Effects of Fluctuating Thermal Regimes on Life History Parameters and Body Size of Ophraella communa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Plants
2.2. Insect Culture
2.3. Fluctuating Thermal Regimes
2.4. Data Analysis
3. Results
3.1. Population Development of Ophraella communa under Thermal Fluctuation
3.2. Age-Specific Survival Rate and Population Parameters of Ophraella communa under Thermal Fluctuation
3.3. Population Dynamics of Ophraella communa under Thermal Fluctuation
3.4. Population Projection
3.5. Adult Fitness of Ophraella communa
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaffner, U.; Steinbach, S.; Sun, Y.; Skjøth, C.A.; de Weger, L.A.; Lommen, S.T.; Augustinus, B.A.; Bonini, M.; Karrer, G.; Šikoparija, B.; et al. Biological weed control to relieve millions from Ambrosia allergies in Europe. Nat. Commun. 2020, 11, 1745. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Chen, G.; Zhang, Y.; Ma, C.; Tian, Z.; Gao, X.; Chen, H.; Guo, J.; Zhou, Z. Rapid evolution of Ophraella communa cold tolerance in new low-temperature environments. J. Pest Sci. 2022, 95, 1233–1244. [Google Scholar] [CrossRef]
- Essl, F.; Biro, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A.; et al. Biological Flora of the British Isles: Ambrosia artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, F.; Chen, H.; Wan, F.; Guo, J.; Zhou, Z. Heritability and evolutionary potential drive cold hardiness in the overwintering Ophraella communa beetles. Front. Physiol. 2018, 9, 666. [Google Scholar] [CrossRef]
- Servick, K. Control freaks. Science 2018, 361, 542–545. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, H.; Zheng, X.; Guo, J.; Guo, W.; Li, M.; Luo, M.; Wan, F. Control of the invasive weed Ambrosia artemisiifolia with Ophraella communa and Epiblema strenuana. Biocontrol Sci. Technol. 2014, 24, 950–964. [Google Scholar] [CrossRef]
- Mouttet, R.; Augustinus, B.; Bonini, M.; Chauvel, B.; Desneux, N.; Gachet, E.; Le Bourgeois, T.; Müller-Schärer, H.; Thibaudon, M.; Schaffner, U. Estimating economic benefits of biological control of Ambrosia artemisiifolia by Ophraella communa in southeastern France. Basic Appl. Ecol. 2018, 33, 14–28. [Google Scholar] [CrossRef]
- Cardarelli, E.; Musacchio, A.; Montagnani, C.; Bogliani, G.; Citterio, S.; Gentili, R. Ambrosia artemisiifolia control in agricultural areas: Effect of grassland seeding and herbivory by the exotic leaf beetle Ophraella communa. NeoBiota 2018, 38, 55–76. [Google Scholar] [CrossRef][Green Version]
- Araújo, M.B.; Ferri-Yáñez, F.; Bozinovic, F.; Marquet, P.A.; Valladares, F.; Chown, S.L. Heat freezes niche evolution. Ecol. Lett. 2013, 16, 1206–1219. [Google Scholar] [CrossRef]
- Önder, B.Ş.; Aksoy, C.F. Seasonal variation in wing size and shape of Drosophila melanogaster reveals rapid adaptation to environmental changes. Sci. Rep. 2022, 12, 14622. [Google Scholar] [CrossRef]
- Chen, H.; Solangi, G.S.; Guo, J.; Wan, F.; Zhou, Z. Antioxidant Responses of Ragweed Leaf Beetle Ophraella communa (Coleoptera: Chrysomelidae) Exposed to Thermal Stress. Front. Physiol. 2018, 9, 808. [Google Scholar] [CrossRef]
- Sandel, B.; Dangremond, E.M. Climate change and the invasion of California by grasses. Glob. Chang. Biol. 2012, 18, 277–289. [Google Scholar] [CrossRef]
- Chen, H.; Solangi, G.S.; Zhao, C.; Yang, L.; Guo, J.; Wan, F.; Zhou, Z. Physiological Metabolic Responses of Ophraella communa to High Temperature Stress. Front. Physiol. 2019, 10, 1053. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, X.; Luo, M.; Guo, J.; Solangi, G.S.; Wan, F.; Zhou, Z. Effect of short-term high-temperature exposure on the life history parameters of Ophraella communa. Sci. Rep. 2018, 8, 13969. [Google Scholar] [CrossRef]
- Tian, Z.; Ma, C.; Zhao, C.; Zhang, Y.; Gao, X.; Tian, Z.; Chen, H.; Guo, J.; Zhou, Z. Heat wave event facilitates defensive responses in invasive C3 plant Ambrosia artemisiifolia L. under elevated CO2 concentration to the detriment of Ophraella communa. Front. Plant Sci. 2022, 13, 907764. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, H.; Ma, C.; Guo, J.; Wan, F.; Zhou, Z. Effects of age at mating of both sexes on female longevity and fecundity performance in Ophraella communa LeSage (Coleoptera: Chrysomelidae). Biocontrol Sci. Technol. 2017, 27, 1145–1152. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, X.; Luo, M.; Guo, J.; Luo, Y.; Zhou, Z.; Wan, F. Effects of high temperature on body size and weight of Ophraella communa. Biocontrol Sci. Technol. 2014, 24, 882–890. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Ma, C. Sporadic short temperature events cannot be neglected in predicting impacts of climate change on small insects. J. Insect Physiol. 2019, 112, 48–56. [Google Scholar] [CrossRef]
- Ma, C.S.; Chen, Y.W. Effects of constant temperature, exposure period, and age on diapause induction in Trichogramma dendrolimi. Biol. Control 2006, 36, 267–273. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, J.; Chen, H.; Wan, F. Effects of temperature on survival, development, longevity, and fecundity of Ophraella communa (Coleoptera: Chrysomelidae), a potential Biological Control agent Against Ambrosia artemisiifolia (Asterales: Asteraceae). Environ. Entomol. 2010, 39, 1021–1027. [Google Scholar] [CrossRef]
- Chen, H. Reproductive and physiological responses of a biocontrol agent of common ragweed, Ophraella communa LeSage (Coleoptera: Chrysomelidae) to Abnormal High Temperature. Ph.D. Thesis, Hunan Agricultural University, Changsha, China, 2012. [Google Scholar]
- Zhang, Y.; Zhao, C.; Ma, W.; Cui, S.; Chen, H.; Ma, C.; Guo, J.; Wan, F.; Zhou, Z. Larger males facilitate population expansion in Ophraella communa. J. Anim. Ecol. 2021, 90, 2782–2792. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Life-Table analysis Incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Mignani, S.; Rosa, R. The moving block bootstrap to assess the accuracy of statistical estimates in Ising model simulations. Computer Phys. Commun. 1995, 92, 203–213. [Google Scholar] [CrossRef]
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Franken, O.; Huizinga, M.; Ellers, J.; Berg, M.P. Heated communities: Large inter- and intraspecific variation in heat tolerance across trophic levels of a soil arthropod community. Oecologia 2018, 186, 311–322. [Google Scholar] [CrossRef]
- Berger, M.; Bastl, K.; Bastl, M.; Dirr, L.; Hutter, H.-P.; Moshammer, H.; Gstöttner, W. Impact of air pollution on symptom severity during the birch, grass and ragweed pollen period in Vienna, Austria: Importance of O3 in 2010–2018. Environ. Pollut. 2020, 263, 114526. [Google Scholar] [CrossRef]
- Sun, Y.; Bossdorf, O.; Grados, R.D.; Liao, Z.; Muller-Scharer, H. Rapid genomic and phenotypic change in response to climate warming in a widespread plant invader. Glob. Chang. Biol. 2020, 26, 6511–6522. [Google Scholar] [CrossRef]
- Zhao, C.; Yue, L.; Wang, Y.; Guo, J.; Zhou, Z.; Wan, F. Relationship between copulation and cold hardiness in Ophraella communa (Coleoptera: Chrysomelidae). J. Integr. Agric. 2019, 18, 900–906. [Google Scholar] [CrossRef]
- Du, Y.; Ma, C.; Zhao, Q.; Gang, M. Effects of heat stress on physiological and biochemical mechanisms of insects: A literature review. Acta Ecol. Sin. 2007, 27, 1565–1572. [Google Scholar]
- Hsu, A.-L.; Murphy, C.T.; Kenyon, C. Regulation of aging and age-Related disease by DAF-16 and heat-shock factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef]
- Angilletta, M. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009; pp. 1–302. [Google Scholar]
- Ma, C.; Wang, L.; Zhang, W.; Rudolf, V.H.W. Resolving biological impacts of multiple heat waves: Interaction of hot and recovery days. Oikos 2018, 127, 622–633. [Google Scholar] [CrossRef]
- Bowler, K.; Terblanche, J.S. Insect thermal tolerance: What is the role of ontogeny, ageing and senescence? Biol. Rev. Camb. Philos. Soc. 2008, 83, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Ju, R.T.; Chen, G.B.; Wang, F.; Li, B. Effects of heat shock, heat exposure pattern, and heat hardening on survival of the sycamore lace bug, Corythucha ciliata. Entomol. Exp. Appl. 2011, 141, 168–177. [Google Scholar] [CrossRef]
- Duyck, P.F.; Sterlin, J.F.; Quilici, S. Survival and development of different life stages of Bactrocera zonata (Diptera: Tephritidae) reared at five constant temperatures compared to other fruit fly species. Bull. Entomol. Res. 2004, 94, 89–93. [Google Scholar] [CrossRef]
- Klockmann, M.; Gunter, F.; Fischer, K. Heat resistance throughout ontogeny: Body size constrains thermal tolerance. Glob. Chang. Biol. 2017, 23, 686–696. [Google Scholar] [CrossRef]
- Davis, J.A.; Radcliffe, E.B.; Ragsdale, D.W. Effects of high and fluctuating temperatures on Myzus persicae (Hemiptera: Aphididae). Environ. Entomol. 2006, 35, 1461–1468. [Google Scholar] [CrossRef]
- Garcia-Ruiz, E.; Marco, V.; Perez-Moreno, I. Effects of variable and constant temperatures on the embryonic development and survival of a new grape pest, Xylotrechus arvicola (Coleoptera: Cerambycidae). Environ. Entomol. 2011, 40, 939–947. [Google Scholar] [CrossRef]
- Kersting, U.; Satar, S.; Uygun, N. Effect of temperature on development rate and fecundity of apterous Aphis gossypii Glover (Hom. Aphididae) reared on Gossypium hirsutum L. J. Appl. Entomol. 1999, 123, 23–27. [Google Scholar] [CrossRef]
- Chown, S.L.; Terblanche, J.S. Physiological dversity in insects: Ecological and evolutionary contexts. Adv. Insect Physiol. 2006, 33, 50–152. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Hercus, M.J.; Loeschcke, V.; Rattan, S.I.S. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology 2003, 3, 149–156. [Google Scholar] [CrossRef]
- Cônsoli, F.L.; Parra, J.R.P. Effects of constant and alternating temperatures on Trichogramma galloiZucchi (Hym. Trichogrammatidae) biology. I. Development and thermal requirements. J. Appl. Entomol. 1995, 119, 415–418. [Google Scholar] [CrossRef]
- Butler, C.D.; Trumble, J.T. Predicting population dynamics of the parasitoid Cotesia marginiventris (Hymenoptera: Braconidae) resulting from novel interactions of temperature and selenium. Biocontrol Sci. Technol. 2010, 20, 391–406. [Google Scholar] [CrossRef]
- Kjaersgaard, A.; Pertoldi, C.; Loeschcke, V.; Blanckenhorn, W.U. The effect of fluctuating temperatures during development on fitness-related traits of Scatophaga stercoraria (Diptera: Scathophagidae). Environ. Entomol. 2013, 42, 1069–1078. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef]
- Calabrese, E.J. Converging concepts: Adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res. Rev. 2008, 7, 8–20. [Google Scholar] [CrossRef]
- Silbermann, R.; Tatar, M. Reproductive costs of heat shock protein in transgenic Drosophila melanogaster. Evolution 2000, 54, 2038. [Google Scholar] [CrossRef]
- Honěk, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U. The evolution of body size: What keeps organisms small? Q. Rev. Biol. 2000, 75, 385–407. [Google Scholar] [CrossRef]
- Gruntenko, N.E.; Bownes, M.; Terashima, J.; Sukhanova, M.; Raushenbach, I.Y. Heat stress affects oogenesis differently in wild-type Drosophila virilis and a mutant with altered juvenile hormone and 20-hydroxyecdysone levels. Insect Mol. Biol. 2003, 12, 393–404. [Google Scholar] [CrossRef]
- Colinet, H.; Hance, T. Male reproductive potential of Aphidius colemani (Hymenoptera: Aphidiinae) exposed to constant or fluctuating thermal regimens. Environ. Entomol. 2009, 38, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.P.; Yocum, G.D.; Denlinger, D.L. Thermotolerance and rapid cold hardening ameliorate the negative effects of brief exposures to high or low temperatures on fecundity in the flesh fly, Sarcophaga crassipalpis. Physiol. Entomol. 2010, 25, 330–336. [Google Scholar] [CrossRef]
- Santidrian Tomillo, P.; Spotila, J.R. Temperature-dependent sex determination in sea turtles in the context of climate change: Uncovering the adaptive significance. Bioessays 2020, 42, e2000146. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.C.; Spinks, P.Q.; Shaffer, H.B. A global phylogeny of turtles reveals a burst of climate-associated diversification on continental margins. Proc. Natl. Acad. Sci. USA 2021, 118, e2012215118. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.J.; Martinez-Lopez, M.; Morales-Garduza, M.A.; Garcia-Morales, R.; Charruau, P.; Gallardo-Cruz, J.A. The sex-determination pattern in crocodilians: A systematic review of three decades of research. J. Anim. Ecol. 2019, 88, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Morales Merida, A.; Helier, A.; Cortes-Gomez, A.A.; Girondot, M. Hatching success rather than temperature-dependent sex determination as the main driver of Olive Ridley (Lepidochelys olivacea) nesting activity in the Pacific Coast of Central America. Animals 2021, 11, 3168. [Google Scholar] [CrossRef] [PubMed]
- Holleley, C.E.; O’Meally, D.; Sarre, S.D.; Marshall Graves, J.A.; Ezaz, T.; Matsubara, K.; Azad, B.; Zhang, X.; Georges, A. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 2015, 523, 79–82. [Google Scholar] [CrossRef]
- Lockley, E.C.; Eizaguirre, C. Effects of global warming on species with temperature-dependent sex determination: Bridging the gap between empirical research and management. Evol. Appl. 2021, 14, 2361–2377. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Insect temperature–body size trends common to laboratory, latitudinal and seasonal gradients are not found across altitudes. Funct. Ecol. 2018, 32, 948–957. [Google Scholar] [CrossRef]
- Tseng, M.; Kaur, K.M.; Soleimani Pari, S.; Sarai, K.; Chan, D.; Yao, C.H.; Porto, P.; Toor, A.; Toor, H.S.; Fograscher, K. Decreases in beetle body size linked to climate change and warming temperatures. J. Anim. Ecol. 2018, 87, 647–659. [Google Scholar] [CrossRef]
- CaraDonna, P.J.; Cunningham, J.L.; Iler, A.M. Experimental warming in the field delays phenology and reduces body mass, fat content and survival: Implications for the persistence of a pollinator under climate change. Funct. Ecol. 2018, 32, 2345–2356. [Google Scholar] [CrossRef]
- Forster, J.; Hirst, A.G.; Atkinson, D. Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proc. Natl. Acad. Sci. USA 2012, 109, 19310–19314. [Google Scholar] [CrossRef]
- Shelomi, M. Where are we now? Bergmann’s rule sensu lato in insects. Am. Nat. 2012, 180, 511–519. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and organism size-A biological law for ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Huey, R. Size, temperature, and fitness: Three rules. Evol. Ecol. Res. 2008, 10, 251–268. [Google Scholar]
- Pereboom, J.J.; Biesmeijer, J.C. Thermal constraints for stingless bee foragers: The importance of body size and coloration. Oecologia 2003, 137, 42–50. [Google Scholar] [CrossRef]
- Teder, T.; Tammaru, T. Sexual size dimorphism within species increases with body size in insects. Oikos 2005, 108, 321–334. [Google Scholar] [CrossRef]
- Guo, W.; Zhou, Z.; Guo, J.; Ma, J. Morphological characteristics of Ophraella communa adults. Plant Prot. 2010, 36, 179–182. [Google Scholar]
- Stillwell, R.C.; Blanckenhorn, W.U.; Teder, T.; Davidowitz, G.; Fox, C.W. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: From physiology to evolution. Annu. Rev. Entomol. 2010, 55, 227–245. [Google Scholar] [CrossRef]
- Karan, D.; Morin, J.P.; Moreteau, B.; David, J.R. Body size and developmental temperature in drosophila melanogaster: Analysis of body weight reaction norm. J. Therm. Biol. 1998, 23, 301–309. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Ragland, G.J.; Diamond, S.E. Evolution in a constant environment: Thermal fluctuations and thermal sensitivity of laboratory and field populations of Manduca sexta. Evolution 2009, 63, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Czarnoleski, M.; Cooper, B.S.; Kierat, J.; Angilletta, M.J., Jr. Flies developed small bodies and small cells in warm and in thermally fluctuating environments. J. Exp. Biol. 2013, 216, 2896–2901. [Google Scholar] [CrossRef] [PubMed]
- Feldheim, K.A.; Gruber, S.H.; Ashley, M.V. Reconstruction of parental microsatellite genotypes reveals female polyandry and philopatry in the lemon shark, Negaprion brevirostris. Evolution 2004, 58, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Petavy, G.; David, J.R.; Gibert, P.; Moreteau, B. Viability and rate of development at different temperatures in Drosophila: A comparison of constant and alternating thermal regimes. J. Therm. Biol. 2001, 26, 29–39. [Google Scholar] [CrossRef]
- Sun, Y.; Muller-Scharer, H.; Schaffner, U. Fighting neobiota with neobiota: Consider it more often and do it more rigorously. Biol. Conserv. 2022, 268, 109506. [Google Scholar] [CrossRef]
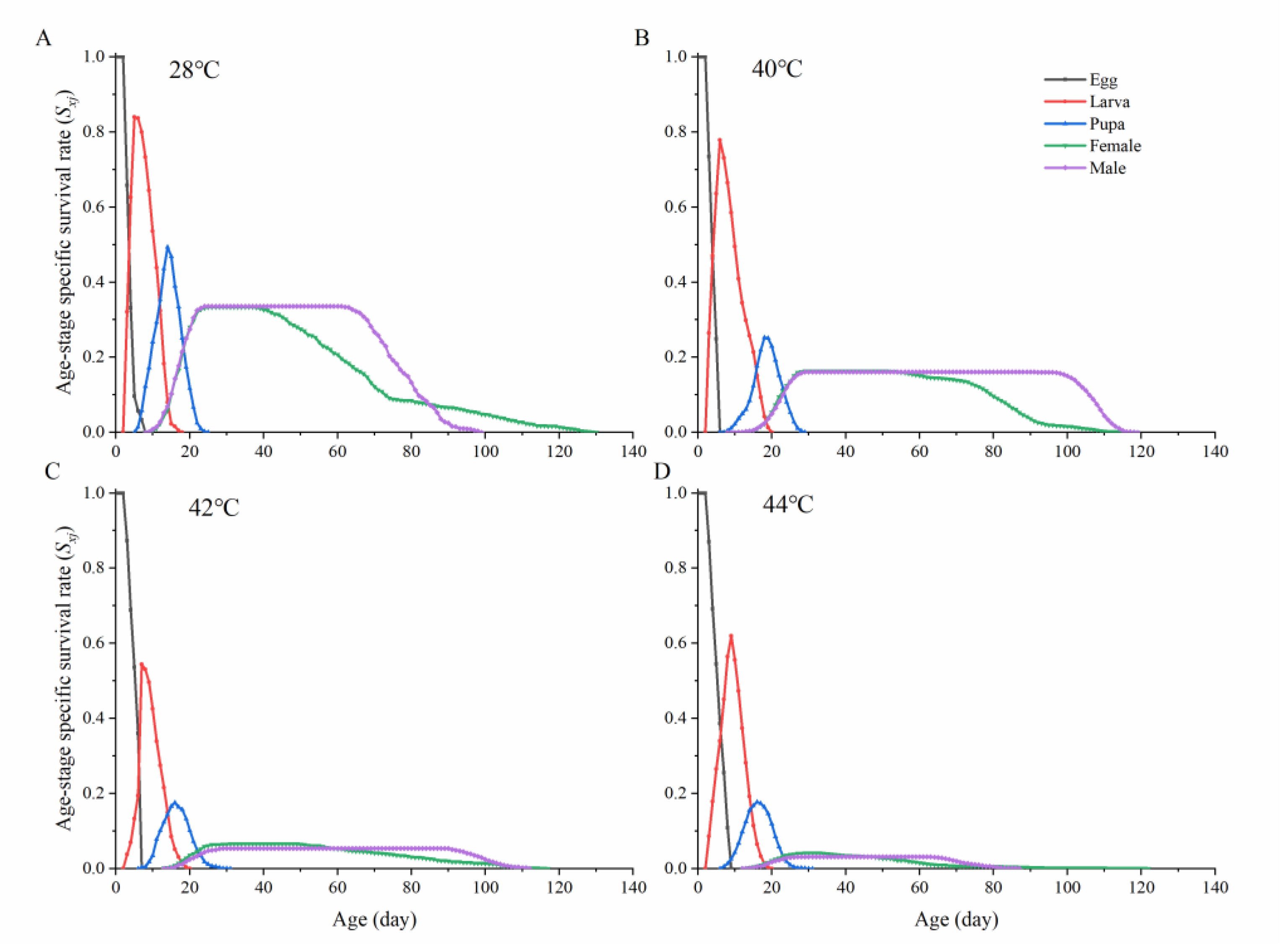
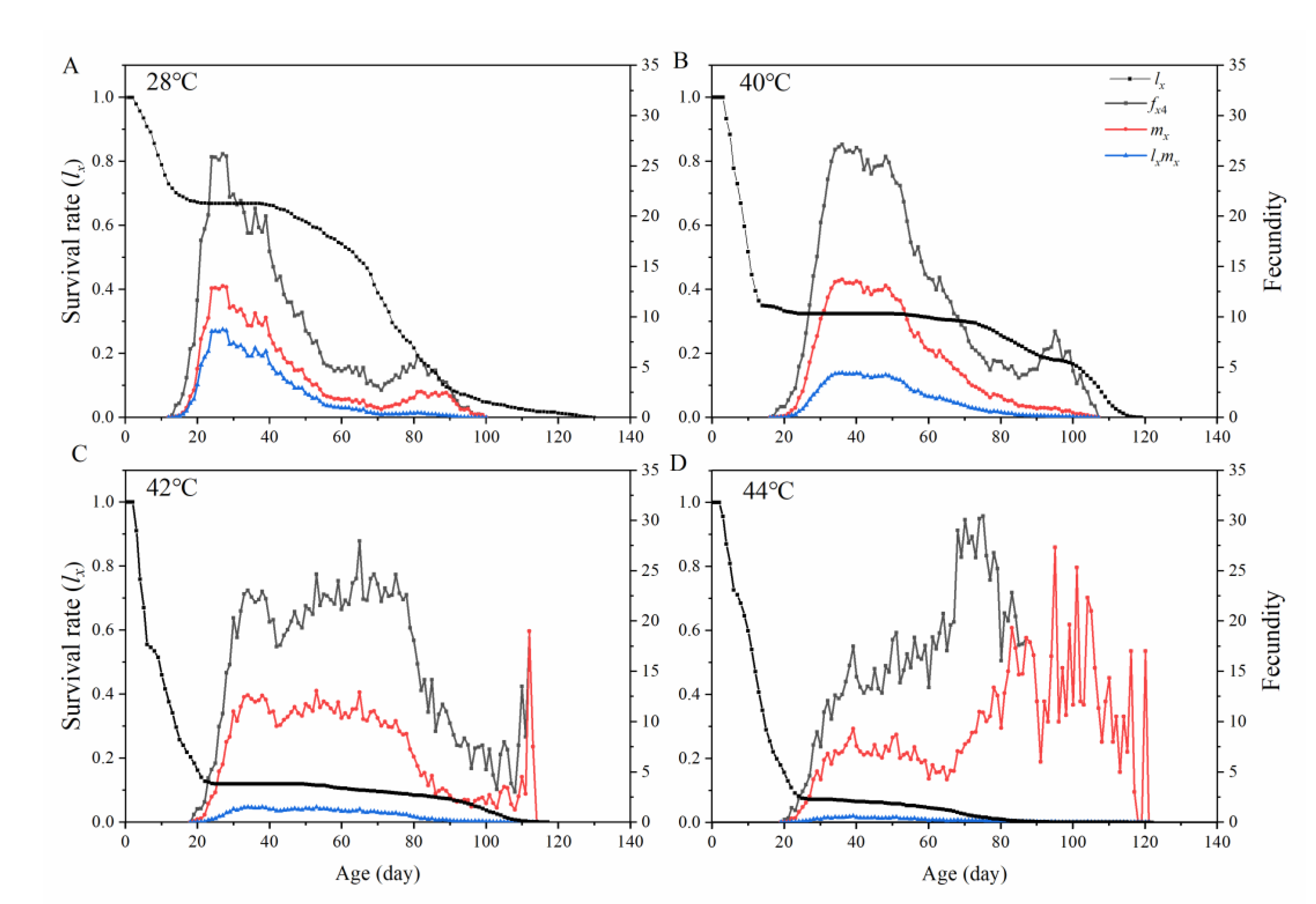
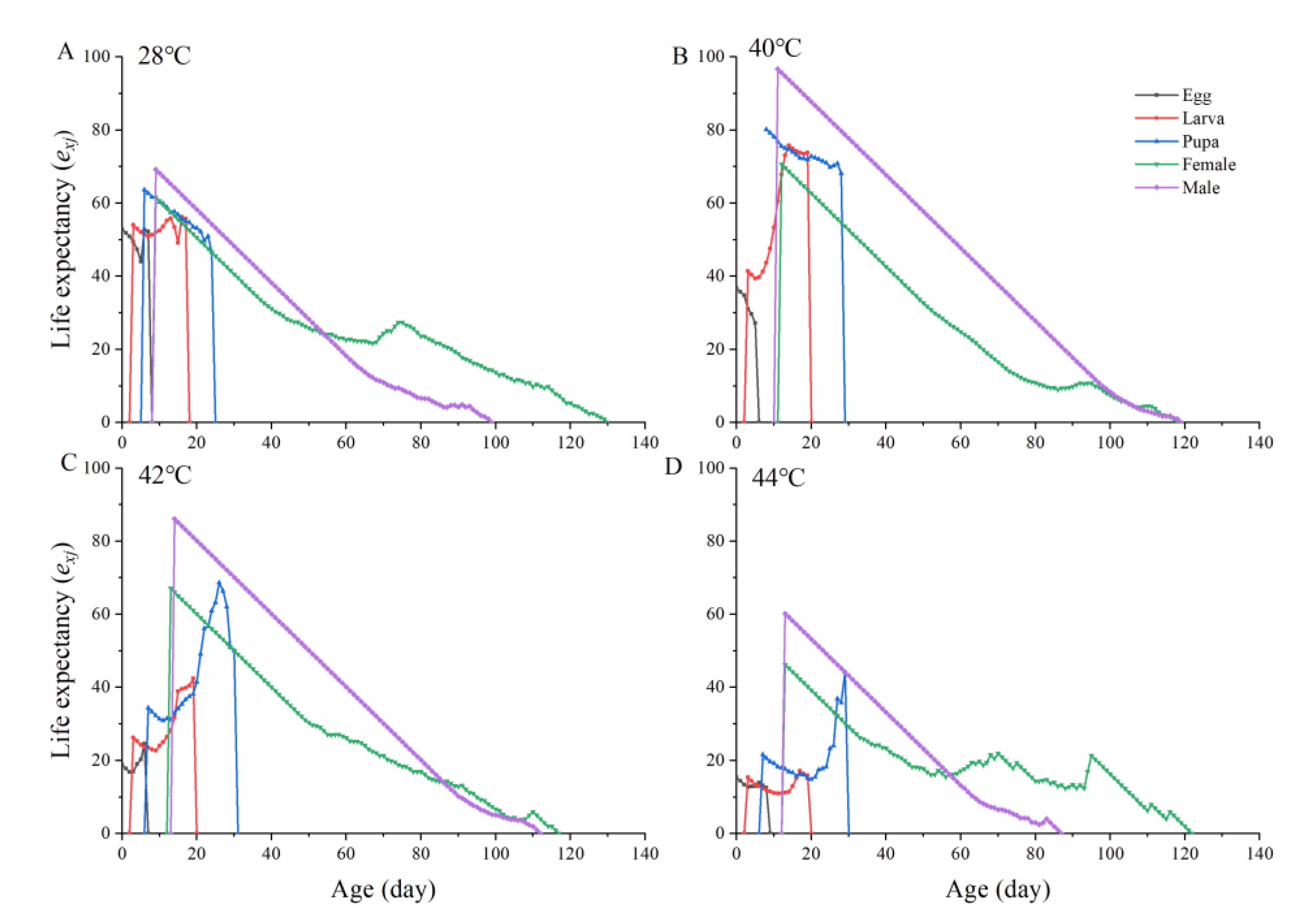



| Population Parameters | Developmental Period and Longevity (Days) | |||
|---|---|---|---|---|
| 28 °C (n) | 40 °C (n) | 42 °C (n) | 44 °C (n) | |
| Egg development duration | 4.15 ± 0.04 a (936) | 4.29 ± 2.33 b (3020) | 6.21 ± 0.04 c (1575) | 6.30 ± 0.05 c (2311) |
| Larva development duration | 7.34 ± 0.09 a (668) | 11.33 ± 0.07 b (1060) | 7.58 ± 0.12 a (459) | 7.31 ± 0.09 a (660) |
| Pupa development duration | 5.76 ± 0.08 a (626) | 6.36± 0.07 b (980) | 6.80 ± 0.14 c (188) | 6.68 ± 0.16 bc (168) |
| Pre-adult development duration | 17.22 ± 0.13 a (626) | 22.01± 0.11 c (980) | 20.79 ± 0.26 b (188) | 20.35 ± 0.27 b (168) |
| Adult development duration | 57.21 ± 0.66 b (626) | 73.07 ± 0.49 c (980) | 68.33 ± 1.22 d (188) | 44.97 ± 1.20 a (168) |
| Female adult development duration | 53.28 ± 1.24 b (312) | 60.76 ± 0.58 c (495) | 59.68 ± 1.81 c (103) | 38.85 ± 1.87 a (95) |
| Male adult development duration | 61.11 ± 0.43 b (314) | 85.64 ± 0.14 d (485) | 78.82 ± 0.46 c (85) | 52.93 ± 0.59 a (73) |
| Overall longevity 1 | 52.87 ± 1.12 a (936) | 36.78 ± 0.76 b (3020) | 18.82 ± 0.68 c (1575) | 15.43 ± 0.33 d (2311) |
| Longevity 2 | 74.43 ± 0.68 b (626) | 95.08 ± 0.51 d (980) | 89.13 ± 1.25 c (188) | 65.32 ± 1.22 a (168) |
| Longevity (female adult) | 70.59 ± 1.26 b (312) | 82.69 ± 0.59 c (495) | 80.07 ± 1.82 c (103) | 59.24 ± 1.89 a (95) |
| Longevity (male adult) | 78.25 ± 0.46 b (314) | 107.73 ± 0.20 d (485) | 100.11 ± 0.61 c (85) | 73.20 ± 0.69 a (73) |
| APOP | 4.80 ± 0.04 a (312) | 5.76± 0.05 b (495) | 6.54 ± 0.08 c (103) | 8.02 ± 0.12 d (95) |
| TPOP | 22.11 ± 0.18 a (312) | 27.69 ± 0.15 bc (495) | 26.93 ± 0.36 b (103) | 28.43 ± 0.39 c (95) |
| Population Parameters | Temperature (°C) | |||
|---|---|---|---|---|
| 28 °C (n) | 40 °C (n) | 42 °C (n) | 44 °C (n) | |
| Oviposition days | 24.96 ± 0.70 a (312) | 35.53 ± 0.71 b (495) | 44.39 ± 1.65 b (103) | 23.15 ± 1.69 a (95) |
| Fecundity (offspring/female) | 637.5 ± 12.97 b (312) | 940.1 ± 19.37 c (495) | 1059.8 ± 41.32 d (103) | 480.0 ± 41.33 a (95) |
| Developmental Stage | Survival Rate and Sex Ratio (%) | |||
|---|---|---|---|---|
| 28 °C (n) | 40 °C (n) | 42 °C (n) | 44 °C (n) | |
| Egg | 91.88 ± 0.88 a (936) | 80.00 ± 0.73 b (3020) | 55.75 ± 1.27 d (1575) | 73.82 ± 0.90 c (2311) |
| Larva | 77.69 ± 1.42 a (668) | 43.88 ± 1.01 c (1060) | 52.28 ± 1.69 b (459) | 38.69 ± 1.18 d (660) |
| Pupa | 93.71 ± 0.94 a (626) | 92.45 ± 0.82 a (980) | 40.96 ± 2.30 b (188) | 25.45 ± 1.67 c (168) |
| Pre-adult | 66.88 ± 1.59 a (936) | 32.45 ± 0.85 b (3020) | 11.94 ± 0.81 c (1575) | 7.27 ± 0.54 d (2311) |
| Sex ratio (female-to-male) | 0.99 ± 0.08 a (936) | 1.02 ± 0.07 a (3020) | 1.21 ± 0.18 a (1575) | 1.30 ± 0.21 a (2311) |
| Population Parameters | Temperature (℃) | |||
|---|---|---|---|---|
| 28 °C | 40 °C | 42 °C | 44 °C | |
| r (d−1) | 0.181 ± 0.002 a | 0.125 ± 0.001 b | 0.098 ± 0.003 c | 0.067 ± 0.003 d |
| λ (d−1) | 1.199 ± 0.003 a | 1.134 ± 0.002 b | 1.103 ± 0.003 c | 1.070 ± 0.003 d |
| Ro | 212.50 ± 10.69 a | 154.08 ± 7.07 b | 69.31± 7.06 c | 19.73 ± 2.62 d |
| T (d) | 29.58 ± 0.24 a | 40.16 ± 0.23 b | 43.45 ± 0.61 c | 44.37 ± 0.19 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Chen, H.; Guo, J.; Zhou, Z. Effects of Fluctuating Thermal Regimes on Life History Parameters and Body Size of Ophraella communa. Insects 2022, 13, 821. https://doi.org/10.3390/insects13090821
Zhao C, Chen H, Guo J, Zhou Z. Effects of Fluctuating Thermal Regimes on Life History Parameters and Body Size of Ophraella communa. Insects. 2022; 13(9):821. https://doi.org/10.3390/insects13090821
Chicago/Turabian StyleZhao, Chenchen, Hongsong Chen, Jianying Guo, and Zhongshi Zhou. 2022. "Effects of Fluctuating Thermal Regimes on Life History Parameters and Body Size of Ophraella communa" Insects 13, no. 9: 821. https://doi.org/10.3390/insects13090821
APA StyleZhao, C., Chen, H., Guo, J., & Zhou, Z. (2022). Effects of Fluctuating Thermal Regimes on Life History Parameters and Body Size of Ophraella communa. Insects, 13(9), 821. https://doi.org/10.3390/insects13090821








