Simple Summary
The red flour beetle, Tribolium castaneum, and lesser grain borer, Rhyzopertha dominica, cause significant damage to stored commodities, including grains. The most widely used fumigant to control stored-product insects is phosphine. However, resistance to this fumigant is worldwide problem. In this study, we examined the lipid content of phosphine resistant and susceptible strains of T. castaneum and R. dominica. The results showed that the resistant strains of both species contained more lipids than the susceptible strains. The finding will contribute to the understanding of the mechanisms of phosphine resistance and provide additional information for developing strategies for managing the resistance problem.
Abstract
Insects rely on lipids as an energy source to perform various activities, such as growth, flight, diapause, and metamorphosis. This study evaluated the role of lipids in phosphine resistance by stored-grain insects. Phosphine resistant and susceptible strains of the two main stored-grain insects, Tribolium castaneum and Rhyzopertha dominica, were analyzed using liquid chromatography-mass spectroscopy (LC-MS) to determine their lipid contents. Phosphine resistant strains of both species had a higher amount of lipids than susceptible stains. Significant variance ratios between the resistant and susceptible strains of T. castaneum were observed for glycerolipids (1.13- to 53.10-fold) and phospholipids (1.05- to 20.00-fold). Significant variance ratios between the resistant and susceptible strains of R. dominica for glycerolipids were 1.04- to 31.50-fold and for phospholipids were 1.04- to 10.10-fold. Glycerolipids are reservoirs to face the long-term energy shortage. Phospholipids act as a barrier to isolate the cells from the surrounding environment and allow each cell to perform its specific function. Thus, lipids offer a consistent energy source for the resistant insect to survive under the stress of phosphine fumigation and provide a suitable environment to protect the mitochondria from phosphine. Hence, it was proposed through this study that the lipid content of phosphine-resistant and phosphine-susceptible strains of T. castaneum and R. dominica could play an important role in the resistance of phosphine.
Keywords:
phosphine; insect resistance; T. castaneum; R. dominica; insect lipid; glycerolipids; phospholipids 1. Introduction
The red flour beetle, Tribolium castaneum (Tenebrionidae: Coleoptera), and the lesser grain borer, Rhyzopertha dominica (Bostrichidae: Coleoptera), are stored-product insect pests that can cause serious damage to various commodities, including grain [1,2]. In addition to feeding on the grains, the damage by these species derives from contamination of the products with insect parts, ecdysis skin and individuals at each life stage [3], which can severely reduce grain quality and economic value [4].
Phosphine is one of the most widely used fumigants currently approved to control stored-product insect pests [5]. However, long-term use and ineffective applications have led to resistance to this fumigant by strains of stored-grain insect species, particularly T. castaneum and R. dominica [6,7]. Resistant insects usually absorb less phosphine than susceptible insects [8]. Suggesting that the exclusion of phosphine is a resistance mechanism [8]. Therefore, it was proposed that the metabolic and physiological variations between the resistant and susceptible strains are strongly associated with the resistance phenotypes [8,9,10]. A genetic study on dihydrolipoamide dehydrogenase (DLD) showed that it is a flavin-dependent oxidoreductase crucial for energy metabolism [11] and is important in phosphine resistance [9]. Dihydrolipoamide dehydrogenase comprises a reactive disulfide and a flavin adenine dinucleotide (FAD) cofactor directly involved in electron transfer in mitochondria [12]. As a by-product of its role in aerobic respiration, the DLD enzyme (rph2) generates reactive oxygen species (ROS), which contribute to phosphine toxicity to target insects [9]. In cellular membranes, fatty acid desaturase (FADS (rph1)) produces desaturated fatty acids that are targets of ROS [13]. Exposure to phosphine exacerbates ROS production, which damages fatty acids [9,13]. Therefore, rph1 and rph2 interact synergistically since FADS (rph1) sensitizes animals to ROS (13), whereas DLD (rph2) generates large amounts of ROS [14], which is exacerbated by phosphine exposure [5]. A homozygous for resistance alleles of rph1 reduces cellular membrane sensitivity to ROS, while homozygous for resistance alleles of rph2 reduces ROS production, resulting in extremely high levels of phosphine resistance [13].
Lipids comprise the largest component of some insect bodies, reaching 75% based on their dry weight [15]. The ability to store fat is essential for insects to adapt to their environment and undergo normal development and reproduction [16]. Additionally, lipids are structural components in cell membranes, have roles in intracellular signaling, and are the main reserved form of energy that insects use for diapause [17], growth [18], flight [19], and metamorphosis [16]. The composition of lipids is influenced by many factors, including genetic, ecological, and nutritional status, and varies across insect species [20]. Lipids are stored in the form of triglycerides (TGs) inside the fat bodies responsible for meeting the energy requirements of insects [21]. TGs can be stored in an anhydrous form, thereby allowing lipids as an essential substance for metabolism, enabling the accumulation of a large reservoir of energy that can be used during long periods of energy demand [22]. Diglycerides (DGs), on the contrary, are the major lipids in insect hemolymph [23]. The importance of DG was described in a study about locust flight when the amount of DG was increased in the hemolymph to threefold of its average concentration to supply the energy requirements [24,25]. Phospholipids are a large group of lipids that contain a polar and non-polar end, consisting of two layers: a hydrophobic layer that has two fatty acids and a hydrophilic layer of the phosphate group connected by glycerol or alcohol [26,27]. The significance of phospholipids is derived from their primary function as a significant part of cellular membranes, which acts as a barrier to isolate the cells from the surrounding environment and allows each cell to perform its specific function [28].
Current extensive research on pesticide resistance focuses on target site and metabolic resistance, but other mechanisms of resistance exist [29]. An example is modifying body parts in order to reduce insecticide penetration into the body, mainly by enhancing the deposit of structural components, such as epicuticular lipids [30]. As compared to pyrethroid-susceptible populations, pyrethroid-resistant Triatoma infestans had a significant increase (more than 50%) in cuticular lipid (CHCs), resulting in reducing the uptake of pyrethroids [31].
Both respiratory and metabolic factors may contribute to phosphine resistance [32]. Resistant strains have a lower respiratory rate than susceptible strains enabling resistant insects to receive less phosphine [33]. The importance of DLD in resistant strains is related to electron transfer resulting in enhancing the energy metabolism [9] and in regulating energy supply in resistant individuals [34]. The possibility of different metabolism between resistant and susceptible strains could lead us to explore the differences in the lipid composition between susceptible and resistant strains of T. castaneum and R. dominica. As mentioned above, lipids have a significant role in insecticide resistance by reducing the penetration of the insecticides into the target insect cells. Therefore, this study aimed to evaluate the role of lipids in two stored-grain insects to resist phosphine.
2. Materials and Methods
2.1. Insect Cultures
One-month-old adult insects were used in the experiments. Susceptible and resistant adult insects of T. castaneum (MUWTCSS-6000 and MUWTCSR) and R. dominica (MUWRDSS-7 and MUWRDSR-675) were obtained from the Department of Primary Industries and Regional Development (DPIRD), Perth, Australia, in 2016. The strains had been regularly treated with phosphine in the laboratory to promote homozygosity for phosphine resistance. Insects were cultured by incubating approximately 3000 2–3-day-old adults with 1000 g of food. The food for T. castaneum was wheat flour/yeast in a 12:1 ratio. The flour was made from newly harvested Australian standard wheat. To avoid contamination, the wheat was stored at −20 °C for 7 d before being transferred to storage at 4 °C until milling. The grain was milled with a Wonder Mill (Model WM2000, WonderMill Co., Seoul, South Korea), and the flour was stored at 4 °C until used. Before feeding to insects, the flour was brought up to room temperature overnight. For R. dominica, the food consisted of broken wheat. All cultures were maintained in 2 L glass jars sealed with mesh. Adult insects were allowed to mate and lay eggs for 4 days, after which they were removed, and the remaining cultural medium was incubated at 28 ± 1 °C, 70 ± 2% relative humidity (RH) and a photoperiod of 14:10 (L:D) h. As adult insects emerged, they were transferred to a new vessel to keep insects of similar ages together [10].
2.2. Preparation of Phosphine Gas for Determination of Resistance Factor
Phosphine was produced by adding Quickphos commercial tablets (United Phosphorus Limited Pty Ltd. (UPL), Adelaide, SA, Australia) of aluminum phosphide in 10% sulphuric acid solution to produce phosphine with final purity of 86% [35]. To obtain the resistance factor, bioassays were employed using the following concentrations of phosphine: 0, 0.005, 0.01, 0.02, 0.03, 0.04, 0.1, 0.3, 0.6, 1, 2, 3, and 4 mg/L, with three replicates of each concentration. Fifty adult insects in 1000 mL flasks for each replicate were fumigated with phosphine for 20 h for the susceptible and resistant strains, followed by a one-week recovery period at 25 ± 1 °C and 65 ± 5% RH. Based on probit model concentration-mortality analysis, the LC50 of 0.009 mg/L were considered the susceptible strain of both species, while the LC50 of 0.26 and 1.042 mg/L for T. castaneum and R. dominica, respectively, were considered as resistant strains [35]. Consequently, the resistance ratio was calculated according to LC50 of the susceptible insects (RR = 28.8-fold for T. castaneum) and (RR = 115.77-fold for R. dominica).
2.3. Chemicals and Apparatuses
The extraction and analysis of lipids were performed using the following materials: acetonitrile ≥99.9% v/v (Fisher Scientific, Glee, Belgium), methanol ≥99.9% v/v, 2-propanol ≥99.9% v/v, and chloroform ≥99.9% v/v (Sigma- Aldrich, Bellefonte, PA, USA). Apparatuses used were a 2 mL micro tube (Benchmark Scientific Inc., Sayreville, NJ, USA), 2 mL clear screw HPLC vials (Agilent Technologies, Santa Clara, CA, USA), different volumes of micropipettes (Dragon Laboratory Instruments Ltd., Beijing, China), bead bug micro tube homogenizer (Model DI030-E, Benchmark Scientific Ltd, Sayreville, NJ, USA), Dynamica Velocity 13µ micro centrifuge (Dynamica Pty Ltd., Mablethorpe, Lincolnshire, LN, United Kingdom), and an ultrasonic cleaner (Model PS-20A, Omegasonics, Simi Valley, CA, USA).
2.4. Extraction Procedures
All the insects used in this study were cleaned by allowing them to crawl on a wet tissue paper for 15 min, and then the insects were transferred into a clean dry tissue paper for 10 min. The cleaned insects were frozen to death and stored using liquid nitrogen. Adult insects were collected in a 2 mL micro tube using a small clean brush.
Fifteen adult insects of resistant and susceptible strains of T. castaneum and R. dominica were homogenized in 1 mL of chloroform/methanol (2:1, v/v) after adding three milling balls using the bead bug micro tube homogenizer at 400 rpm for 1 min [36]. The supernatant was filtered using a 3 mL syringe (Terumo Australia Pty Limited (TAUS), Sydney, NSW Australia) coupled with 13 mm 0.2 µm Agilent Captiva Econo Filters (Agilent Technologies, Santa Clara, CA, USA). To separate the non-lipids substances, the filtered homogenate was washed with 0.2 mL distilled water and centrifuged at 2000 rpm using Dynamica Velocity micro centrifuge. After centrifuging, the upper phase, which contains non-lipids substances, was removed. The lower chloroform phase, which contains lipids, was transferred into a 2 mL GC clear vial, which was already weighed as (Wvial). The extract was blown to dryness under nitrogen flow. The same vial was weighed as (Wvial+ lipids) for calculating total lipids weight (Wlipids) according to the following equation (Equation (1)):
Wlipids = Wvial+ lipids − Wvial
After calculating the total lipids, 600 µL of HPLC solvent (Isopropanol/Acetonitrile/Water (2:1:1, w/w/w) was added to reconstitute the dried lipid components for UPLC-Q-ToF analysis. The ultrasonic cleaner was also used to assist the dissolution of the lipids.
2.5. Alalysis of Lipids with Ultra Performance Liquid Chromatography-Quadrupole-Mass Spectrometry (UPLC-Q-ToF-MS) and Analytical Conditions
Samples were analyzed using Waters Acquity UPLC-Q-Tof. Data acquisition and processing were performed using the Masslynx software (version 4.1, Waters Corporation, Milford, MA, USA).
For analysis of lipids, separation of lipid compounds was performed on a Waters Acquity BEH C18 column (2.1 × 100 mm, 1.7 μm). The binary gradient consisted of eluents A (60% acetonitrile: 40% water w/w) and B (90% 2-propanol: 10% acetonitrile w/w) with 10 µM ammonium formate and 0.1% formic acid at room temperature with a flow rate at 0.25 mL/min and a 2 μL injection volume. Optimal separation was accomplished using the following solvent gradient elution: mobile B started with 40%, increased to 92.1% (1−16 min), then ramped back to 40% (17–17.5 min), followed by 2.5 min of re-equilibration with a total run time of 20 min. All features were analyzed in a positive ionization mode and were monitored in a full scan mode. The optimum MS parameters were capillary voltage 3.1 kV, sample cone 45 V, extraction 5.0 V, ion guide voltage 3.0 V, desolvation gas temperature 350 °C with 350 L/min, collision cell 0.6 mL/min of UHP Argon, and detector voltage 1820 V.
2.6. Data Processing and Analysis
All measurements were conducted according to completely randomized design (CRD). All the samples were analyzed in four biological replicates. The LC-MS data samples were analyzed as one batch to ensure that the parameters would be applied equally in all the samples. Peak deconvolution, filtering, scaling, and integration were extracted and aligned using the MassLynx software (version 4.1, Waters Corporation, Milford, MA, USA). Chromatographic peaks were extracted from 1 to 20 min with a retention time error window of 0.2 min, and the mass spectral peaks detected ranged from 50 to 2000 m/z with a mass error window of 7 ppm. The resulting data matrix extracted from total ion consisted of retention time, and m/z was generated together with peak intensity based on peak area for all features.
The mass spectra of the lipids were loaded into LIPID MAPS Lipidomics Gateway (http://www.lipidmaps.org/tools/ms/lmmassform.php, accessed on 30 August 2018). The identification search was restricted to two main lipids categories, which included glycerolipids and phospholipids. The following parameters were applied for an appropriate identification: mass tolerance ±0.1 m/z and ion adducts of positive mode [M+H]+ and [M+NH4]+. The loaded spectra were compared with the matched spectra, which were obtained from the lipid maps to identify the compounds. The compounds with the highest spectrum match factor were chosen as the lipid compound candidates.
Data were normalized with internal standards before statistical evaluation. Statistical analysis was employed to evaluate and visualize the data through MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/MetaboAnalyst/upload/StatUploadView.xhtml, accessed on 30 August 2018) using volcano plot analysis and t-test [37]. Samples were uploaded to Metaboanalyst as columns (unpaired); data filtering was characterized by using the mean intensity value. Sample normalization, data transformation, and data scaling were specified as a “NONE” mode. Volcano plot was analyzed at a p-value threshold of 0.05 and fold change threshold ≥2. Figure 1 was generated using IPM SPSS statistics 24 (Murdoch University version).
3. Results and Discussion
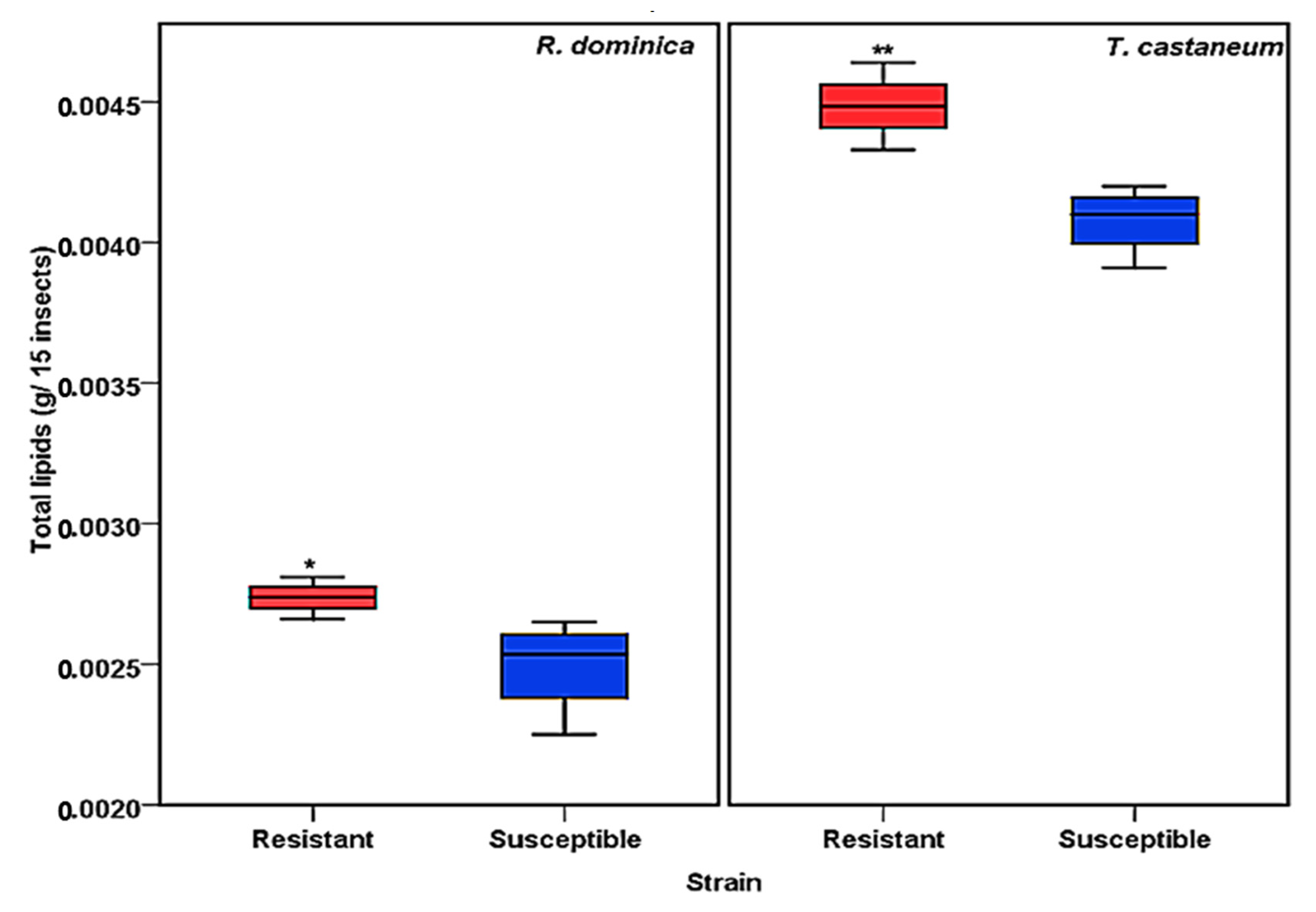
The total lipid contents of susceptible and resistant strains of T. castaneum and R. dominica were evaluated according to the Floch method [36]. The results showed a significant difference in the quantity of total lipids (Figure 1). The resistant strains of both T. castaneum and R. dominica species contained a significantly greater amount (p values <0.001 for T. castaneum and <0.01 for R. dominica) of lipids compared to the susceptible strains of both species (Figure 1).
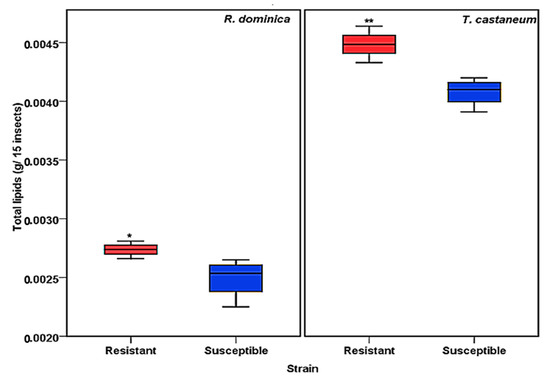
Figure 1.
Total lipid content obtained from phosphine-susceptible and -resistant strains of R. dominica and T. castaneum. Each value in the figure represents the average of four biological insect sets. The values were statistically analyzed by t-test. * = p ≤ 0.01, ** = p ≤ 0.001.
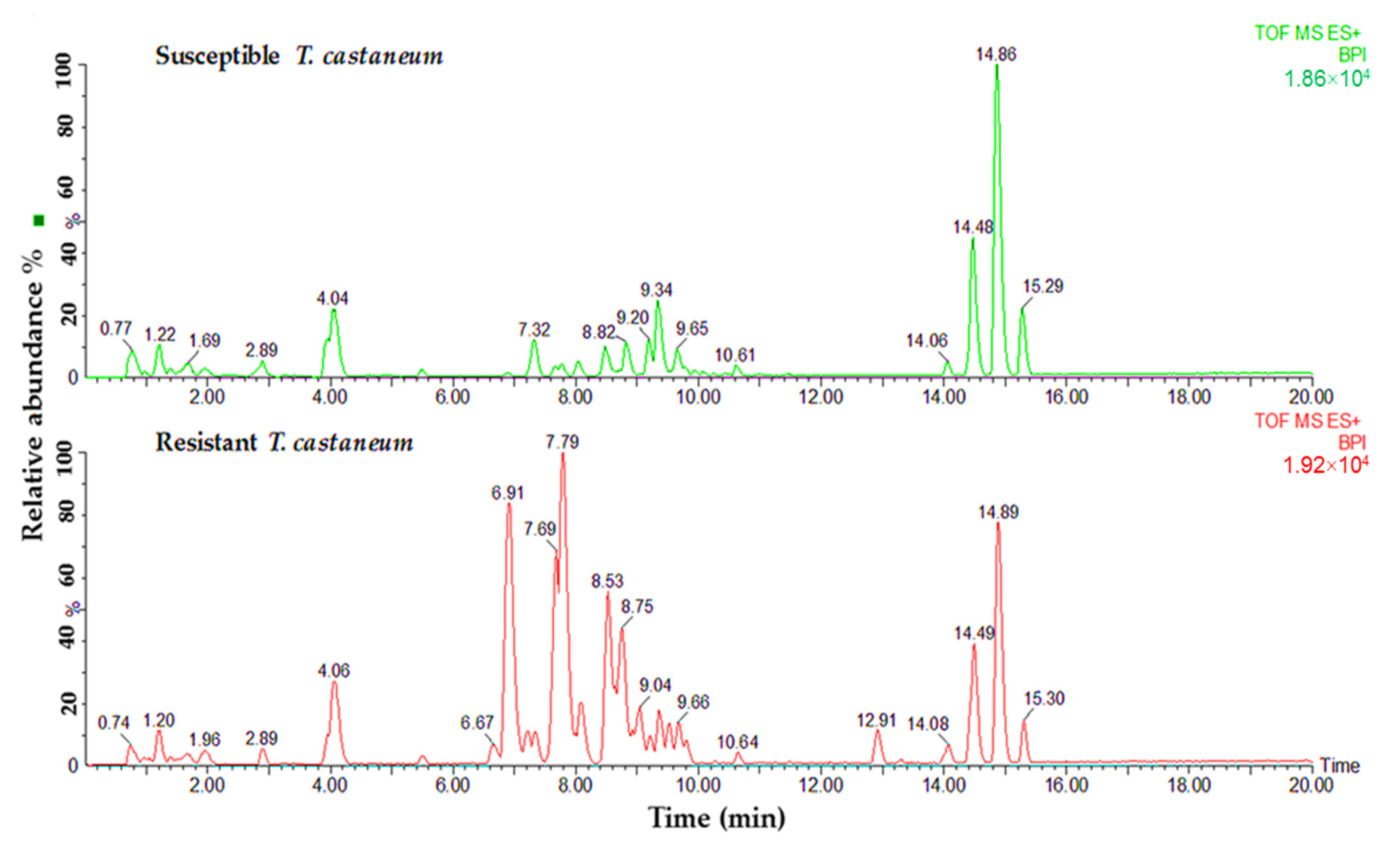
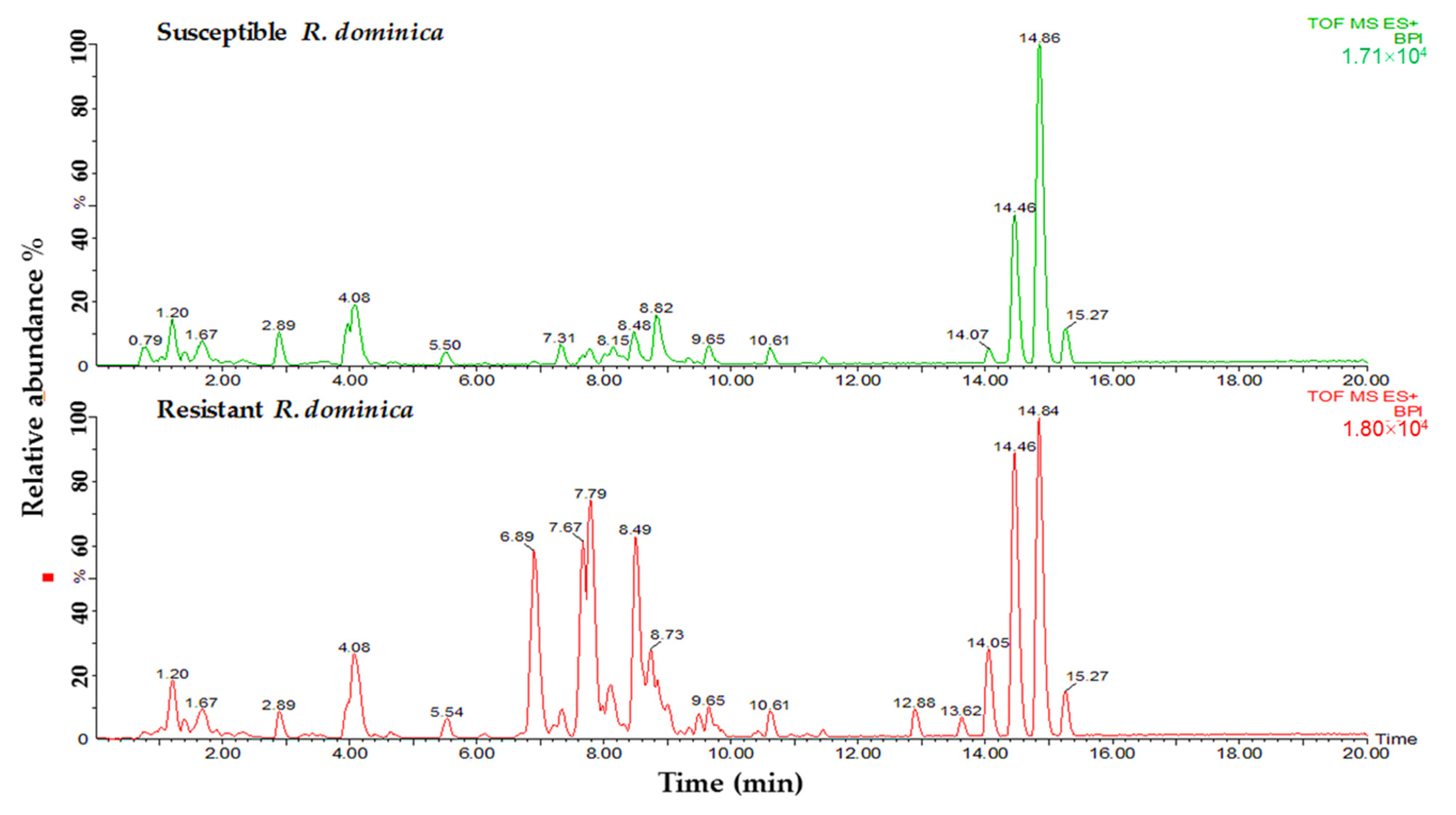
Lipid samples from both susceptible and resistant strains of insects were tested to determine the differences in phospholipids and glycerolipids for their predicted role in phosphine resistance. Variances were observed in relation to the major peaks of LC-MS base peak intensities chromatograms when comparing susceptible and resistant strains of both insect species. A comparison of the lipids data obtained from the profiles of susceptible and resistant insects of the two studied species is shown in Figure 2 and Figure 3. The main difference between the susceptible and resistant strains of T. castaneum in the chromatograms was between RT = 6.67 to 10.64 min (Figure 2). In contrast, the differences between the two strains of R. dominica included the majority of the peaks in the base peak intensity chromatogram (Figure 3).
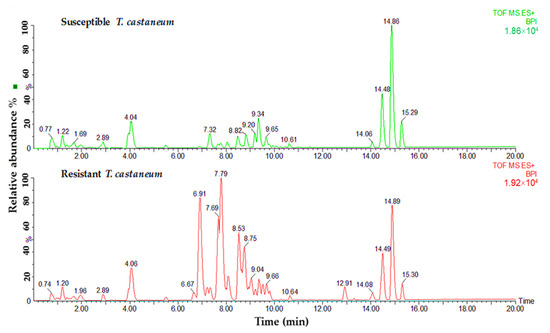
Figure 2.
Base peak intensity (BPI) chromatograms show the differences in the lipid content obtained from susceptible (green) and resistant (red) strains of T. castaneum.
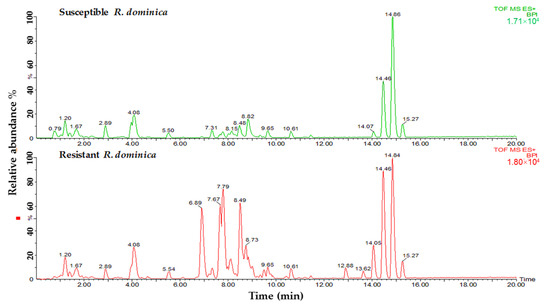
Figure 3.
Base peak intensity (BPI) chromatograms show the differences in the lipid content obtained from susceptible (green) and resistant (red) strains of R. dominica.
The lipids separated from the insect samples were further characterized by MS detector. In the identification of the lipid compounds, only glycerolipids and phospholipids were studied for their role in the energy production and structuring of the cell membranes. The fold changes results (using volcano plot statistical analysis) revealed a higher quantity of lipids obtained from resistant strains than susceptible insects in both species. High difference ratios were obtained for most lipids obtained from T. castaneum, ranging for glycerolipids from 1.13- to 53.10-fold and phospholipids from 1.05- to 20.00-fold (Table 1). In comparison, the fold changes in glycerolipids for R. dominica were between 1.04- to 31.50-fold and for phospholipids from 1.04- to 10.10-fold (Table 2).

Table 1.
Glycerolipids and phospholipids candidates obtained from T. castaneum.

Table 2.
Glycerolipids and phospholipids candidates obtained from R. dominica.
A total of 45 features from T. castaneum and 67 from R. dominica were identified as lipids belonging to either glycerolipids or phospholipids (Table 1 and Table 2). The statistical analysis revealed significant differences between the two lipids categories. The resistant insects contained more lipid compounds in abundance in both lipid categories than the susceptible strains (Table 1 and Table 2).
Volcano plot statistical analysis identified 17 glycerolipid and 8 phospholipid features from T. castaneum as significant (p-value ≤ 0.05 and fold change ≥ 2). The highest significant difference between resistant and susceptible strains for the glycerolipids was recorded for the compound’s ID 6.89_782.485 (p-value < 0.0005). In contrast, the compound’s ID 7.77_758.494 (p-value < 0.0005) recorded the highest significant difference for the phospholipids. A statistical analysis of volcano plot selected 18 features associated with glycerolipids and 8 associated with phospholipids as significant features in comparing resistant and susceptible strains of R dominica. The compound’s ID 14.48_874.714 recorded the highest significant difference between resistant and susceptible strains in the glycerolipids category (p-value < 0.0005). The compound’s ID 7.81_806.504 recorded the highest significant difference in the phospholipid category (p-value < 0.0005). In addition, some other lipids were significantly higher in resistant individuals than the susceptible strains in both T. castaneum and R. dominica. These included lipid IDs 6.89_782.485 (DGDG(23:1)), 8.77_786.518 (MGDG(36:9)), 8.48_760.505 (PG(34:4)), 8.73_577.469 (DG(33:3)), 7.27_599.455 (DG(35:6)), 7.20_740.45 (MGDG(32:4)), 8.03_575.455 (DG(33:4)), 6.88_601.468 (MGDG(23:2)), 8.77_718.469 (PC(30:2(OH))), and 14.06_900.704 (TG(55:11)) (Table 1 and Table 2).
Our examination of total lipid content aimed to provide an overview of the variances of the lipid amounts presented in insect bodies of phosphine-resistant and phosphine-susceptible strains of two insect species. The greater amount of lipids in phosphine-resistant insects of both T. castaneum and R. dominica led us to hypothesize that lipids may have a significant survival role with regard to phosphine resistance. We considered the importance of lipids for resistant insects as a factor required to tolerate more effectively the toxic effect of phosphine. We also considered the strong link that was reported between greater amounts of lipids, specifically cuticular lipids, and higher resistance to pesticides in a variety of insect species, such as Drosophila melanogaster to DDT [38] and Triatoma infestans to pyrethroids [31].
Insects rely more on lipids in severe long-term conditions, such as exposure to lack of energy [39]. This is because lipids are reserved for recovering from the lack of energy for long periods [20]. Strains with high-energy demands might be more susceptible to phosphine because of the increased mitochondrial activity levels that are necessary to sustain energy production [9]. This observation is because phosphine, a respiratory inhibitor in the mitochondria of insects and rats [40,41], disturbs the energy production of mitochondria [42]. The inhibitory effect on the mitochondria explains why lack of energy is one of the plausible reasons for mortality in insects due to phosphine [32,42]. This result is consistent with research results that showed that artificially raising the energy demand increased the sensitivity of the nematode Caenorhabditis elegance toward phosphine [43]. Thus, more lipids in resistant insects can provide survival factors to resist the negative effect of phosphine, that is, a decrease in energy by affecting mitochondria.
In our study, the outcome further supports the hypothesis that increased lipids provide advantages to survive the toxic effect of phosphine. The negligible impact of phosphine on resistant insects compared to susceptible insects indicated that metabolic factors contribute to resistance to phosphine [44]. A study on roundworm C. elegans revealed that phosphine affected both structure and function of mitochondria; however, phosphine-resistant C. elegans had a substantial increase in the mitochondrial membrane potential and less oxygen consumption (43). Consistent with that, reduced levels of respiration were acquired for resistant strains with high resistance ratios from different insect species, such as T. castaneum, R. dominica, and O. surinamensis (33). It may be that the higher mitochondrial membrane potential gives resistant strains an advantage in using energy resources to avoid the effect of phosphine.
The results indicate an increase in glycerolipids, which are considered major energy sources. Glycerolipids contain triglycerides, which, along with glycogen, are the main sources of stored energy in insect bodies [45]. Glycogen is consumed in the short-term [46]. While triglycerides have a higher caloric content than glycogen and are the main source of releasing fatty acids, which can be used for energy production [47]. Stored fatty acids are utilized in different forms for many purposes, such as energy provision to perform metabolic activities [20]. Another advantage of lipids is that fatty acids play a role in synthesizing energy components, such as trehalose [47] and proline, which is oxidized during endothermic pre-flight warm-up and during flight after prolonged starvation [48]. That is why higher concentrations of triglycerides that were found in this study may help resistant insects avoid the effect of phosphine on the mitochondria, which causes a reduction in the energy that causes death.
Significantly higher content of diglycerides obtained from different metabolic pathways in resistant insect strains were compared with susceptible strains, as this plays an essential role in being the main source for triglycerides synthesis [20]. Diglycerides are also important because they are the core lipids in insect hemolymph after the triglycerides degradation [49]. According to their importance, as explained above, the significant differences in the triglycerides and diglycerides between the resistant and susceptible strains obtained in this study indicate that these compounds are being utilized by the insects to survive from phosphine exposure, especially after long-term exposure.
Phospholipids levels were significantly higher in the resistant insects than in the susceptible insects. This finding supports the assumption that resistant insects rely on lipids to survive the phosphine effect. Phospholipids exist in organisms as essential compounds to maintain life activity and are also vital components of cellular and semi-cellular membranes [27]. Furthermore, they are crucial parts of cellular membranes, which act as barriers between cells and their surrounding environment and enable each cell to perform its specific function [28]. This characteristic may reduce or prevent phosphine penetration to the cells, thereby causing more exclusion of phosphine. This is consistent with one of the accepted explanations that the exclusion of phosphine is a possible resistance mechanism [8].
Additionally, phospholipids are essential for improving nerve cell function [50]. Moreover, phosphine also affects the neural system [51]; therefore, the higher concentration of phospholipids in resistant insects may improve the functions of the neural system in these insects. Furthermore, phospholipid of the mitochondrial membrane that is rich in unsaturated fatty acids plays an essential part in mitochondrial energy by affecting the activity of proteins of the mitochondrial inner membrane [52]. Phospholipids also contribute a significant amount to the mitochondrial membrane lipid environment. They have a substantial role in the mitochondrial respiratory chain by affecting the physical properties of the mitochondrial membrane [53]. Hence, respiration was found to be affected by the reduction in the mitochondrial phospholipids [54]. Therefore, a higher concentration of phospholipids in resistant strains may enhance mitochondria function and reduce the impact of phosphine toxicity.
In addition to its effect on the mitochondrial respiratory chain, the reduction in phospholipids was also observed to be synchronous with a significant reduction in adenosine triphosphate (ATP) [44]. A study by Price and Walter [55] on lesser grain borer R. dominica, demonstrated that ATPs were reduced from 2.75 to 1.64 nmoles/insect after treating the insect with phosphine. Phosphine causes a severe reduction in cytochrome oxidase activities and affects nicotinamide adenine dinucleotide (NAD) and succinic dehydrogenase activities, thereby leading to a reduction in respiration and causing a decline in the synthesis and ATP level [42]. Therefore, improving the mitochondrial energy and raising the mitochondria function by a higher content of phospholipids will certainly affect phosphine toxicity and energy production. Another advantage of the higher levels of phospholipids is increasing the production of phosphatidic acids from the glycerophosphate pathway. Phosphatidic acids are also considered as one of the main sources of triglycerides composition that allows the presence of more energy sources [20], which can provide more energy to the resistant insects to resist phosphine.
Finally, as reported in previous studies, both rph1 and rph2 contribute to phosphine toxicity by damaging fatty acids [9,13]. In resistant insects, which have homozygous for resistance alleles of rph1 or rph2, the damage of fatty acids is extremely reduced due to the reduction in the sensitivity of cell membranes to reactive oxygen species (ROS) [13]. This, in turn, might lead to an abundance of fatty acids, which are the primary components of lipid formation.
4. Conclusions
The levels of lipids and contents were investigated and compared between phosphine-resistant and -susceptible strains of R. dominica and T. castaneum. The total lipid content was found to be higher in the resistant strains than in the susceptible strains. Results showed that most glycerolipids and phospholipids in the resistant insects were more abundant than in the susceptible insects. Both glycerolipids and phospholipids play a significant role in tolerating the harmful effect of phosphine in phosphine-resistant insects by their contribution to providing energy sources and building cell walls. This research will benefit in developing a strategy for managing phosphine resistant insect pests based on understanding the role of lipids in phosphine resistance of the stored-grain insect pests, T. castaneum and R. dominica. We recommend conducting further studies on isogenic phosphine-resistant and -susceptible strains of insects.
Author Contributions
I.A., Y.R. and M.A. provided methodology and experiment design. I.A. and X.D. completed all experimental procedures. I.A., T.L., and N.A. implemented the data analysis. M.A. and Y.R. were in charge of supervision. I.A., X.D., Y.R., M.A., T.L., and N.A. conducted the original draft preparation. I.A., Y.R., and M.A. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
We thank the Iraqi government for its support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Donahaye, E.J.; Bell, C.; Jayes, D.; Noyas, R.; Phillips, T.W. Integrated pest management strategies used in stored grains in Brazil to manage phosphine resistance. In Proceedings of the International Conference Controlled Atmosphere and Fumigation in Stored Product, Gold Coast, Australia, 8–13 August 2007; pp. 293–300. [Google Scholar]
- Edde, P.A. A review of the biology and control of Rhyzopertha dominica (F.) the lesser grain borer. J. Stored Prod. Res. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Hameed, A.; Freed, S.; Hussain, A.; Iqbal, M.; Hussain, M.; Naeem, M.; Sajjad, A.; Hussnain, H.; Sadiq, M.A.; Tipu, A.L. Toxicological effects of neem (Azadirachta indica), Kanair (Nerium oleander) and spinosad (Tracer 240 SC) on the red flour beetle (Tribolium castaneum) (Herbst.). Afr. J. Agric. Res. 2012, 7, 555–560. [Google Scholar]
- Oppert, B.; Guedes, R.N.; Aikins, M.J.; Perkin, L.; Chen, Z.; Phillips, T.W.; Zhu, K.Y.; Opit, G.P.; Hoon, K.; Sun, Y. Genes related to mitochondrial functions are differentially expressed in phosphine-resistant and-susceptible Tribolium castaneum. BMC Genom. 2015, 16, 968. [Google Scholar] [CrossRef]
- Chaudhry, M. A Review of the Mechanisms Involved in the Action of Phosphine as an Insecticide and Phosphine Resistance in Stored-Product Insects. Pestic. Sci. 1997, 49, 213–228. [Google Scholar] [CrossRef]
- Benhalima, H.; Chaudhry, M.; Mills, K.; Price, N. Phosphine resistance in stored-product insects collected from various grain storage facilities in Morocco. J. Stored Prod. Res. 2004, 40, 241–249. [Google Scholar] [CrossRef]
- Pimentel, M.A.; Faroni, L.R.; Batista, M.D.; Silva, F.H. Resistance of stored-product insects to phosphine. Pesqui. Agropecu. Bras. 2008, 43, 1671–1676. [Google Scholar] [CrossRef]
- Price, N. Active exclusion of phosphine as a mechanism of resistance in Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae). J. Stored Prod. Res. 1984, 20, 163–168. [Google Scholar] [CrossRef]
- Schlipalius, D.I.; Valmas, N.; Tuck, A.G.; Jagadeesan, R.; Ma, L.; Kaur, R.; Goldinger, A.; Anderson, C.; Kuang, J.; Zuryn, S. A core metabolic enzyme mediates resistance to phosphine gas. Science 2012, 338, 807–810. [Google Scholar] [CrossRef]
- Alnajim, I.; Agarwal, M.; Liu, T.; Du, X.; Ren, Y.L. Preliminary Study on the Differences in Hydrocarbons Between Phosphine-Susceptible and-Resistant Strains of Rhyzopertha dominica (Fabricius) and Tribolium castaneum (Herbst) Using Direct Immersion Solid-Phase Microextraction Coupled with GC-MS. Molecules 2020, 25, 1565. [Google Scholar] [CrossRef]
- Patel, M.S.; Roche, T.E. Molecular biology and biochemistry of pyruvate dehydrogenase complexes 1. FASEB J. 1990, 4, 3224–3233. [Google Scholar] [CrossRef]
- Williams, C.H. Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and mercuric ion reductase-a family of flavoenzyme transhydrogenases. Chem. Biochem. Flavoenzymes 1992, 3, 121–211. [Google Scholar]
- Schlipalius, D.I.; Tuck, A.G.; Jagadeesan, R.; Nguyen, T.; Kaur, R.; Subramanian, S.; Barrero, R.; Nayak, M.; Ebert, P.R. Variant linkage analysis using de novo transcriptome sequencing identifies a conserved phosphine resistance gene in insects. Genetics 2018, 209, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahara, E.B.; Barros, M.H.; Oliveira, G.A.; Netto, L.E.; Kowaltowski, A.J. Dihydrolipoyl dehydrogenase as a source of reactive oxygen species inhibited by caloric restriction and involved in Saccharomyces cerevisiae aging. FASEB J. 2007, 21, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Pino Moreno, J.; Ganguly, A. Determination of fatty acid content in some edible insects of Mexico. J. Insects Food Feed. 2016, 2, 37–42. [Google Scholar] [CrossRef]
- Gilbert, L.I.; Chino, H. Transport of lipids in insects. J. Lipid Res. 1974, 15, 439–456. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insect diapause: Nutrient storage and utilisation. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef]
- Ziegler, R.; Van Antwerpen, R. Lipid uptake by insect oocytes. Insect Biochem. Mol. Biol. 2006, 36, 264–272. [Google Scholar] [CrossRef]
- Beenakkers, A.T.; Van der Horst, D.; Van Marrewijk, W. Insect flight muscle metabolism. Insect Biochem. 1984, 14, 243–260. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Ad, M.T.; Van der Horst, D.J.; Van Marrewijk, W.J. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lipid Res. 1985, 24, 19–67. [Google Scholar]
- Downer, R.; Matthews, J. Patterns of lipid distribution and utilisation in insects. Am. Zool. 1976, 16, 733–745. [Google Scholar] [CrossRef]
- Chang, F. Effects of vertebrate adipokinetic hormones on the rate of in vitro lipid release in insects. Comp. Biochem. Physiol. B Biochem. Mol. Biol. Comp. Biochem. B 1974, 49, 567–578. [Google Scholar] [CrossRef]
- Arrese, E.L.; Wells, M.A. Adipokinetic hormone-induced lipolysis in the fat body of an insect, Manduca sexta: Synthesis of sn-1, 2-diacylglycerols. J. Lipid Res. 1997, 38, 68–76. [Google Scholar] [CrossRef]
- Jutsum, A.; Goldsworthy, G. Fuels for flight in Locusta. J. Insect Physiol. 1976, 22, 243–249. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Singh, R.P.; Gangadharappa, H.; Mruthunjaya, K. Phospholipids: Unique carriers for drug delivery systems. J. Drug Deliv. Sci. Technol. 2017, 39, 166–179. [Google Scholar] [CrossRef]
- Bohdanowicz, M.; Grinstein, S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol. Rev. 2013, 93, 69–106. [Google Scholar] [CrossRef]
- Puinean, A.M.; Foster, S.P.; Oliphant, L.; Denholm, I.; Field, L.M.; Millar, N.S.; Williamson, M.S.; Bass, C. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010, 6, e1000999. [Google Scholar] [CrossRef]
- Balabanidou, V.; Kampouraki, A.; MacLean, M.; Blomquist, G.J.; Tittiger, C.; Juárez, M.P.; Mijailovsky, S.J.; Chalepakis, G.; Anthousi, A.; Lynd, A. Cytochrome P450 associated with insecticide resistance catalyses cuticular hydrocarbon production in Anopheles gambiae. Proc. Natio. Acad. Sci. USA 2016, 113, 9268–9273. [Google Scholar] [CrossRef]
- Pedrini, N.; Mijailovsky, S.J.; Girotti, J.R.; Stariolo, R.; Cardozo, R.M.; Gentile, A.; Juárez, M.P. Control of pyrethroid-resistant Chagas disease vectors with entomopathogenic fungi. PLoS Negl. Trop. Dis. 2009, 3, e434. [Google Scholar] [CrossRef]
- Nath, N.S.; Bhattacharya, I.; Tuck, A.G.; Schlipalius, D.I.; Ebert, P.R. Mechanisms of phosphine toxicity. J. Toxicol. 2011, 2011, 494168. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.A.; Faroni, L.R.; Tótola, M.R.; Guedes, R.N. Phosphine resistance, respiration rate and fitness consequences in stored product insects. Pest Manag. Sci. Former. Pestic. Sci. 2008, 63, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T. Genetics of Phosphine Resistance in the Rice Weevil Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2016; p. 151. [Google Scholar]
- FAO. Recommended methods for the detection and measurement of resistance of agricultural pests to pesticides. Tentative method for adults of some major pest species of stored cereals, with methyl bromide and phosphine. FAO Method No. 16. FAO Plant Prot. Bull. 1975, 23, 12–25. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 1–14. [Google Scholar] [CrossRef]
- Strycharz, J.P.; Lao, A.; Li, H.; Qiu, X.; Lee, S.H.; Sun, W.; Yoon, K.S.; Doherty, J.J.; Pittendrigh, B.R.; Clark, J.M. Resistance in the highly DDT-resistant 91-R strain of Drosophila melanogaster involves decreased penetration, increased metabolism, and direct excretion. Pestic. Biochem. Physiol. 2013, 107, 207–217. [Google Scholar] [CrossRef]
- Ziegler, R. Changes in lipid and carbohydrate metabolism during starvation in adult Manduca sexta. J. Comp. Physiol. B 1991, 161, 125–131. [Google Scholar] [CrossRef]
- Dua, R.; Sunkaria, A.; Kumar, V.; Gill, K.D. Impaired mitochondrial energy metabolism and kinetic properties of cytochrome oxidase following acute aluminium phosphide exposure in rat liver. Food Chem. Toxicol. 2010, 48, 53–60. [Google Scholar] [CrossRef]
- Price, NR Dance, SJ Some biochemical aspects of phosphine action and resistance in three species of stored product beetles. Comp. Biochem. Physiol. B Comp. Pharmacol. Toxicol. 1983, 76, 277–281. [CrossRef]
- Price, N. Some aspects of the inhibition of cytochrome c oxidase by phosphine in susceptible and resistant strains of Rhyzopertha dominicia. Insect Biochem. 1980, 10, 147–150. [Google Scholar] [CrossRef]
- Zuryn, S.; Kuang, J.; Ebert, P. Mitochondrial modulation of phosphine toxicity and resistance in Caenorhabditis elegans. Toxicol. Sci. 2008, 102, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.Q.; Price, N.R. Insect mortality at doses of phosphine which produce equal uptake in susceptible and resistant strains of Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae). J. Stored Prod. Res. 1990, 26, 101–107. [Google Scholar] [CrossRef]
- Steele, J. Glycogen phosphorylase in insects. Insect Biochem. 1982, 12, 131–147. [Google Scholar] [CrossRef]
- Athenstaedt, K.; Daum, G. The life cycle of neutral lipids: Synthesis, storage and degradation. Cell. Mol. Life Sci. 2006, 63, 1355–1369. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.; Steele, J. Free fatty acids as a source of energy for trehalose synthesis in the fat body of the American cockroach (Periplaneta americana). Insect Biochem. 1988, 18, 591–597. [Google Scholar] [CrossRef]
- Gäde, G.; Auerswald, L. Beetles’ choice proline for energy output: Control by AKHs. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 117–129. [Google Scholar] [CrossRef]
- Tsuchida, K.; Arai, M.; Tanaka, Y.; Ishihara, R.; Ryan, R.O.; Maekawa, H. Lipid transfer particle catalyses transfer of carotenoids between lipophorins of Bombyx mori. Insect Biochem. Mol. Biol. 1998, 28, 927–934. [Google Scholar] [CrossRef]
- Pepeu, G.; Pepeu, I.M.; Amaducci, L. A review of phosphatidylserine pharmacological and clinical effects. Is phosphatidylserine a drug for the ageing brain? Pharmacol. Res. 1996, 33, 73–80. [Google Scholar] [CrossRef]
- Al-Azzawi, M.J.; Al-Hakkak, Z.S.; Al-Adhami, B.W. In vitro inhibitory effects of phosphine on human and mouse serum cholinesterase. Toxicol. Environ. Chem. 1990, 29, 53–56. [Google Scholar] [CrossRef]
- Hoch, F.L. Cardiolipins and biomembrane function. Biochim. Biophys. Acta Biomembr. 1992, 1113, 71–133. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.D.; Basu Ball, W.; Pryce, E.N.; Gohil, V.M. Specific requirements of nonbilayer phospholipids in mitochondrial respiratory chain function and formation. J. Mol. Cell Biol. 2016, 27, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Price, N.R.; Walter, C.M. A comparison of some effects of phosphine, hydrogen cyanide and anoxia in the lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae). Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1987, 86, 33–36. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).