Genome-Wide Analysis of Odorant and Gustatory Receptors in Six Papilio Butterflies (Lepidoptera: Papilionidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics
2.1.1. Genome Resource
2.1.2. Gene Annotation
2.1.3. Gene Nomenclature
2.1.4. Intron Analysis and Genomic Arrangement
2.1.5. Phylogenetic Analysis
2.2. Molecular Biology
2.2.1. Insect Rearing and Tissue Collection
2.2.2. Total RNA Extraction and First-Strand cDNA Synthesis
2.2.3. Expression Profiling Analysis of ORs and GRs in P. xuthus
3. Results
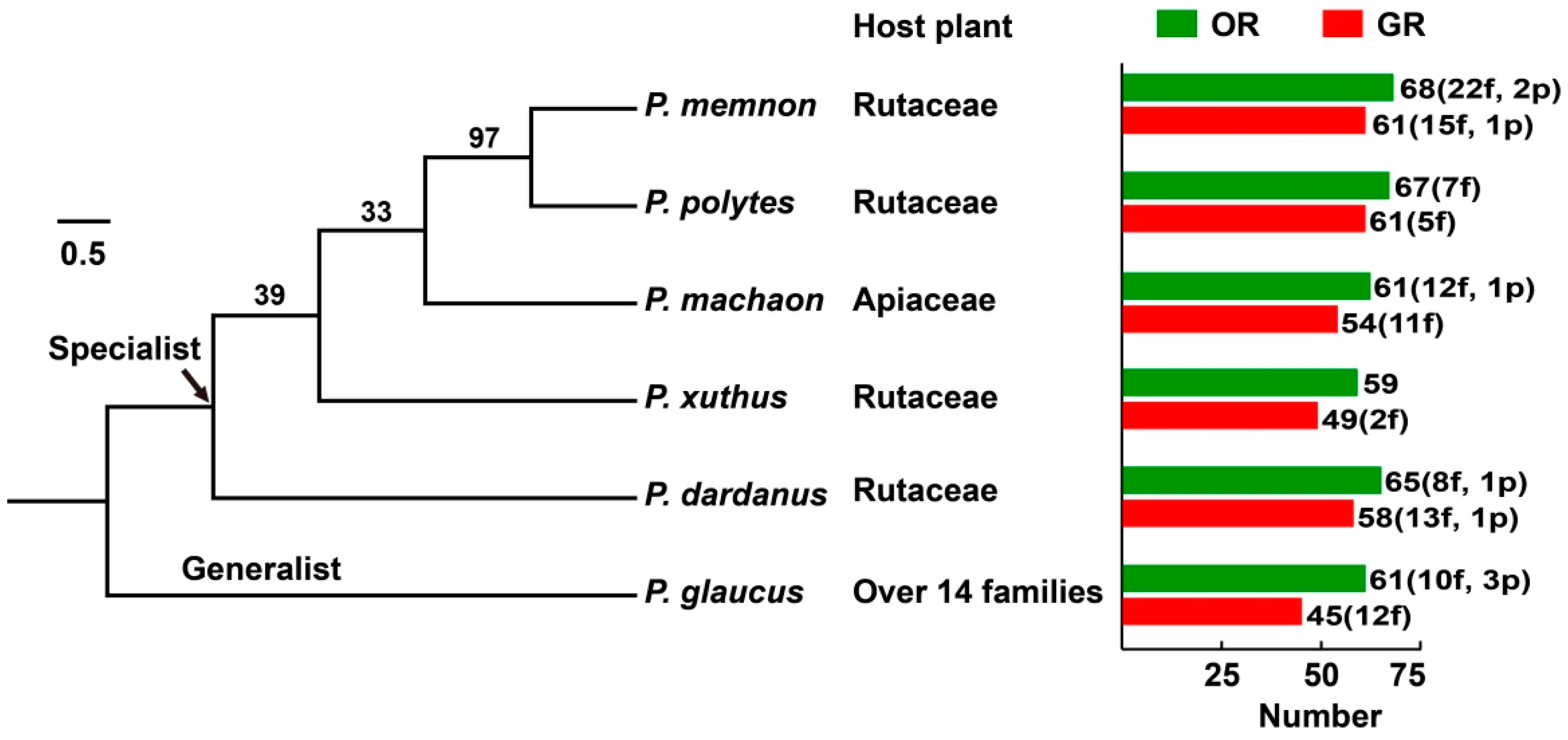
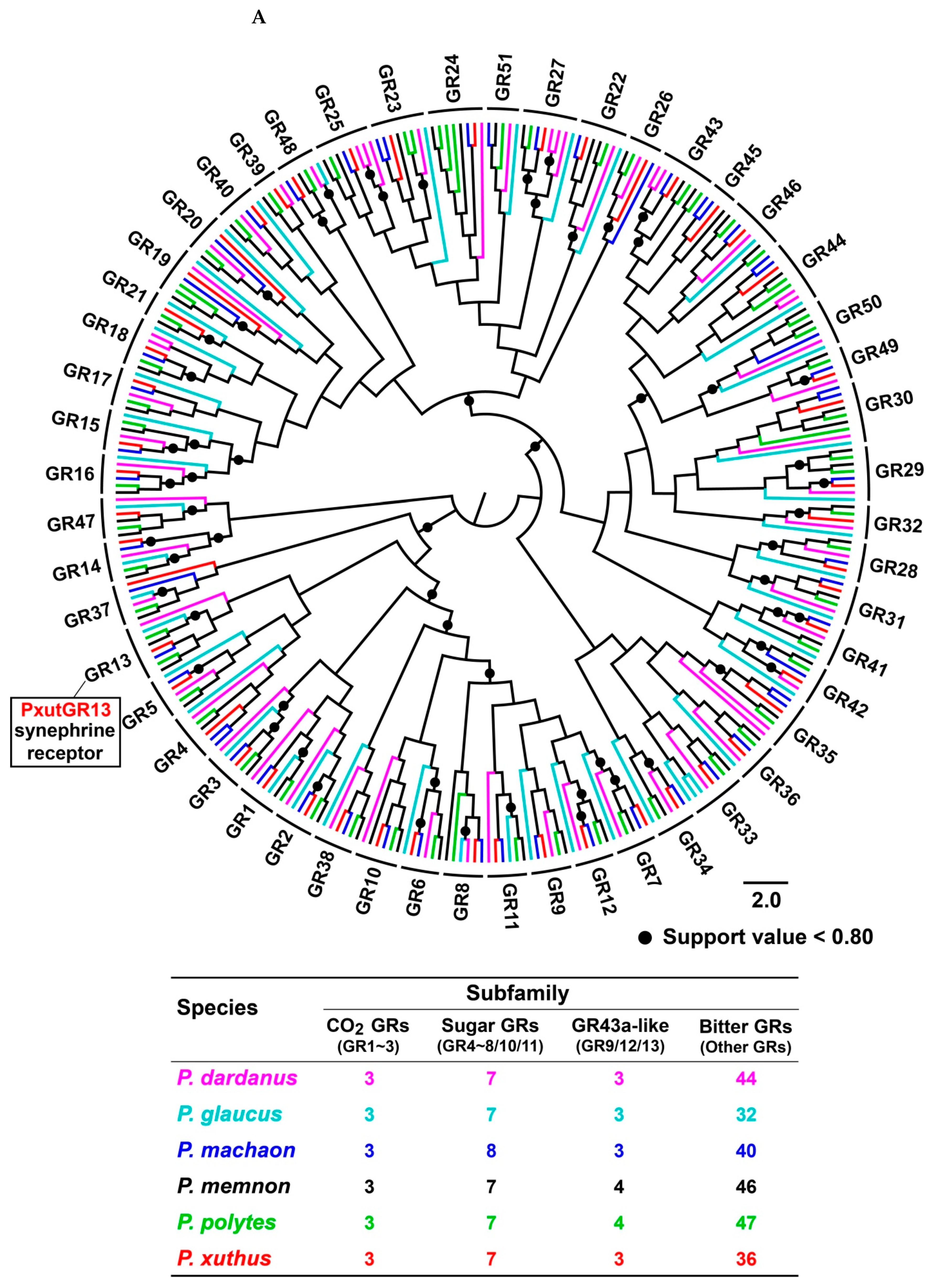
3.1. Identification of Papilio ORs and GRs
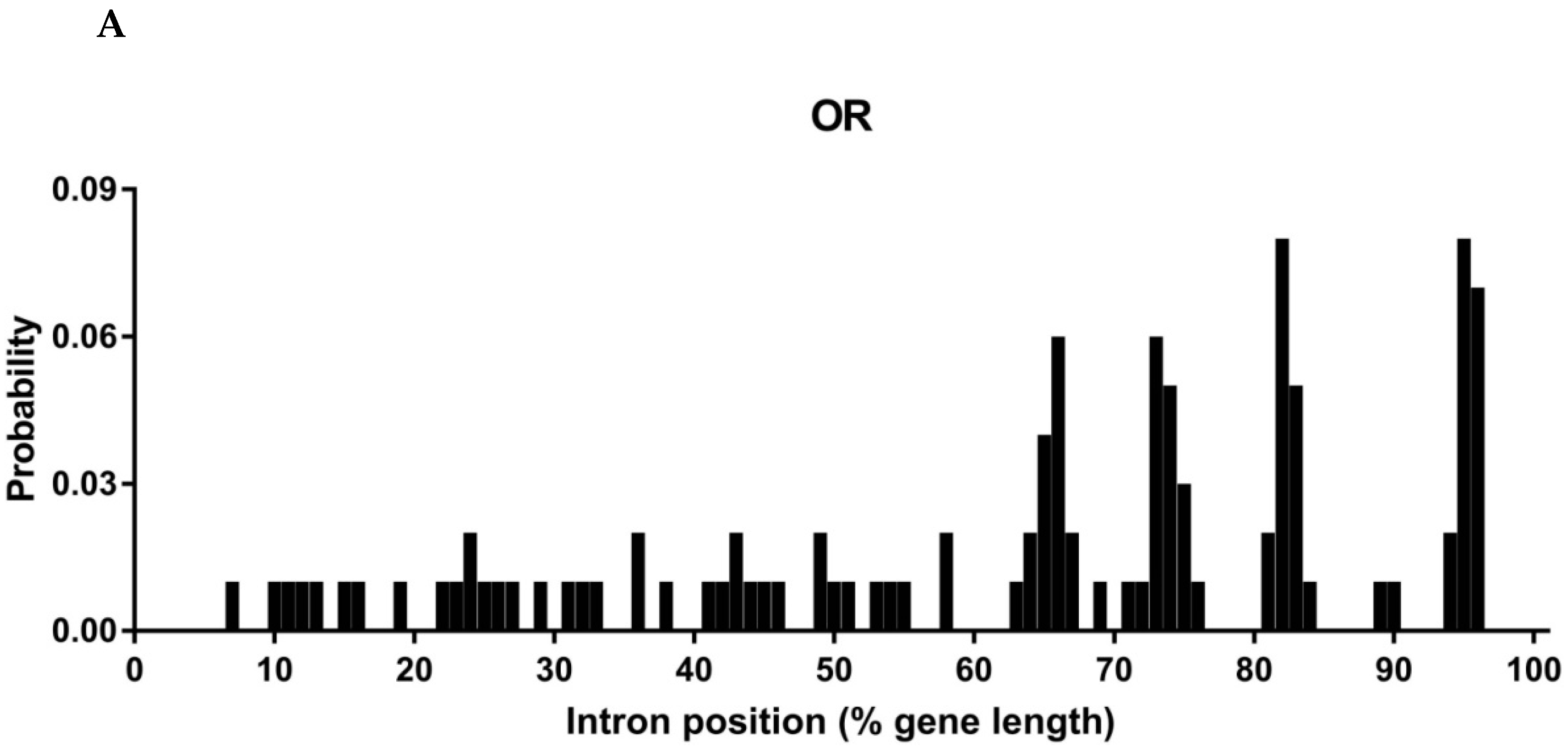
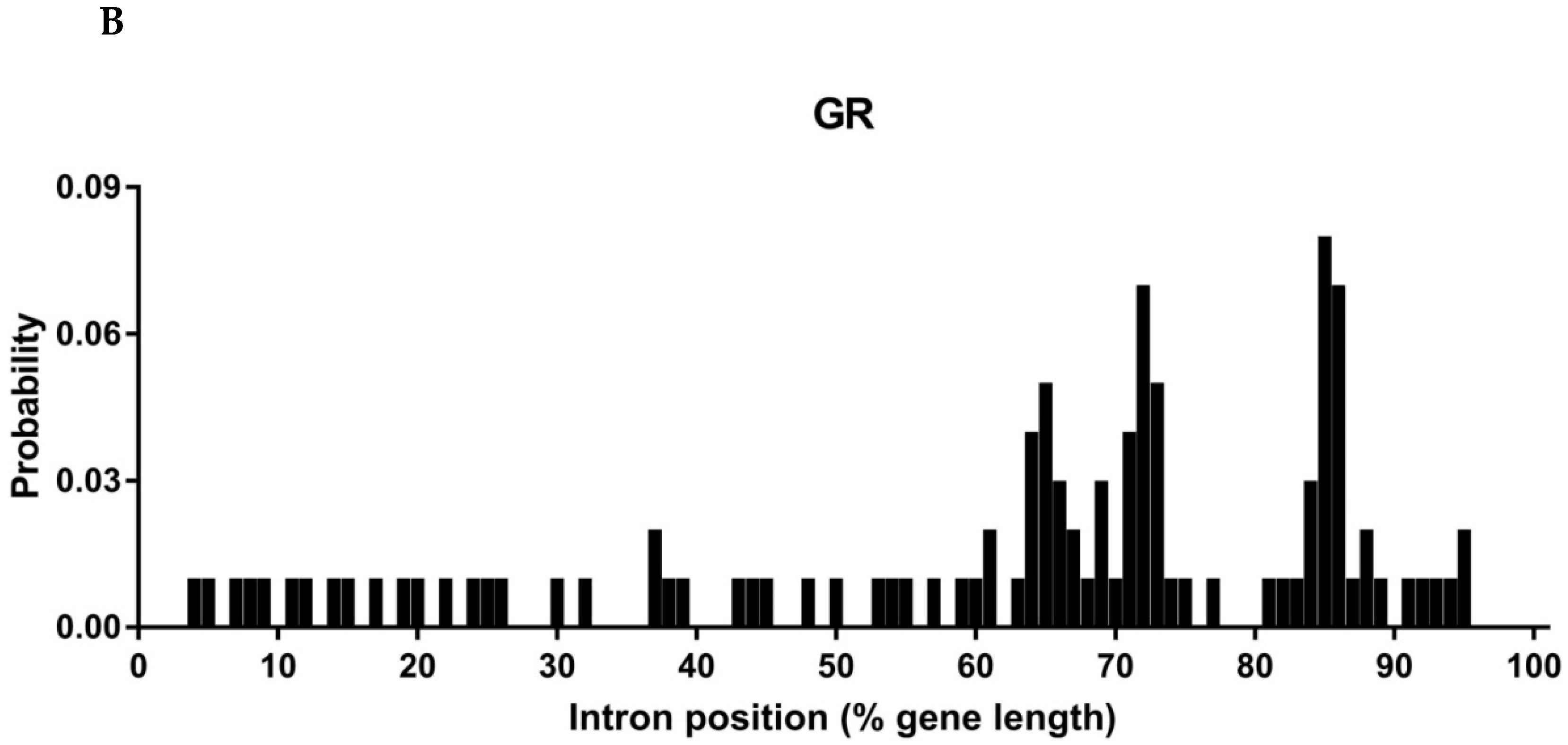
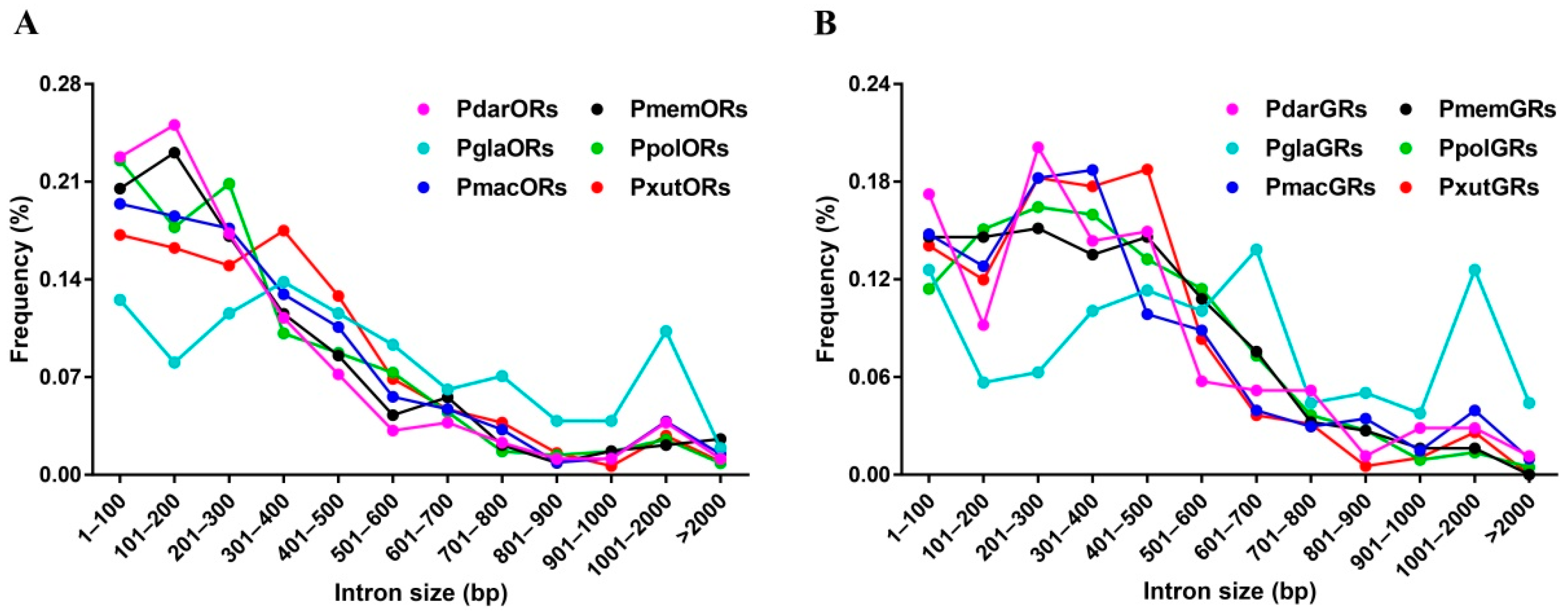
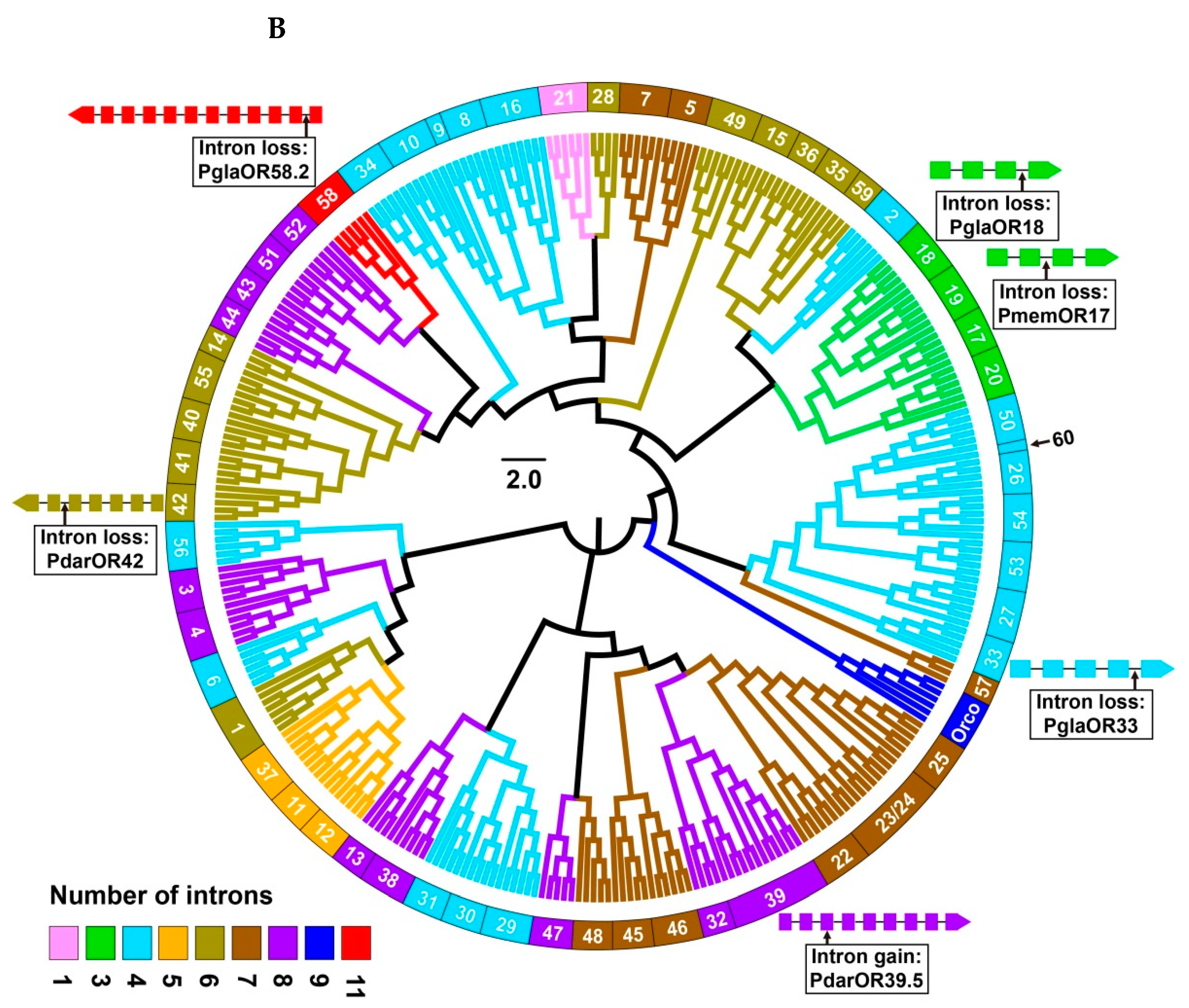
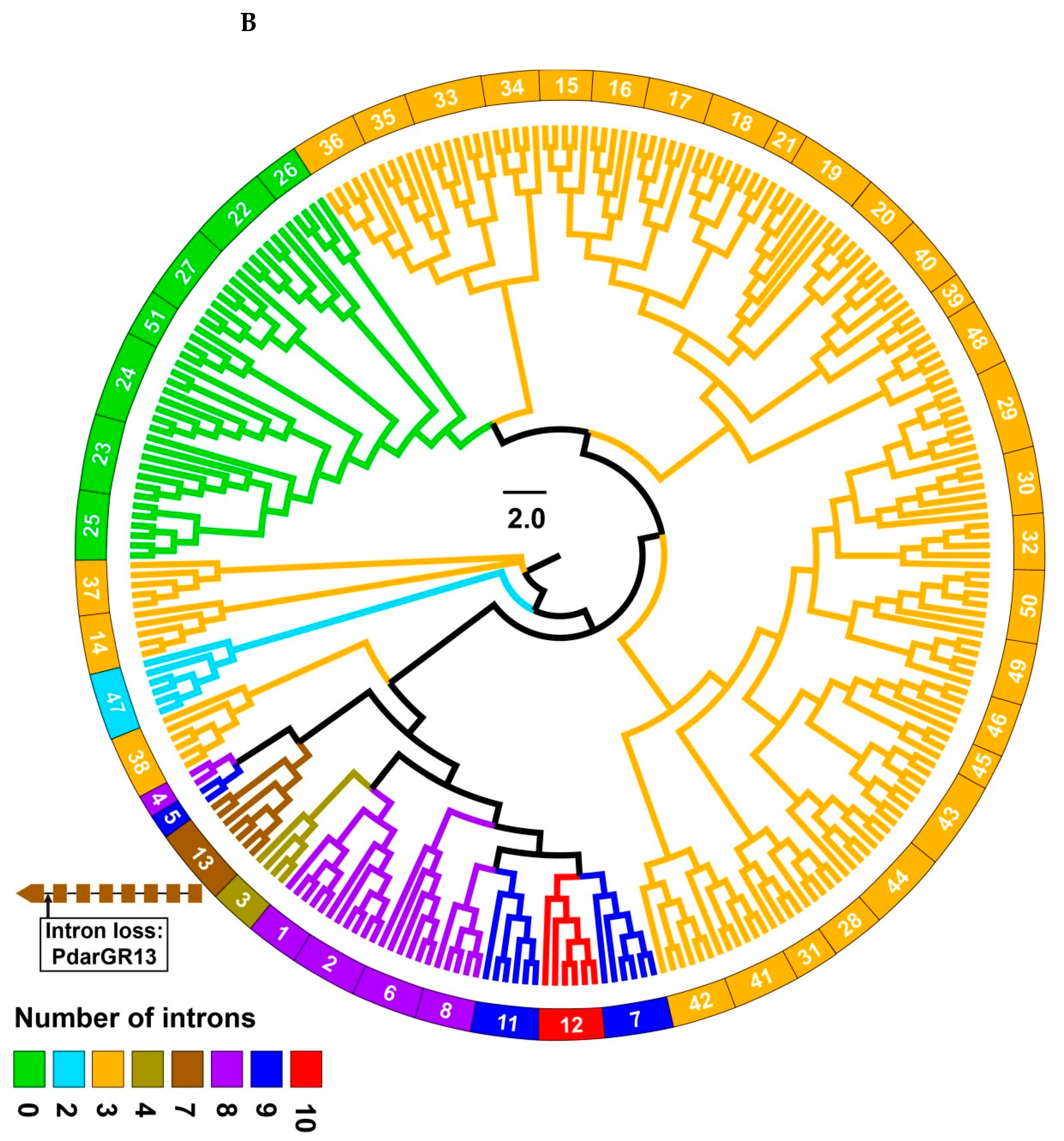
3.2. Intron Analysis of Papilio ORs and GRs
3.3. Phylogenetic Analysis of Papilio ORs
3.4. Phylogenetic Analysis of Papilio GRs
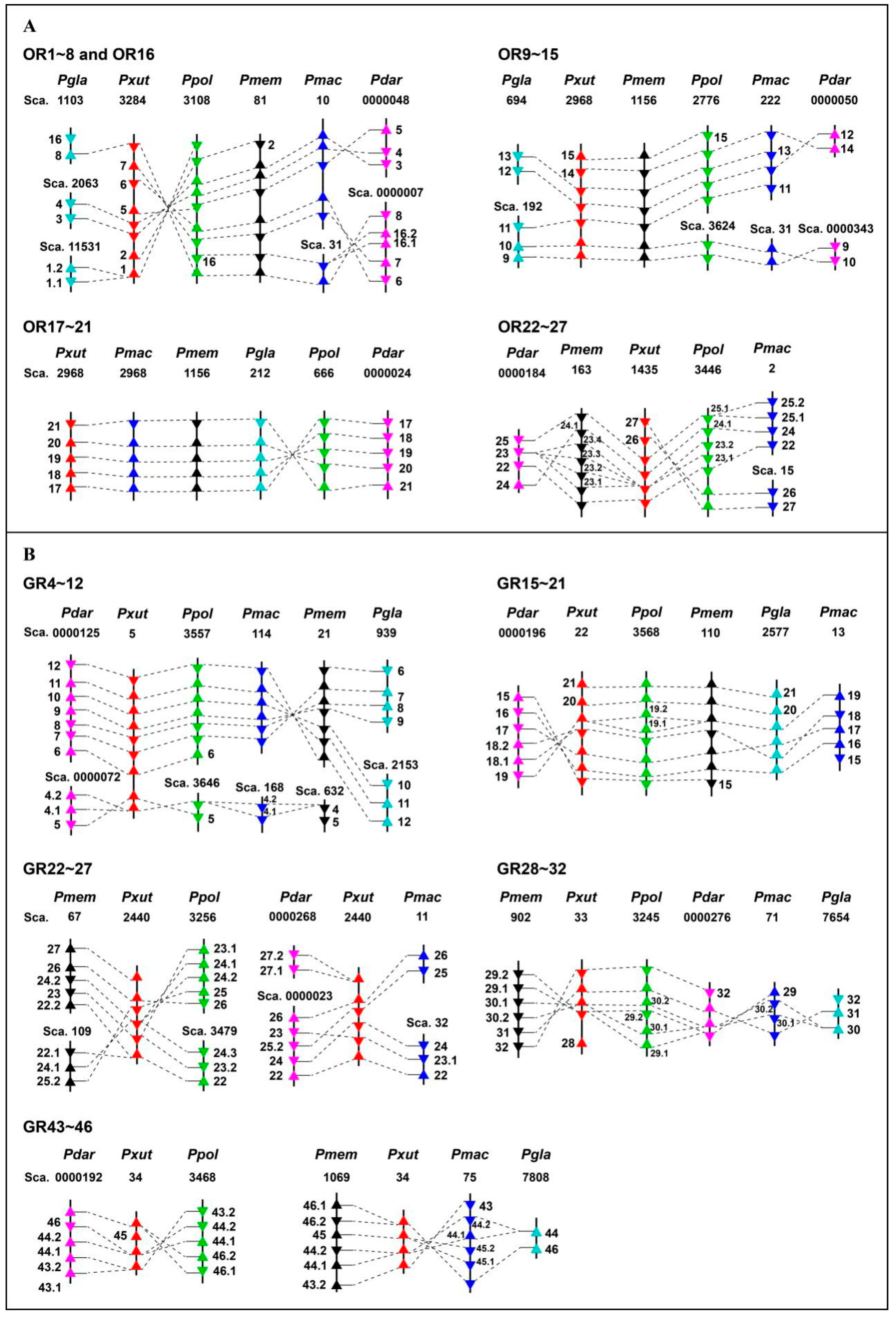
3.5. Genomic Arrangement of Papilio ORs and GRs
3.6. Expression Profiling Analysis of P. xuthus ORs
3.7. Expression Profiling Analysis of P. xuthus GRs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Nieukerken, E.; Kaila, L.; Kitching, I.; Kristensen, N.; Lees, D.; Minet, J.; Mitter, C.; Mutanen, M.; Regier, J.; Simonsen, T.; et al. Order Lepidoptera Linnaeus, 1758. In Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness; Zhang, Z.Q., Ed.; Magnolia Press: Auckland, New Zealand, 2011. [Google Scholar]
- Häuser, C.; De Jong, R.; Lamas, G.; Robbins, R.; Smith, C.; Vane-Wright, R. Papilionidae–Revised GloBIS/GART Species Checklist, 2nd Draft. 2005. Available online: https://www.insectsonline.de/frames/papilio.htm (accessed on 12 May 2021).
- Yukawa, J.; Nishida, R.; Fukuda, H.; Inoue, R. Aristolochiaceae- and Asteraceae-feeding by larvae of Papilio xuthus L. (Lepidoptera: Papilionidae) in Japan: A review. Entomol. Sci. 2019, 22, 355–364. [Google Scholar] [CrossRef]
- Robinson, G.; Ackery, P.; Kitching, I.; Beccaloni, G.; Hernandez, L. Hostplants of the Moth and Butterfly Caterpillars of the Oriental Region; United Selangor Press: Kuala Lumpur, Malaysia, 2001. [Google Scholar]
- Scriber, J.M. Origins of the regional feeding abilities in the tiger swallowtail butterfly: Ecological monophagy and the Papilio glaucus australis subspecies in Florida. Oecologia 1986, 71, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Scriber, J.M. Latitudinal gradients in larval feeding specialization of the world Papilionidae (Lepidoptera). Psyche 1973, 80, 052610. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Schneider, T.M.; Schwartz, A.M.; Andersson, M.N.; McKenna, D.D. The diversity and evolution of odorant receptors in beetles (Coleoptera). Insect Mol. Biol. 2020, 29, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Auer, T.O.; Khallaf, M.A.; Silbering, A.F.; Zappia, G.; Ellis, K.; Álvarez-Ocaña, R.; Arguello, J.R.; Hansson, B.S.; Jefferis, G.S.X.E.; Caron, S.J.C.; et al. Olfactory receptor and circuit evolution promote host specialization. Nature 2020, 579, 402–408. [Google Scholar] [CrossRef]
- Robertson, H.M. Molecular evolution of the major Arthropod chemoreceptor gene families. Annu. Rev. Entomol. 2019, 64, 227–242. [Google Scholar] [CrossRef]
- Suzuki, H.C.; Ozaki, K.; Makino, T.; Uchiyama, H.; Yajima, S.; Kawata, M. Evolution of gustatory receptor gene family provides insights into adaptation to diverse host plants in nymphalid butterflies. Genome Biol. Evol. 2018, 10, 1351–1362. [Google Scholar] [CrossRef]
- Engsontia, P.; Sangket, U.; Chotigeat, W.; Satasook, C. Molecular evolution of the odorant and gustatory receptor genes in lepidopteran insects: Implications for their adaptation and speciation. J. Mol. Evol. 2014, 79, 21–39. [Google Scholar] [CrossRef]
- McBride, C.S. Rapid evolution of smell and taste receptor genes during host specialization in Drosophila sechellia. Proc. Natl. Acad. Sci. USA 2007, 104, 4996–5001. [Google Scholar] [CrossRef]
- Matsunaga, T.; Reisenman, C.E.; Goldman-Huertas, B.; Brand, P.; Miao, K.; Suzuki, H.C.; Verster, K.I.; Ramírez, S.R.; Whiteman, N.K. Evolution of olfactory receptors tuned to mustard oils in herbivorous Drosophilidae. Mol. Biol. Evol. 2021, 39, msab362. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef] [PubMed]
- De Fouchier, A.; Walker, W.B.; Montagné, N.; Steiner, C.; Binyameen, M.; Schlyter, F.; Chertemps, T.; Maria, A.; François, M.-C.; Monsempes, C.; et al. Functional evolution of Lepidoptera olfactory receptors revealed by deorphanization of a moth repertoire. Nat. Commun. 2017, 8, 15709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-D.; Löfstedt, C. Moth pheromone receptors: Gene sequences, function, and evolution. Front. Ecol. Evol. 2015, 3, 105. [Google Scholar] [CrossRef]
- Yang, K.; Wang, C.Z. Review of pheromone receptors in heliothine species: Expression, function, and evolution. Entomol. Exp. Appl. 2021, 169, 156–171. [Google Scholar] [CrossRef]
- Bastin-Héline, L.; De Fouchier, A.; Cao, S.; Koutroumpa, F.; Caballero-Vidal, G.; Robakiewicz, S.; Monsempes, C.; Francois, M.C.; Ribeyre, T.; Maria, A.; et al. A novel lineage of candidate pheromone receptors for sex communication in moths. Elife 2019, 8, e49826. [Google Scholar] [CrossRef]
- Yuvaraj, J.K.; Corcoran, J.A.; Andersson, M.N.; Newcomb, R.D.; Anderbrant, O.; Löfstedt, C. Characterization of odorant receptors from a non-ditrysian moth, Eriocrania semipurpurella sheds light on the origin of sex pheromone receptors in Lepidoptera. Mol. Biol. Evol. 2017, 34, 2733–2746. [Google Scholar] [CrossRef]
- Andersson, M.N.; Keeling, C.I.; Mitchell, R.F. Genomic content of chemosensory genes correlates with host range in wood-boring beetles (Dendroctonus ponderosae, Agrilus planipennis, and Anoplophora glabripennis). BMC Genom. 2019, 20, 690. [Google Scholar] [CrossRef]
- Tian, J.; Dewer, Y.; Hu, H.; Li, F.; Yang, S.; Luo, C. Diversity and molecular evolution of odorant receptor in hemipteran insects. Insects 2022, 13, 214. [Google Scholar] [CrossRef]
- He, P.; Engsontia, P.; Chen, G.L.; Yin, Q.; Wang, J.; Lu, X.; Zhang, Y.N.; Li, Z.Q.; He, M. Molecular characterization and evolution of a chemosensory receptor gene family in three notorious rice planthoppers, Nilaparvata lugens, Sogatella furcifera and Laodelphax striatellus, based on genome and transcriptome analyses. Pest. Manag. Sci. 2018, 74, 2156–2167. [Google Scholar] [CrossRef]
- Gouin, A.; Bretaudeau, A.; Nam, K.; Gimenez, S.; Aury, J.M.; Duvic, B.; Hilliou, F.; Durand, N.; Montagné, N.; Darboux, I.; et al. Two genomes of highly polyphagous lepidopteran pests (Spodoptera frugiperda, Noctuidae) with different host-plant ranges. Sci. Rep. 2017, 7, 11816. [Google Scholar] [CrossRef]
- Cheng, T.; Wu, J.; Wu, Y.; Chilukuri, R.V.; Huang, L.; Yamamoto, K.; Feng, L.; Li, W.; Chen, Z.; Guo, H.; et al. Genomic adaptation to polyphagy and insecticides in a major East Asian noctuid pest. Nat. Ecol. Evol. 2017, 1, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.L.; Clarke, D.F.; East, P.D.; Elfekih, S.; Gordon, K.H.J.; Jermiin, L.S.; McGaughran, A.; Oakeshott, J.G.; Papanikolaou, A.; Perera, O.P.; et al. Genomic innovations, transcriptional plasticity and gene loss underlying the evolution and divergence of two highly polyphagous and invasive Helicoverpa pest species. BMC Biol. 2017, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Koenig, C.; Hirsh, A.; Bucks, S.; Klinner, C.; Vogel, H.; Shukla, A.; Mansfield, J.H.; Morton, B.; Hansson, B.S.; Grosse-Wilde, E. A reference gene set for chemosensory receptor genes of Manduca sexta. Insect Biochem. Mol. Biol. 2015, 66, 51–63. [Google Scholar] [CrossRef]
- Tanaka, K.; Uda, Y.; Ono, Y.; Nakagawa, T.; Suwa, M.; Yamaoka, R.; Touhara, K. Highly selective tuning of a silkworm olfactory receptor to a key mulberry leaf volatile. Curr. Biol. 2009, 19, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Moon, S.J.; Montell, C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl. Acad. Sci. USA 2009, 106, 4495–4500. [Google Scholar] [CrossRef]
- Ozaki, K.; Ryuda, M.; Yamada, A.; Utoguchi, A.; Ishimoto, H.; Calas, D.; Marion-Poll, F.; Tanimura, T.; Yoshikawa, H. A gustatory receptor involved in host plant recognition for oviposition of a swallowtail butterfly. Nat. Commun. 2011, 2, 542. [Google Scholar] [CrossRef]
- Yang, K.; Gong, X.L.; Li, G.C.; Huang, L.Q.; Ning, C.; Wang, C.Z. A gustatory receptor tuned to the steroid plant hormone brassinolide in Plutella xylostella (Lepidoptera: Plutellidae). Elife 2020, 9, e64114. [Google Scholar] [CrossRef]
- Xu, W. How do moth and butterfly taste?—Molecular basis of gustatory receptors in Lepidoptera. Insect Sci. 2020, 27, 1148–1157. [Google Scholar] [CrossRef]
- Agnihotri, A.R.; Roy, A.A.; Joshi, R.S. Gustatory receptors in Lepidoptera: Chemosensation and beyond. Insect Mol. Biol. 2016, 25, 519–529. [Google Scholar] [CrossRef]
- Zhang, H.J.; Anderson, A.R.; Trowell, S.C.; Luo, A.R.; Xiang, Z.H.; Xia, Q.Y. Topological and functional characterization of an insect gustatory receptor. PLoS ONE 2011, 6, e24111. [Google Scholar]
- Sato, K.; Tanaka, K.; Touhara, K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 11680–11685. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Yan, Q.; Yang, Y.L.; Hou, W.; Miao, C.L.; Peng, Y.C.; Dong, S.L. A gustatory receptor GR8 tunes specifically to D-fructose in the common cutworm Spodoptera litura. Insects 2019, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, H.J.; Anderson, A. A sugar gustatory receptor identified from the foregut of cotton bollworm Helicoverpa armigera. J. Chem. Ecol. 2012, 38, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, H.; Jiang, N.J.; Tang, R.; Li, G.C.; Huang, L.Q.; Van Loon, J.J.A.; Wang, C.Z. Identification of a gustatory receptor tuned to sinigrin in the cabbage butterfly Pieris rapae. PLoS Genet. 2021, 17, e1009527. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Shii, F.; Tsuneto, K.; Yamagishi, T.; Adegawa, S.; Endo, H.; Sato, R. Insect taste receptors relevant to host identification by recognition of secondary metabolite patterns of non-host plants. Biochem. Biophys. Res. Commun. 2018, 499, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Papanicolaou, A.; Zhang, H.J.; Anderson, A. Expansion of a bitter taste receptor family in a polyphagous insect herbivore. Sci. Rep. 2016, 6, 23666. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, G.; Chen, X.E.; Cao, Y.; Wu, N.; Cui, Q.; Zhu, C.; Qian, L.; Huang, Y.; Zhan, S. The genome of the black cutworm Agrotis ipsilon. Insect Biochem. Mol. Biol. 2021, 139, 103665. [Google Scholar] [CrossRef]
- Cong, Q.; Borek, D.; Otwinowski, Z.; Grishin, N.V. Tiger swallowtail genome reveals mechanisms for speciation and caterpillar chemical defense. Cell Rep. 2015, 10, 910–919. [Google Scholar] [CrossRef]
- Yin, N.N.; Nuo, S.M.; Xiao, H.Y.; Zhao, Y.J.; Zhu, J.Y.; Liu, N.Y. The ionotropic receptor gene family in Lepidoptera and Trichoptera: Annotation, evolutionary and functional perspectives. Genomics 2021, 113, 601–612. [Google Scholar] [CrossRef]
- Nishikawa, H.; Iijima, T.; Kajitani, R.; Yamaguchi, J.; Ando, T.; Suzuki, Y.; Sugano, S.; Fujiyama, A.; Kosugi, S.; Hirakawa, H.; et al. A genetic mechanism for female-limited Batesian mimicry in Papilio butterfly. Nat. Genet. 2015, 47, 405–409. [Google Scholar] [CrossRef]
- Iijima, T.; Kajitani, R.; Komata, S.; Lin, C.-P.; Sota, T.; Itoh, T.; Fujiwara, H. Parallel evolution of Batesian mimicry supergene in two Papilio butterflies, P. polytes and P. memnon. Sci. Adv. 2018, 4, eaao5416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Fan, D.; Zhang, W.; Liu, G.; Zhang, L.; Zhao, L.; Fang, X.; Chen, L.; Dong, Y.; Chen, Y.; et al. Outbred genome sequencing and CRISPR/Cas9 gene editing in butterflies. Nat. Commun. 2015, 6, 8212. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, M.; Srivathsan, A.; Collins, S.; Meier, R.; Vogler, A.P. Mimicry diversification in Papilio dardanus via a genomic inversion in the regulatory region of engrailed-invected. Proc. Biol. Sci. 2020, 287, 20200443. [Google Scholar] [CrossRef]
- Guo, H.; Cheng, T.; Chen, Z.; Jiang, L.; Guo, Y.; Liu, J.; Li, S.; Taniai, K.; Asaoka, K.; Kadono-Okuda, K.; et al. Expression map of a complete set of gustatory receptor genes in chemosensory organs of Bombyx mori. Insect Biochem. Mol. Biol. 2017, 82, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Heliconius Genome Consortium. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 2012, 487, 94–98. [Google Scholar] [CrossRef]
- Zhan, S.; Merlin, C.; Boore, J.L.; Reppert, S.M. The monarch butterfly genome yields insights into long-distance migration. Cell 2011, 147, 1171–1185. [Google Scholar] [CrossRef]
- Birney, E.; Clamp, M.; Durbin, R. GeneWise and Genomewise. Genome Res. 2004, 14, 988–995. [Google Scholar] [CrossRef]
- Xu, W.; Liu, N.; Liao, Y.; Anderson, A. Molecular characterization of sugar taste receptors in the cotton bollworm Helicoverpa armigera. Genome 2017, 60, 1037–1044. [Google Scholar] [CrossRef]
- Xu, W.; Anderson, A. Carbon dioxide receptor genes in cotton bollworm Helicoverpa armigera. Naturwissenschaften 2015, 102, 1260. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Simon, P. Q-Gene: Processing quantitative real-time RT–PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.Y.; Janovjak, H.; Miserez, A.R.; Dobbie, Z. Processing of gene expression data generated by quantitative real-time RT–PCR. Biotechniques 2002, 32, 1372–1374, 1376, 1378–1379. [Google Scholar] [PubMed]
- Liu, N.Y.; Xu, W.; Dong, S.L.; Zhu, J.Y.; Xu, Y.X.; Anderson, A. Genome-wide analysis of ionotropic receptor gene repertoire in Lepidoptera with an emphasis on its functions of Helicoverpa armigera. Insect Biochem. Mol. Biol. 2018, 99, 37–53. [Google Scholar] [CrossRef]
- Wanner, K.W.; Robertson, H.M. The gustatory receptor family in the silkworm moth Bombyx mori is characterized by a large expansion of a single lineage of putative bitter receptors. Insect Mol. Biol. 2008, 17, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Obiero, G.F.; Pauli, T.; Geuverink, E.; Veenendaal, R.; Niehuis, O.; Große-Wilde, E. Chemoreceptor diversity in apoid wasps and its reduction during the evolution of the pollen-collecting lifestyle of bees (Hymenoptera: Apoidea). Genome Biol. Evol. 2021, 13, evaa269. [Google Scholar] [CrossRef]
- Ryuda, M.; Calas-List, D.; Yamada, A.; Marion-Poll, F.; Yoshikawa, H.; Tanimura, T.; Ozaki, K. Gustatory sensing mechanism coding for multiple oviposition stimulants in the swallowtail butterfly, Papilio xuthus. J. Neurosci. 2013, 33, 914–924. [Google Scholar] [CrossRef]
- Meslin, C.; Mainet, P.; Montagné, N.; Robin, S.; Legeai, F.; Bretaudeau, A.; Johnston, J.S.; Koutroumpa, F.; Persyn, E.; Monsempès, C.; et al. Spodoptera littoralis genome mining brings insights on the dynamic of expansion of gustatory receptors in polyphagous noctuidae. G3 Genes Genom. Genet. 2022, 12, jkac131. [Google Scholar] [CrossRef]
- McKenna, D.D.; Scully, E.D.; Pauchet, Y.; Hoover, K.; Kirsch, R.; Geib, S.M.; Mitchell, R.F.; Waterhouse, R.M.; Ahn, S.J.; Arsala, D.; et al. Genome of the Asian longhorned beetle (Anoplophora glabripennis), a globally significant invasive species, reveals key functional and evolutionary innovations at the beetle-plant interface. Genome Biol. 2016, 17, 227. [Google Scholar] [CrossRef]
- Ferry, N.; Edwards, M.G.; Gatehouse, J.A.; Gatehouse, A.M. Plant-insect interactions: Molecular approaches to insect resistance. Curr. Opin. Biotechnol. 2004, 15, 155–161. [Google Scholar] [CrossRef]
- Meng, P.S.; Hoover, K.; Keena, M.A. Asian Longhorned Beetle (Coleoptera: Cerambycidae), an Introduced Pest of Maple and Other Hardwood Trees in North America and Europe. J. Integr. Pest Manag. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhang, S.S.; Niu, B.L.; Ji, D.F.; Liu, X.J.; Li, M.W.; Bai, H.; Palli, S.R.; Wang, C.Z.; Tan, A.J. A determining factor for insect feeding preference in the silkworm, Bombyx mori. PLoS Biol. 2019, 17, e3000162. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.M.; Wanner, K.W. The chemoreceptor superfamily in the honey bee, Apis mellifera: Expansion of the odorant, but not gustatory, receptor family. Genome Res. 2006, 16, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.M.; Warr, C.G.; Carlson, J.R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2003, 100, 14537–14542. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, J.; Lin, J.; Zheng, Y.; Yu, X.; Sun, J. Sex Pheromone Receptors of Lepidopteran Insects. Front. Ecol. Evol. 2022, 10, 797287. [Google Scholar] [CrossRef]
- Shiota, Y.; Sakurai, T. Molecular Mechanisms of Sex Pheromone Reception in Moths. In Insect Sex Pheromone Research and Beyond: From Molecules to Robots; Ishikawa, Y., Ed.; Springer: Singapore, 2020; pp. 185–205. [Google Scholar]
- Ômura, H.; Honda, K. Chemical composition of volatile substances from adults of the swallowtail, Papilio polytes (Lepidoptera: Papilionidae). Appl. Entomol. Zool. 2005, 40, 421–427. [Google Scholar] [CrossRef]
- Guo, M.; Du, L.; Chen, Q.; Feng, Y.; Zhang, J.; Zhang, X.; Tian, K.; Cao, S.; Huang, T.; Jacquin-Joly, E.; et al. Odorant receptors for detecting flowering plant cues are functionally conserved across moths and butterflies. Mol. Biol. Evol. 2020, 38, 1413–1427. [Google Scholar] [CrossRef]
- Nishida, R. Chemosensory basis of host recognition in butterflies--multi-component system of oviposition stimulants and deterrents. Chem. Senses 2005, 30, i293–i294. [Google Scholar] [CrossRef]
- Ohsugi, T.; Nishida, R.; Fukami, H. Multi-component system of oviposition stimulants for a Rutaceae-feeding swallowtail butterfly, Papilio xuthus (Lepidoptera: Papilionidae). Appl. Entomol. Zool. 1991, 26, 29–40. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Li, G.C.; Wang, Z.Q.; Guo, Y.R.; Liu, N.Y. Combined transcriptomic, proteomic and genomic analysis identifies reproductive-related proteins and potential modulators of female behaviors in Spodoptera litura. Genomics 2021, 113, 1876–1894. [Google Scholar] [CrossRef]
- Li, G.C.; Nuo, S.M.; Wang, Z.Q.; Yang, A.J.; Liu, N.Y. Identification and expression profiling of chemosensory membrane protein genes in Achelura yunnanensis (Lepidoptera: Zygaenidae). Comp. Biochem. Physiol. D Genom. Proteom. 2021, 40, 100876. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.A.; Rodriguez, S.D.; Drake, L.L.; Price, D.P.; Blakely, B.N.; Hammond, J.I.; Tsujimoto, H.; Monroy, E.Y.; Maio, W.A.; Romero, A. The odorant receptor co-receptor from the bed bug, Cimex lectularius L. PLoS ONE 2014, 9, e113692. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.F.; Zhao, Z.F.; Yan, M.J.; Zhou, W.; Zhang, Z.; Zhang, A.; Lu, Z.X.; Wang, M.Q. Identification and comparative expression profiles of chemoreception genes revealed from major chemoreception organs of the rice leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). PLoS ONE 2015, 10, e0144267. [Google Scholar] [CrossRef] [PubMed]
- Musundi, S.D.; Ochieng, P.J.; Wamunyokoli, F.; Nyanjom, S.G. Expression profile of odorant receptors in brain, gut and reproductive tissues in male and female Glossina morsitans morsitans. Sci. Afr. 2020, 10, e00591. [Google Scholar] [CrossRef]

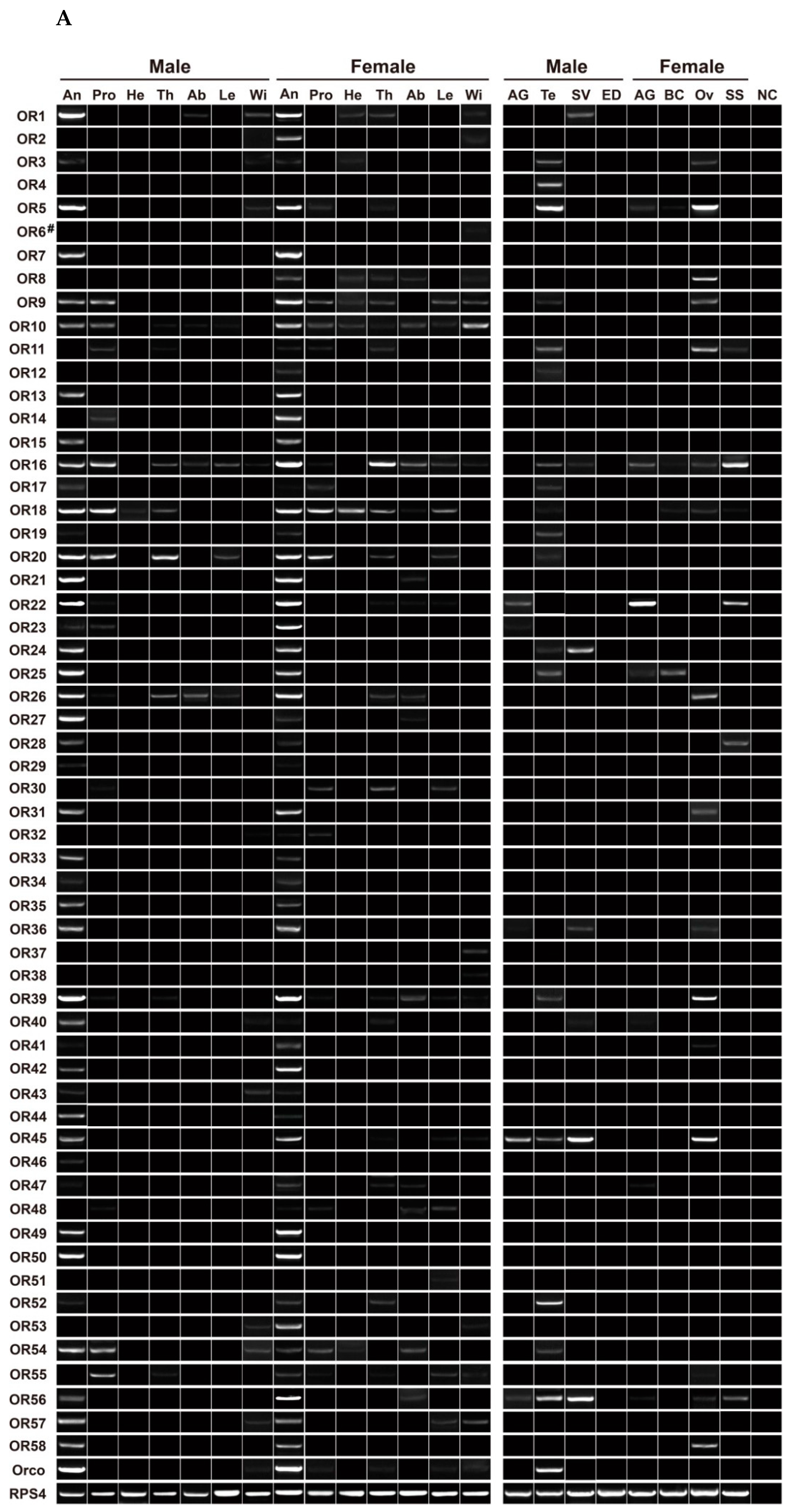
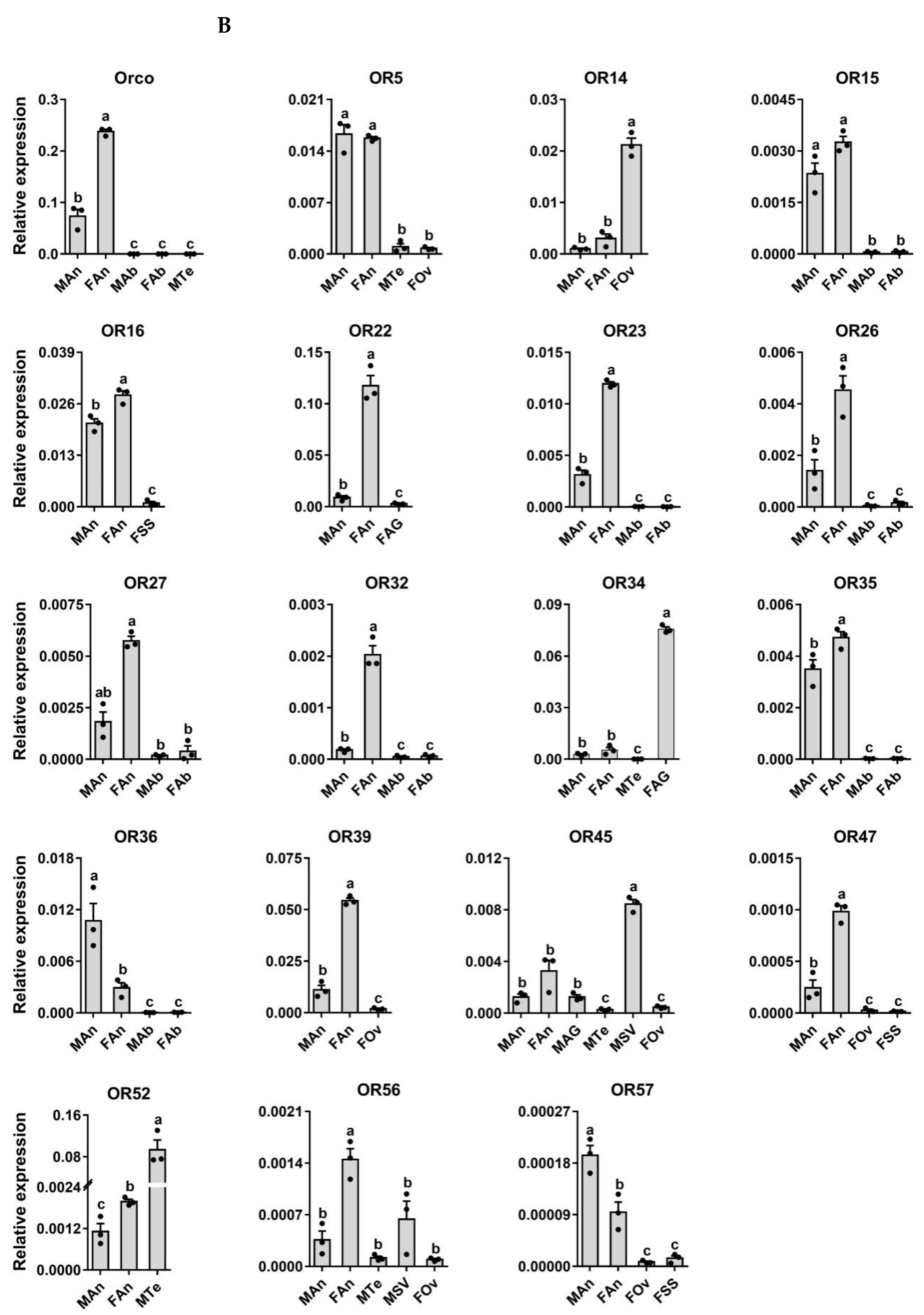
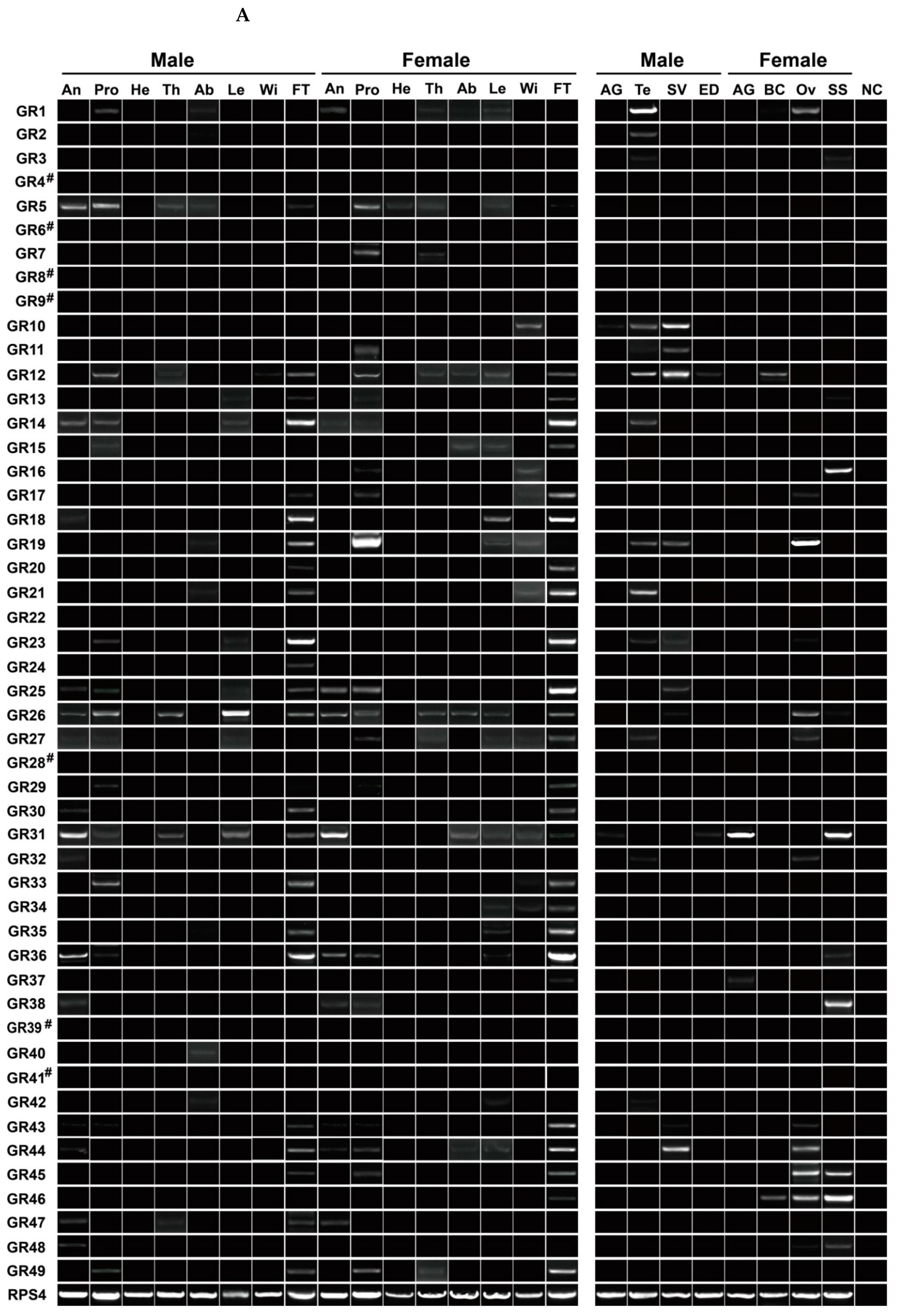
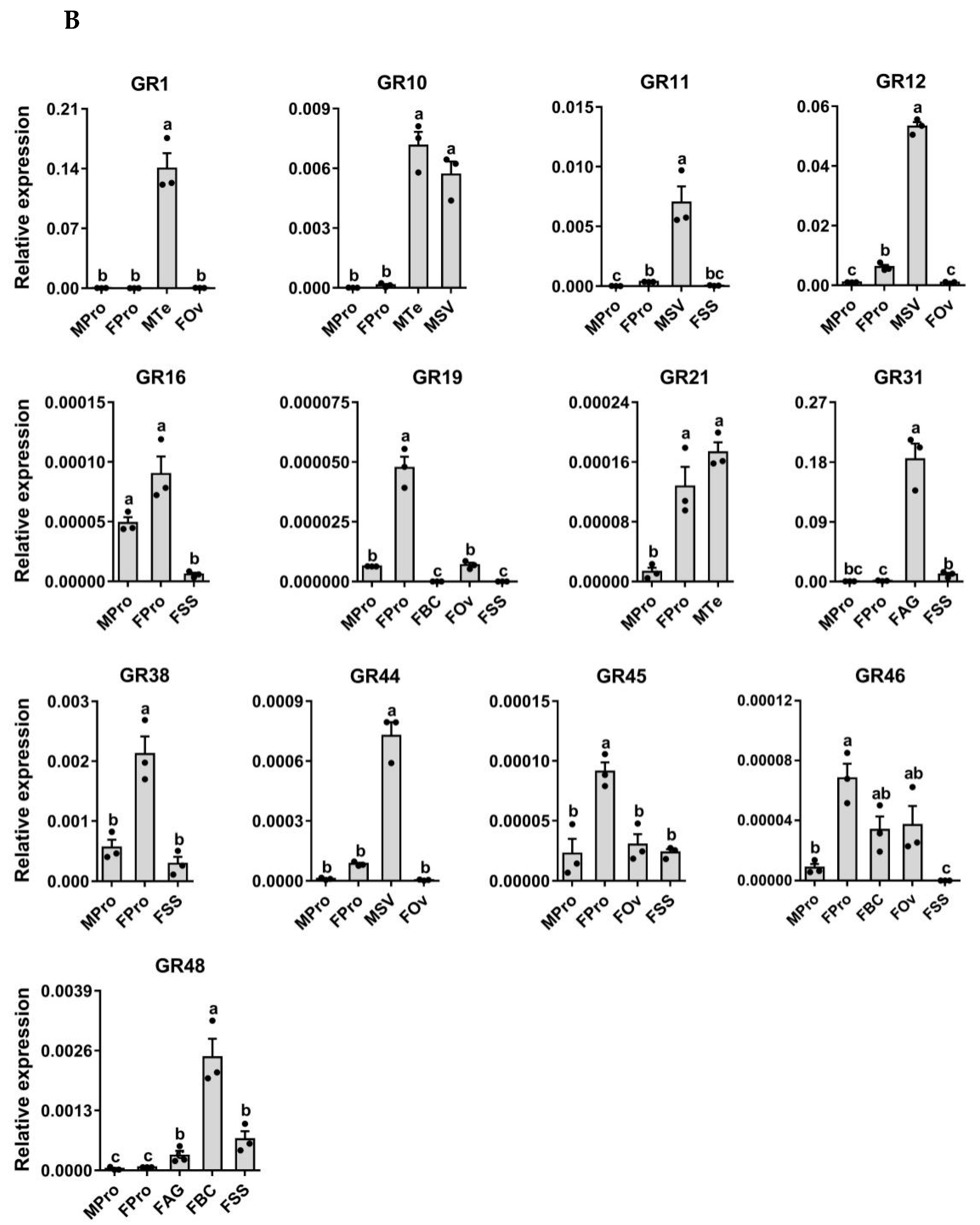
| Ortholog | Species | Ortholog | Species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pdar | Pgla | Pmac | Pmem | Ppol | Pxut | Pdar | Pgla | Pmac | Pmem | Ppol | Pxut | ||
| r | a | c | m | l | t | r | a | c | m | l | t | ||
| OR1/OR41/ OR53/OR58 | 1 | 2 | 1 | 1 | 1 | 1 | GR22 | 1 | 1 | 1 | 2 | 1 | 1 |
| OR11 | 1 | 1 | 1 | 1 | 1 | GR23 | 1 | 1 | 2 | 1 | 2 | 1 | |
| OR16 | 2 | 1 | 1 | 1 | 1 | 1 | GR24 | 1 | 1 | 2 | 3 | 1 | |
| OR23 | 1 | 4 | 2 | 1 | GR25 | 3 | 1 | 2 | 1 | 1 | |||
| OR24 | 1 | 1 | 2 | 2 | 1 | GR26 | 1 | 1 | 1 | 1 | 1 | ||
| OR25 | 1 | 2 | 1 | 2 | 1 | GR27 | 3 | 1 | 1 | 1 | 1 | 1 | |
| OR29 | 1 | 1 | 2 | 1 | 1 | 1 | GR30 | 1 | 1 | 2 | 2 | 2 | 1 |
| OR30 | 1 | 1 | 1 | 2 | 2 | 1 | GR32 | 1 | 1 | 1 | 1 | 1 | |
| OR39 | 6 | 1 | 1 | 2 | 4 | 1 | GR33 | 1 | 3 | 1 | 1 | 1 | 1 |
| OR47 | 1 | 1 | 1 | 1 | 1 | GR35 | 2 | 2 | 1 | 1 | 1 | ||
| OR59 | 1 | 1 | 1 | 1 | GR39 | 1 | 1 | 1 | 1 | ||||
| OR60 | 1 | 1 | 2 | GR43 | 2 | 1 | 2 | 2 | 1 | ||||
| GR13/GR29 /GR46 | 1 | 1 | 1 | 2 | 2 | 1 | GR44 | 2 | 1 | 2 | 2 | 2 | 1 |
| GR4 | 2 | 1 | 2 | 1 | 1 | 1 | GR45 | 2 | 1 | 1 | |||
| GR18 | 2 | 1 | 1 | 1 | 1 | 1 | GR49 | 2 | 1 | 1 | 1 | 1 | |
| GR19 | 1 | 1 | 1 | 1 | 2 | 1 | GR50 | 1 | 1 | 1 | 2 | 2 | |
| GR21 | 1 | 1 | 1 | 1 | GR51 | 1 | 1 | 1 | 1 | 1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, N.; Xiao, H.; Yang, A.; Wu, C.; Liu, N. Genome-Wide Analysis of Odorant and Gustatory Receptors in Six Papilio Butterflies (Lepidoptera: Papilionidae). Insects 2022, 13, 779. https://doi.org/10.3390/insects13090779
Yin N, Xiao H, Yang A, Wu C, Liu N. Genome-Wide Analysis of Odorant and Gustatory Receptors in Six Papilio Butterflies (Lepidoptera: Papilionidae). Insects. 2022; 13(9):779. https://doi.org/10.3390/insects13090779
Chicago/Turabian StyleYin, Ningna, Haiyan Xiao, Anjin Yang, Chun Wu, and Naiyong Liu. 2022. "Genome-Wide Analysis of Odorant and Gustatory Receptors in Six Papilio Butterflies (Lepidoptera: Papilionidae)" Insects 13, no. 9: 779. https://doi.org/10.3390/insects13090779
APA StyleYin, N., Xiao, H., Yang, A., Wu, C., & Liu, N. (2022). Genome-Wide Analysis of Odorant and Gustatory Receptors in Six Papilio Butterflies (Lepidoptera: Papilionidae). Insects, 13(9), 779. https://doi.org/10.3390/insects13090779






