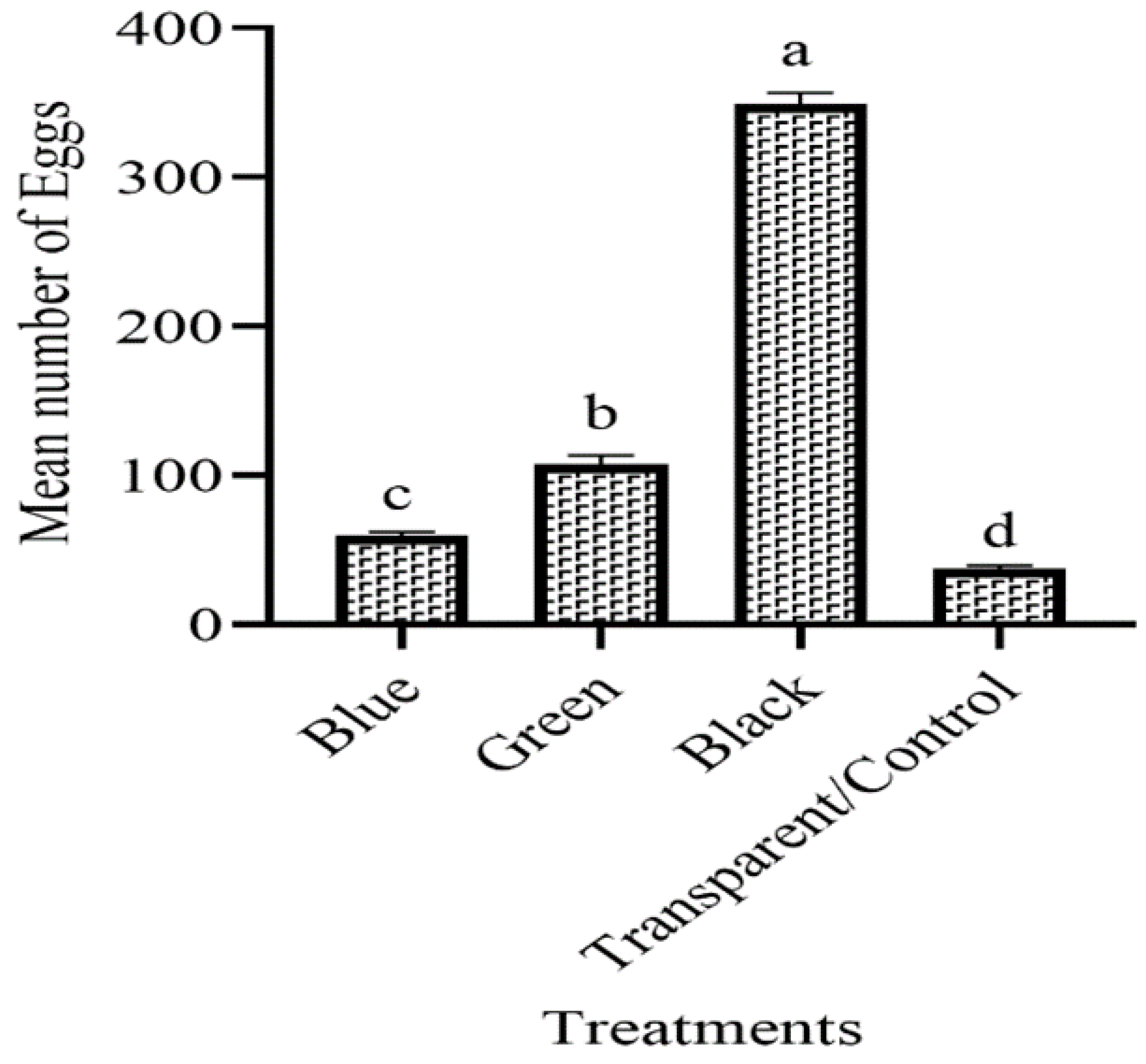
3.1. The Ovipositional Preference of Females to Various Colored Ovitraps in the Laboratory
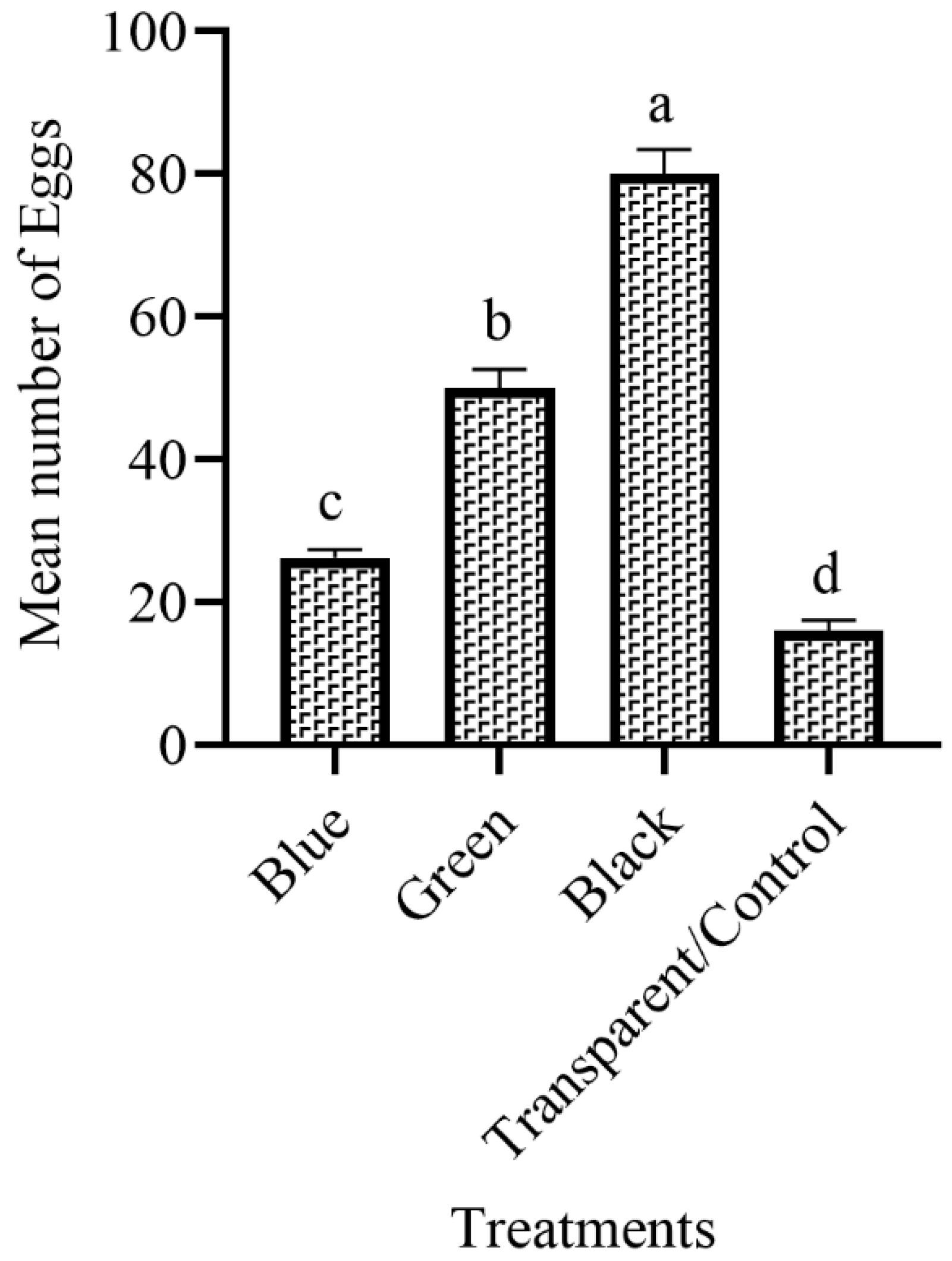
3.2. The Attraction of Ae. aegypti to Different Colored Ovitraps in the Field
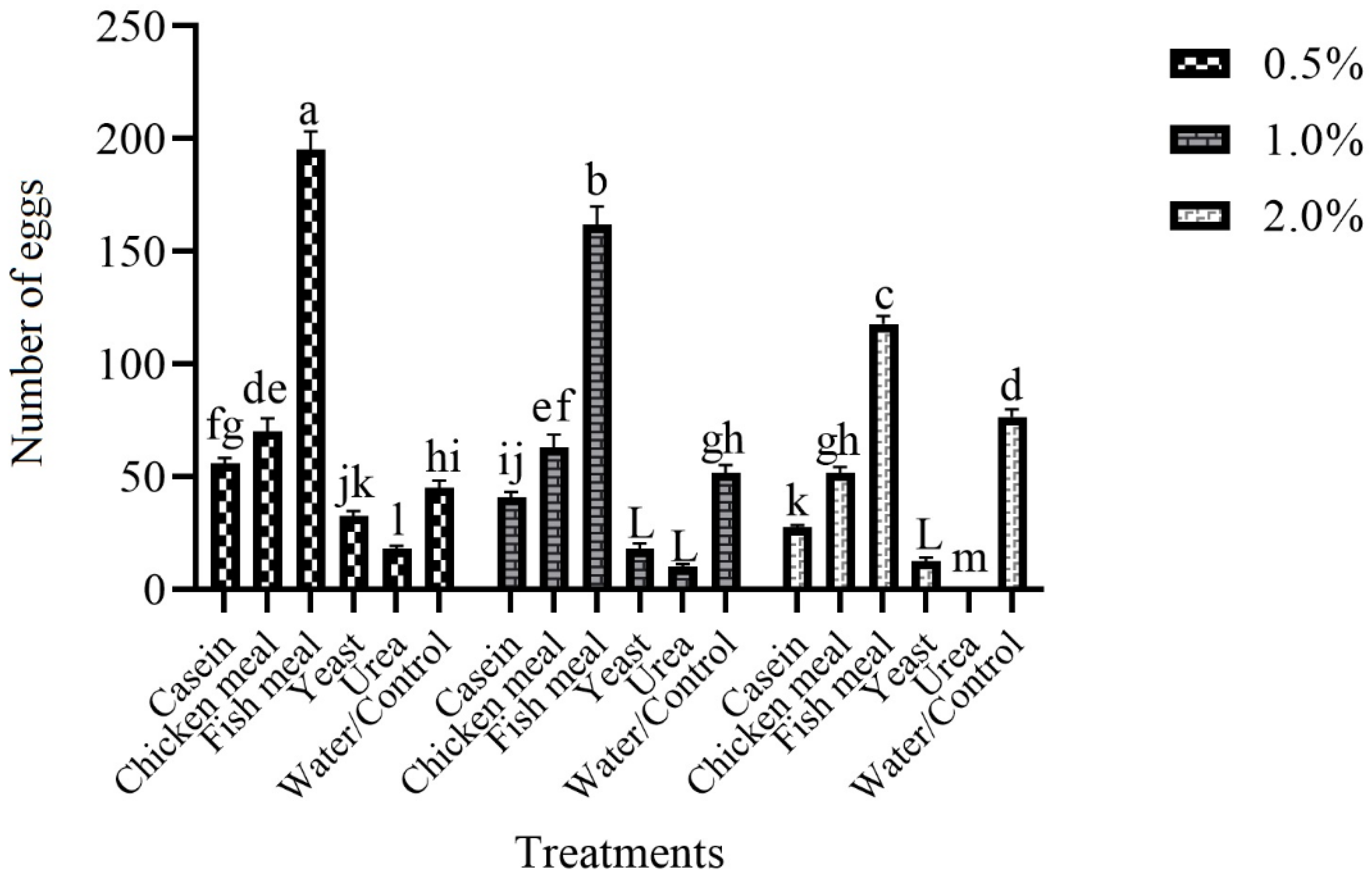
3.3. Effect of Different Attractive Materials with Various Concentrations on Female Oviposition in Black-Colored Ovitraps in Laboratory
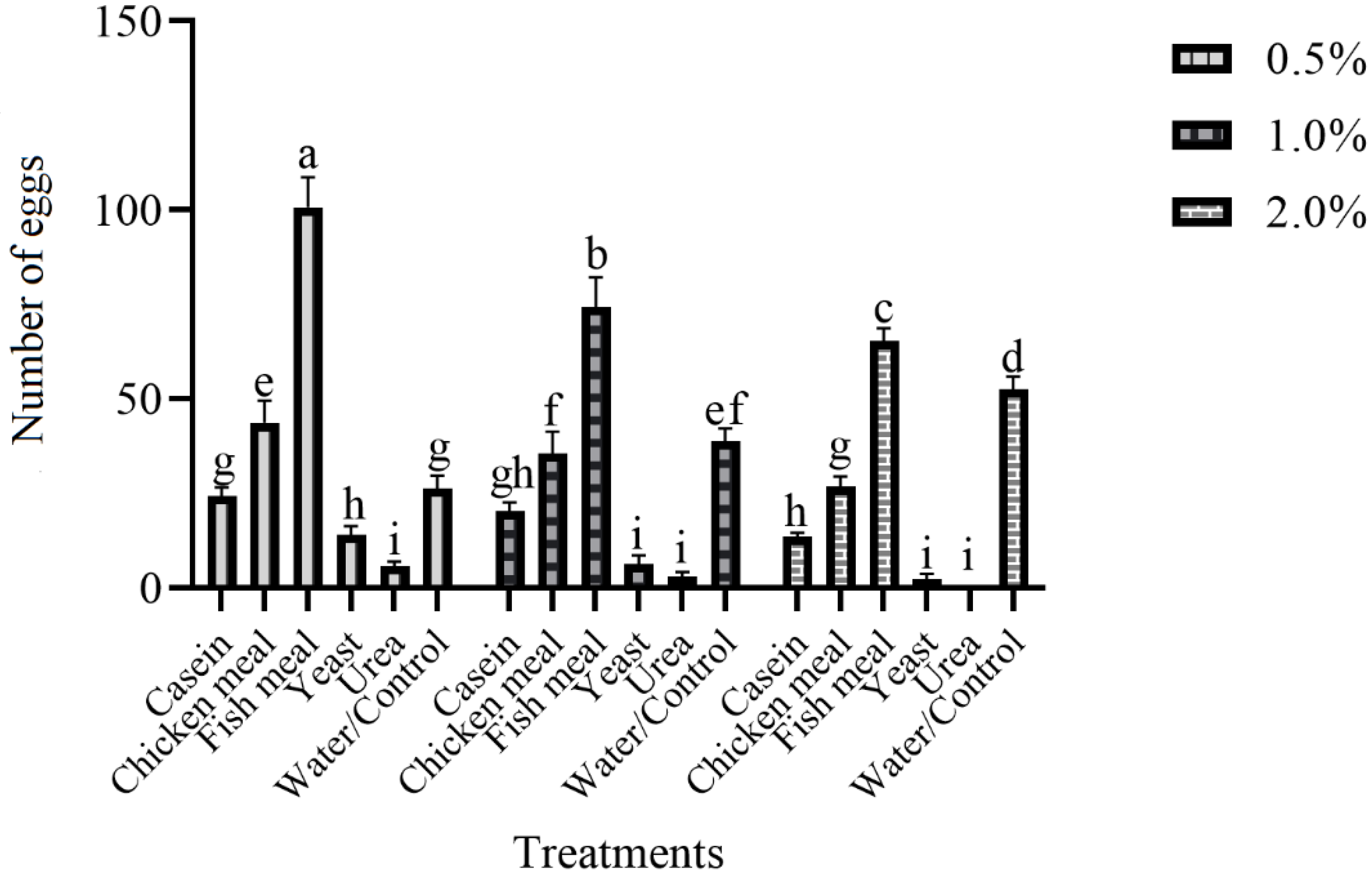
3.4. Ovipositional Response of Gravid Females of Ae. aegypti to Various Attractive Materials with a Different Concentration in the Field
3.5. Lethality of Ovitraps by Treating Them with Insecticides in the Laboratory
3.6. The Effects of Lethal Ovitraps in the Field
3.7. The Population of Adults Ae. aegypti before Installation of Lethal Ovitraps
3.8. Adult Ae. aegypti Density after the Collection of Lethal Ovitraps
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gubler, D.J.; Kuno, G. Dengue and Dengue Hemorrhagic Fever; Cab International: Wallingford, UK, 1997. [Google Scholar]
- Hameed, M.; Ahmed, N.; Khan, I.A.; Shah, B.; Khan, A.; Ali, M.; Rasheed, M.T.; Junaid, K.; Adnan, M.; Zaki, A.B.; et al. Studies on the efficacy of selected insecticides against Anopheles mosquitoes of village Goth Bhoorji (Sindh) Pakistan. J. Entomol. Zool. Stud. 2015, 3, 169–173. [Google Scholar]
- Farrar, J.; Focks, D.; Gubler, D.; Barrera, R.; Guzman, M.G.; Simmons, C.; Kalayanarooj, S.; Lum, L.; McCall, P.J.; Lloyd, L.; et al. Towards a global dengue research agenda. Trop. Med. Int. Health 2007, 12, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.N.; Ritchie, S.A. Outbreaks of dengue in north Queensland, 1990–2008. Commun. Dis. Intell. 2009, 33, 32–33. [Google Scholar]
- Devine, G.J.; Furlong, M.J. Insecticide use: Contexts and ecological consequences. Agric. Hum. Values 2007, 24, 281–306. [Google Scholar] [CrossRef]
- Pucci, T.M.; Lynch, J.; Keiper, J.B. Insect composition of the Mosquito Magnet Pro® mosquito trap in northeastern Ohio. Great Lakes Entomol. 2003, 36, 25–30. [Google Scholar]
- Mills, N.E.; Semlitsch, R.D. Competition and predation mediate the indirect effects of an insecticide on southern leopard frogs. Ecol. Appl. 2004, 14, 1041–1054. [Google Scholar] [CrossRef]
- Williams, C.R.; Ritchie, S.A.; Long, S.A.; Dennison, N.; Russell, R.C. Impact of a bifenthrin-treated lethal ovitrap on Aedes aegypti oviposition and mortality in north Queensland, Australia. J. Med. Entomol. 2007, 44, 256–262. [Google Scholar] [CrossRef]
- Jahan, N.; Sarwar, M.S.; Riaz, T. Field evaluation of lethal ovitraps impregnated with deltamethrin against dengue vectors in Lahore, Pakistan. Biologia 2011, 57, 7–13. [Google Scholar]
- Perich, M.J.; Kardec, A.; Braga, I.A.; Portal, I.F.; Burge, R.; Zeichner, B.C.; Brogdon, W.A.; Wirtz, R.A. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med. Vet. Entomol. 2003, 17, 205–210. [Google Scholar] [CrossRef]
- Rapley, L.P.; Johnson, P.H.; Williams, C.R.; Silcock, R.M.; Larkman, M.; Long, S.A.; Russell, R.C.; Ritchie, S.A. A lethal ovitrap-based mass trapping scheme for dengue control in Australia: II. Impact on populations of the mosquito Aedes aegypti. Med. Vet. Entomol. 2019, 23, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Prokopec, G.M.; Kitron, U.; Montgomery, B.; Horne, P.; Ritchie, S.A. Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Negl. Trop. Dis. 2010, 4, e920. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Pyke, A.T.; Hall-Mendelin, S.; Day, A.; Mores, C.N.; Christofferson, R.C.; Gubler, D.J.; Bennett, S.N.; van den Hurk, A.F. An explosive epidemic of DENV-3 in Cairns, Australia. PLoS ONE 2013, 8, e68137. [Google Scholar] [CrossRef]
- Barrera, R.; Amador, M.; Acevedo, V.; Caban, B.; Felix, G.; Mackay, A.J. Use of the CDC autocidal gravid ovitrap to control and prevent outbreaks of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 145–154. [Google Scholar] [CrossRef]
- Kline, D.L. Mosquito population surveillance techniques. Tech. Bull. Fla. Mosq. Control Assoc. 2006, 7, 2–8. [Google Scholar]
- Zeichner, B.C.; Perich, M.J. Laboratory testing of a lethal ovitrap for Aedes aegypti. Med. Vet. Entomol. 1999, 13, 234–238. [Google Scholar] [CrossRef]
- Jahan, N.; Sarwar, M.S. Field Evaluation of Lethal Ovitraps for the Control of Dengue Vectors in Lahore, Pakistan. Pak. J. Zool. 2013, 45, 2. [Google Scholar]
- Khan, I.; Farid, A.; Zeb, A. Development of inexpensive and globally available larval diet for rearing Anopheles Stephens (Diptera: Culicidae) mosquitoes. Parasites Vectors 2013, 6, 90. [Google Scholar] [CrossRef]
- Khan, I.; Hussain, A.; Khan, A.; Khan, M.A. Surveillance of Aedes Mosquito in Swabi and districts of Khyber Pakhtunkhwa, Pakistan; Centre of Excellence in Marine Biology, University of Karachi: Karachi, Pakistan, 2015; Volume 1, pp. 17–26. [Google Scholar]
- Dibo, M.R.; Chierotti, A.P.; Ferrari, M.S.; Mendonça, A.L.; Chiaravalloti Neto, F. Study of the relationship between Aedes (Stegomyia) aegypti (egg and adult densities, dengue fever and climate in Mirassol, state of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz 2008, 103, 554–560. [Google Scholar] [CrossRef]
- Singh, R.K.; Das, M.K.; Dhiman, R.C.; Mittal, P.K.; Sinha, A.T.S. Preliminary investigation of dengue vectors in Ranchi, India. J. Vector Dis. 2008, 45, 170–173. [Google Scholar]
- Gunathilaka, N.; Ranathunge, T.; Udayanga, L.; Wijegunawardena, A.; Abeyewickreme, W. Oviposition preferences of dengue vectors; Aedes aegypti and Aedes albopictus in Sri Lanka under laboratory settings. Bull. Entomol. Res. 2018, 108, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.D.C. Measures of urban infestation levels for Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus in an entomological surveillance program. SUS Epidemiol. Rep. 1998, 7, 49–57. [Google Scholar] [CrossRef]
- Seenivasagan, T.; Sharma, K.R.; Sekhar, K.; Ganesan, K.; Prakash, S.; Vijayaraghavan, R. Electroantennogram, flight orientation, and oviposition responses of Aedes aegypti to the oviposition pheromone n-heneicosane. Parasitol. Res. 2009, 104, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.E.; Blackwell, A. Colour cues for oviposition behaviour in Toxorhynchites moctezuma and Toxorhynchites amboinensis mosquitoes. J. Vector Ecol. 2000, 25, 127–135. [Google Scholar]
- Kumawat, R.; Singh, K.V.; Bansal, S.K.; Singh, H. Use of different coloured ovitraps in the surveillance of Aedes mosquitoes in an arid-urban area of western Rajasthan, India. J. Vector Dis. 2014, 51, 320. [Google Scholar]
- Marin, G.; Mahiba, B.; Arivoli, S.; Tennyson, S. Does the colour of ovitrap influence the ovipositional preference of Aedes aegypti Linnaeus 1762 (Diptera: Culicidae). Int. J. Mosq. Res. 2020, 7, 11–15. [Google Scholar]
- Gopalakrishnan, R.; Das, M.; Baruah, I.; Veer, V.; Dutta, P. Studies on the ovitraps baited with hay and leaf infusions for the surveillance of dengue vector, Aedes albopictus in northeastern India. Trop. Biomed. 2012, 29, 598–604. [Google Scholar]
- Harwood, J.F.; Rama, V.; Hash, J.M.; Gordon, S.W. The attractiveness of the gravid Aedes trap to dengue vectors in Fiji. J. Med. Entomol. 2018, 55, 481–484. [Google Scholar] [CrossRef]
- Santana, A.L.; Roque, R.A.; Eiras, A.E. Characteristics of grass infusions as oviposition attractants to Aedes (Stegomyia)(Diptera: Culicidae). J. Med. Entomol. 2014, 43, 214–220. [Google Scholar] [CrossRef]
- Trexler, J.D.; Apperson, C.S.; Zurek, L.; Gemeno, C.; Schal, C.; Kaufman, M.; Wallace, L. Role of bacteria in mediating the oviposition responses of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2003, 40, 841–848. [Google Scholar] [CrossRef]
- Polson, A.K.; Curtis, C.; Seng, C.M.; Olson, J.G.; Chantha, N.; Rawlins, S.C. The use of ovitraps baited with hay infusion as a surveillance tool for Aedes aegypti mosquitoes in Cambodia. Den. Bull. 2002, 26, 178–184. [Google Scholar]
- Sithiprasasna, R.; Mahapibul, P.; Noigamol, C.; Perich, M.J.; Zeichner, B.C.; Burge, B.; Norris, S.L.W.; Jones, J.W.; Schleich, S.S.; Coleman, R.E. Field evaluation of a lethal ovitrap for the control of Aedes aegypti (Diptera: Culicidae) in Thailand. J. Med. Entomol. 2003, 40, 455–462. [Google Scholar] [CrossRef] [PubMed]
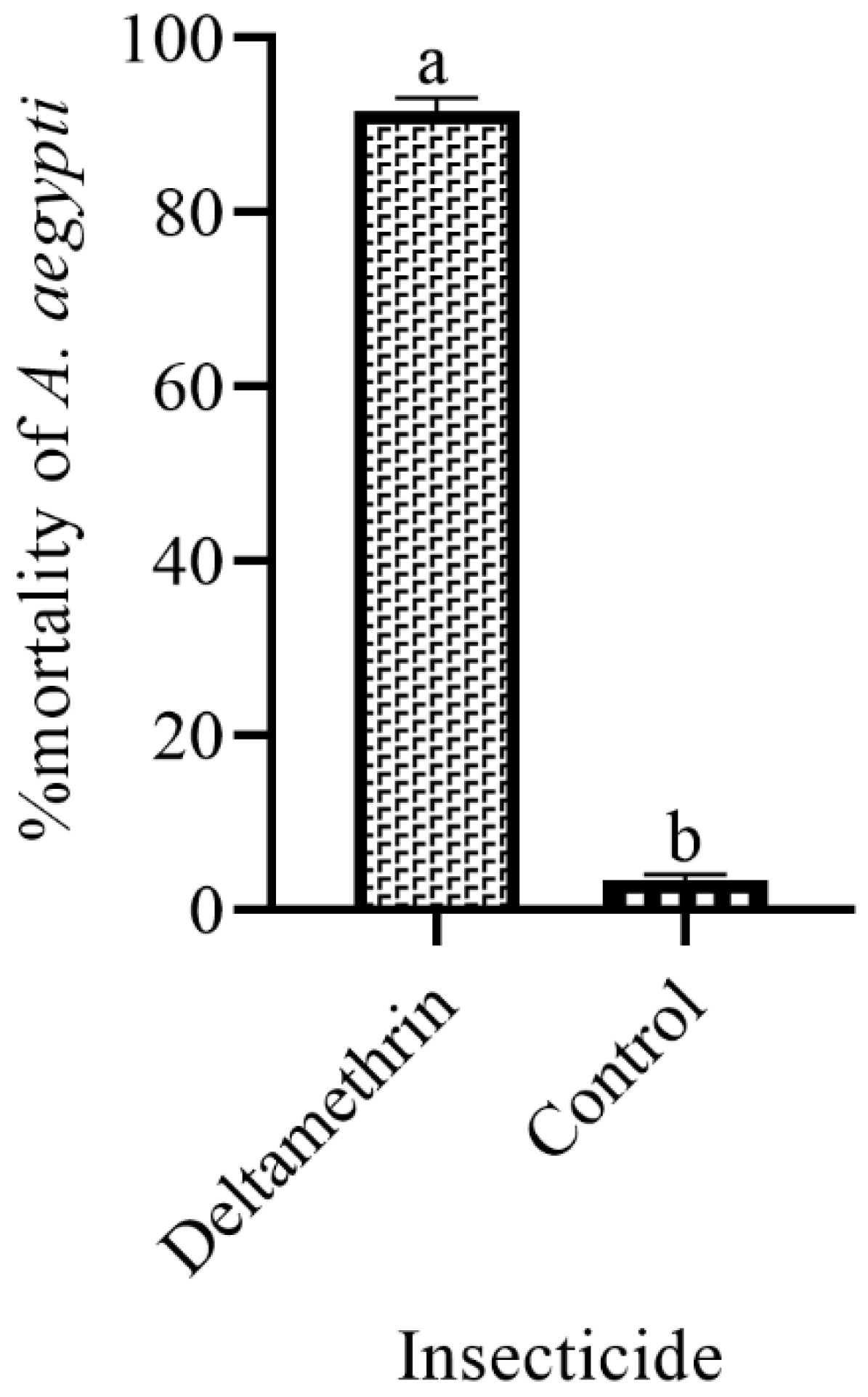
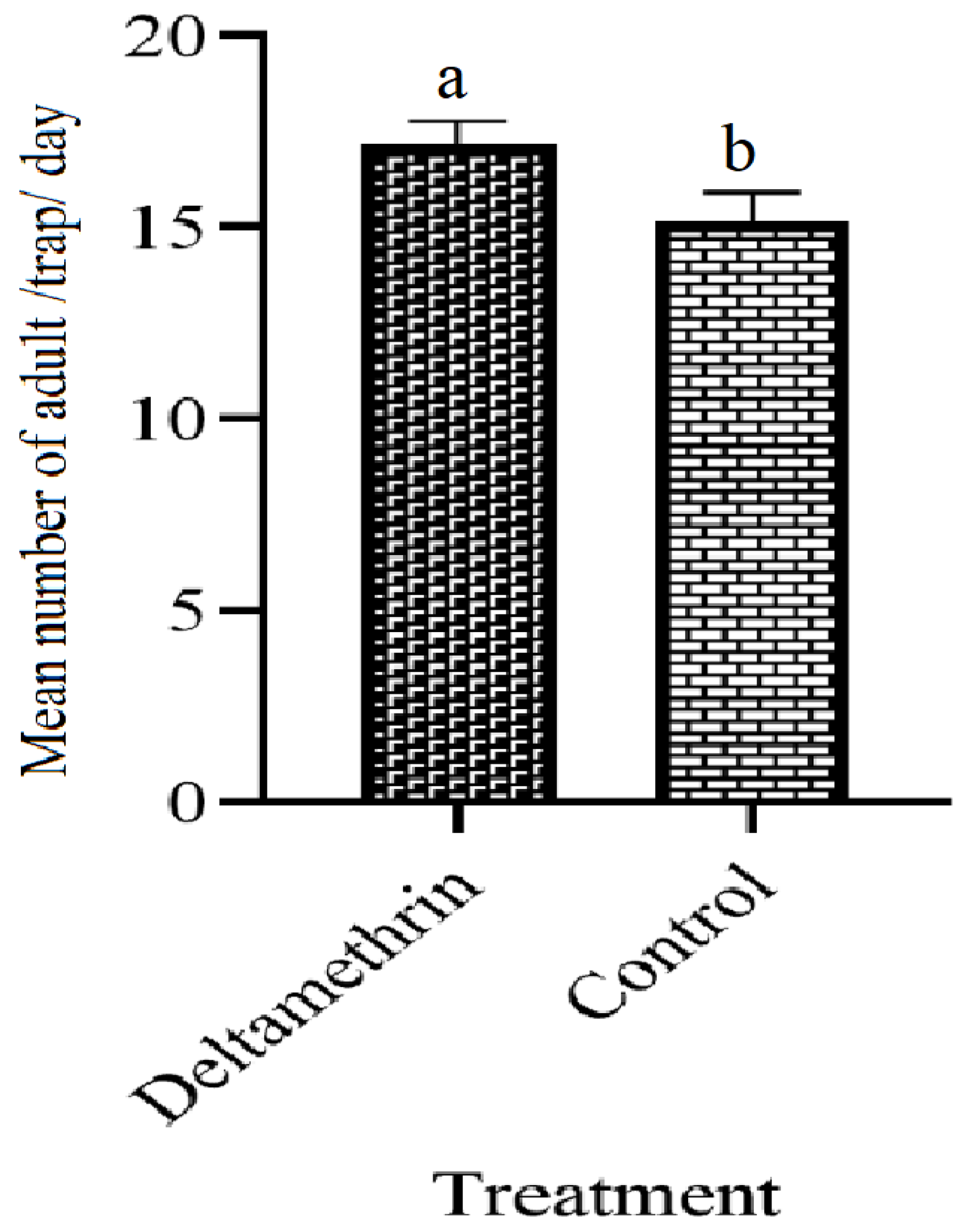
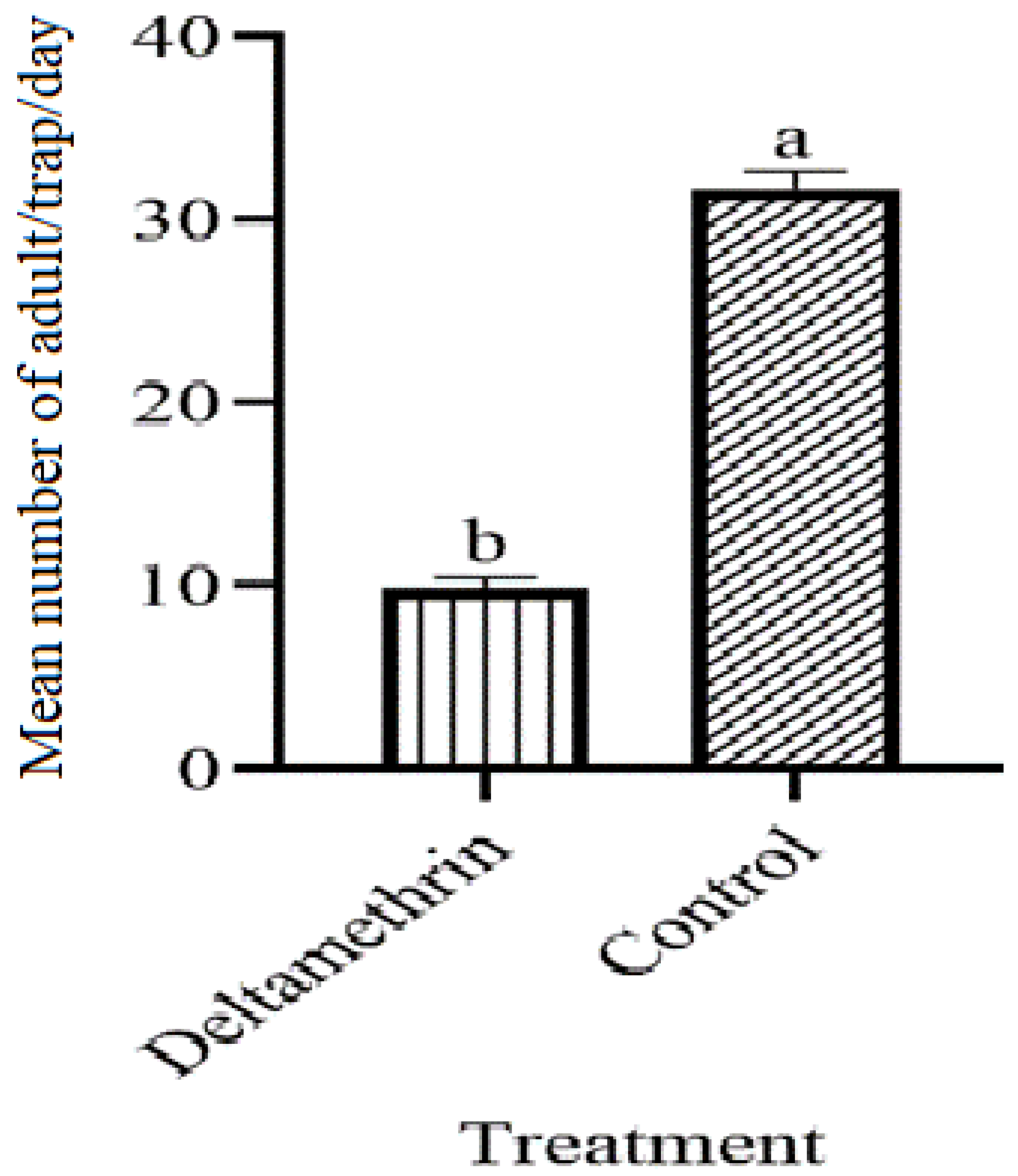
| S. No | Treatments | No. of Los | Positive Los | Total No. of Eggs | Sum of Total % | EDI | |||
|---|---|---|---|---|---|---|---|---|---|
| Installed | Collection | Total | (n) | OPI | |||||
| 1 | LO | 10 | 6 | 60 | 30 | 50 | 727 | 28.04 | 24.2 |
| 2 | Control | 10 | 6 | 60 | 50 | 83.3 | 1865 | 71.95 | 37.3 |
| 3 | Total | 20 | 12 | 120 | 80 | 66.6 | 2592 | 100 | 32.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Ullah, M.; Khan, G.Z.; Ahmed, N.; Shami, A.; El Hadi Mohamed, R.A.; Abd Al Galil, F.M.; Salman, M. Assessment of Various Colors Combined with Insecticides in Devising Ovitraps as Attracting and Killing Tools for Mosquitoes. Insects 2023, 14, 25. https://doi.org/10.3390/insects14010025
Khan A, Ullah M, Khan GZ, Ahmed N, Shami A, El Hadi Mohamed RA, Abd Al Galil FM, Salman M. Assessment of Various Colors Combined with Insecticides in Devising Ovitraps as Attracting and Killing Tools for Mosquitoes. Insects. 2023; 14(1):25. https://doi.org/10.3390/insects14010025
Chicago/Turabian StyleKhan, Adam, Misbah Ullah, Gul Zamin Khan, Nazeer Ahmed, Ashwag Shami, Rania Ali El Hadi Mohamed, Fahd Mohammed Abd Al Galil, and Muhammad Salman. 2023. "Assessment of Various Colors Combined with Insecticides in Devising Ovitraps as Attracting and Killing Tools for Mosquitoes" Insects 14, no. 1: 25. https://doi.org/10.3390/insects14010025
APA StyleKhan, A., Ullah, M., Khan, G. Z., Ahmed, N., Shami, A., El Hadi Mohamed, R. A., Abd Al Galil, F. M., & Salman, M. (2023). Assessment of Various Colors Combined with Insecticides in Devising Ovitraps as Attracting and Killing Tools for Mosquitoes. Insects, 14(1), 25. https://doi.org/10.3390/insects14010025






