Relative Influence of Land Use, Mosquito Abundance, and Bird Communities in Defining West Nile Virus Infection Rates in Culex Mosquito Populations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
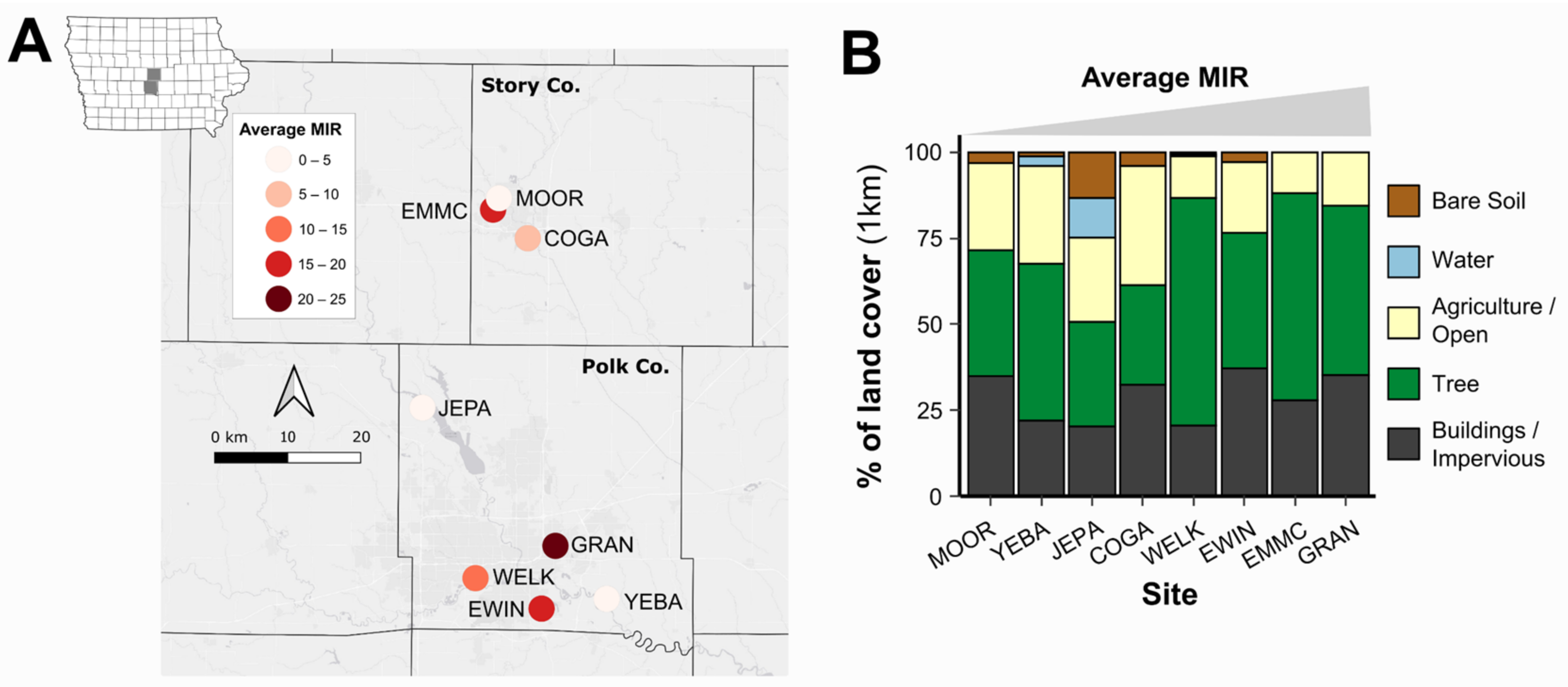
2.1. Study Area
2.2. Mosquito Trapping, Sample Identification, and WNV Testing
2.3. Bird Surveys
2.4. Land Use/Land Cover Analysis
2.5. Statistical Analyses
2.6. Mosquito Community Metrics
2.7. Avian Community Metrics
2.8. Relating Land Use and Community Metrics to WNV Prevalence in Mosquitoes
3. Results
3.1. Study Sites, Land Use, and Mosquito Minimum Infection Rates
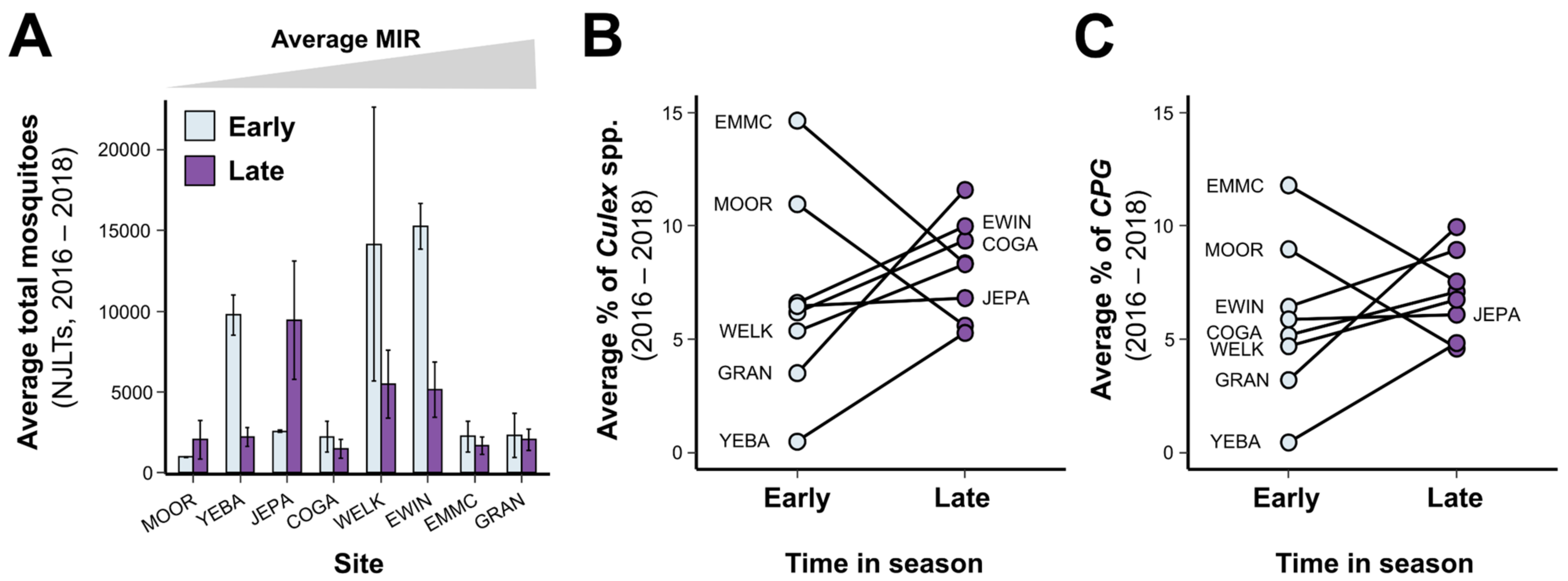
3.2. Mosquito Communities
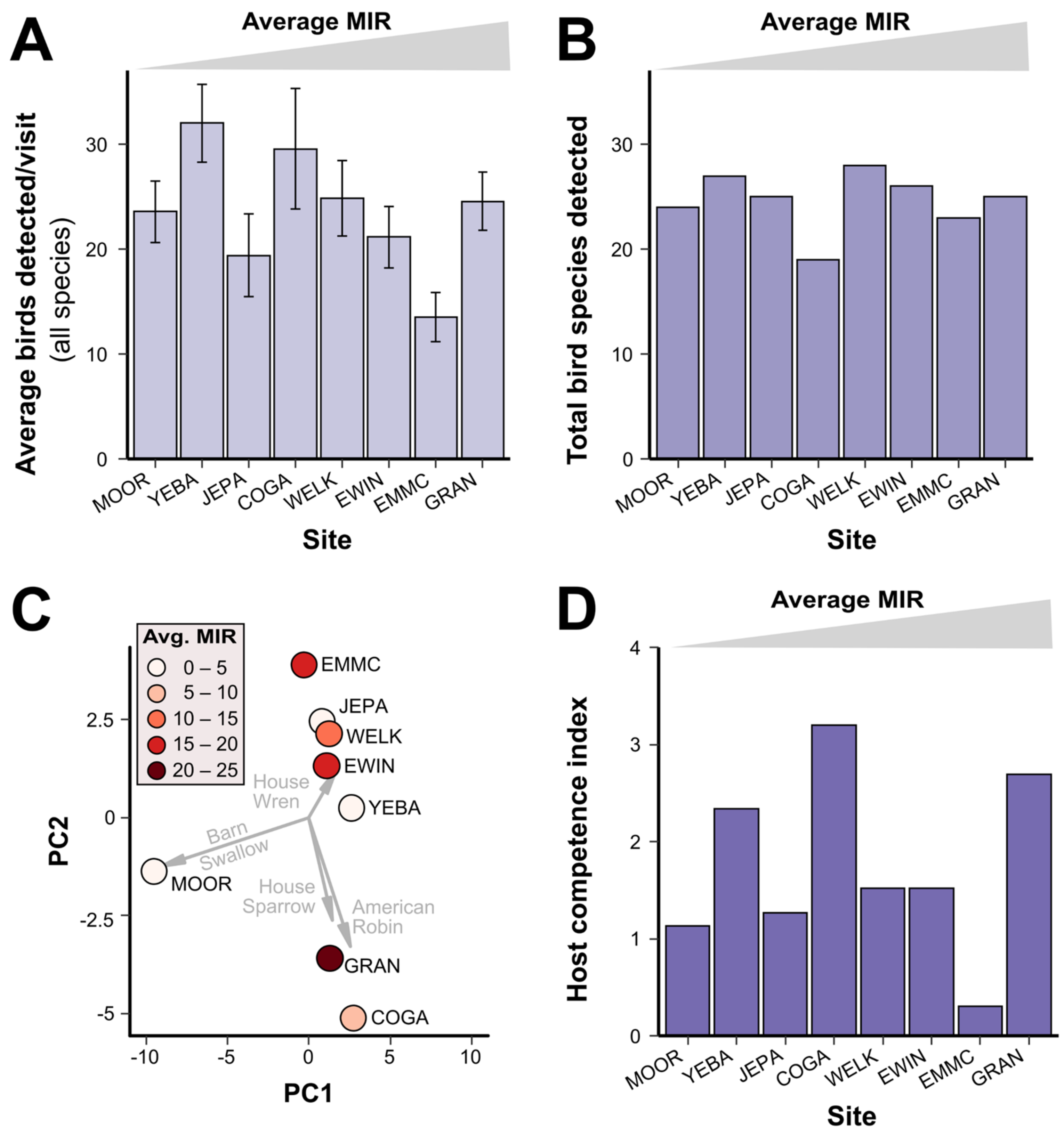
3.3. Bird Communities
3.4. Ecological Factors and Mosquito MIR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- West Nile Virus. Available online: http://www.cdc.gov/westnile/index.html (accessed on 18 July 2022).
- Gray, T.J.; Webb, C.E. A Review of the Epidemiological and Clinical Aspects of West Nile Virus. Int. J. Gen. Med. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A Global Perspective on the Epidemiology of West Nile Virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochlin, I.; Faraji, A.; Healy, K.; Andreadis, T.G. West Nile Virus Mosquito Vectors in North America. J. Med. Entomol. 2019, 56, 1475–1490. [Google Scholar] [CrossRef]
- Duggal, N.K.; Langwig, K.E.; Ebel, G.D.; Brault, A.C. On the Fly: Interactions Between Birds, Mosquitoes, and Environment That Have Molded West Nile Virus Genomic Structure Over Two Decades. J. Med. Entomol. 2019, 56, 1467–1474. [Google Scholar] [CrossRef]
- Ozdenerol, E.; Taff, G.N.; Akkus, C. Exploring the Spatio-Temporal Dynamics of Reservoir Hosts, Vectors, and Human Hosts of West Nile Virus: A Review of the Recent Literature. Int. J. Environ. Res. Public Health 2013, 10, 5399–5432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, A.M. Globalization, Land Use, and the Invasion of West Nile Virus. Science 2011, 334, 323–327. [Google Scholar] [CrossRef] [Green Version]
- Blair, R.B. Land Use and Avian Species Diversity along an Urban Gradient. Ecol. Appl. 1996, 6, 506–519. [Google Scholar] [CrossRef]
- Chapman, K.A.; Reich, P.B. Land Use and Habitat Gradients Determine Bird Community Diversity and Abundance in Suburban, Rural and Reserve Landscapes of Minnesota, USA. Biol. Conser. 2007, 135, 527–541. [Google Scholar] [CrossRef]
- Meyer Steiger, D.B.; Ritchie, S.A.; Laurance, S.G.W. Mosquito Communities and Disease Risk Influenced by Land Use Change and Seasonality in the Australian Tropics. Parasit. Vectors 2016, 9, 387. [Google Scholar] [CrossRef] [Green Version]
- Ferraguti, M.; Martínez-de la Puente, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Effects of Landscape Anthropization on Mosquito Community Composition and Abundance. Sci. Rep. 2016, 6, 29002. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.S.; Hedeen, D.L.; Hedeen, M.W.; Hamer, G.L.; Mead, D.G.; Kitron, U.D. Avian Species Diversity and Transmission of West Nile Virus in Atlanta, Georgia. Parasit. Vectors 2017, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunphy, B.M.; Kovach, K.B.; Gehrke, E.J.; Field, E.N.; Rowley, W.A.; Bartholomay, L.C.; Smith, R.C. Long-Term Surveillance Defines Spatial and Temporal Patterns Implicating Culex tarsalis as the Primary Vector of West Nile Virus. Sci. Rep. 2019, 9, 6637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, E.N.; Tokarz, R.E.; Smith, R.C. Satellite Imaging and Long-Term Mosquito Surveillance Implicate the Influence of Rapid Urbanization on Culex Vector Populations. Insects 2019, 10, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowder, D.W.; Dykstra, E.A.; Brauner, J.M.; Duffy, A.; Reed, C.; Martin, E.; Peterson, W.; Carrière, Y.; Dutilleul, P.; Owen, J.P. West Nile Virus Prevalence across Landscapes Is Mediated by Local Effects of Agriculture on Vector and Host Communities. PLoS ONE 2013, 8, 0055006. [Google Scholar] [CrossRef]
- Levine, R.S.; Mead, D.G.; Hamer, G.L.; Brosi, B.J.; Hedeen, D.L.; Hedeen, M.W.; McMillan, J.R.; Bisanzio, D.; Kitron, U.D. Supersuppression: Reservoir Competency and Timing of Mosquito Host Shifts Combine to Reduce Spillover of West Nile Virus. Am. J. Trop. Med. Hyg. 2016, 95, 1174–1184. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.S.; Mead, D.G.; Kitron, U.D. Limited Spillover to Humans from West Nile Virus Viremic Birds in Atlanta, Georgia. Vector Borne Zoonotic Dis. 2013, 13, 812–817. [Google Scholar] [CrossRef] [Green Version]
- Kramer, L.D.; Bernard, K.A. West Nile Virus Infection in Birds and Mammals. Ann. N. Y. Acad. Sci. 2001, 951, 84–93. [Google Scholar] [CrossRef]
- Molaei, G.; Andreadis, T.G.; Armstrong, P.M.; Anderson, J.F.; Vossbrinck, C.R. Host Feeding Patterns of Culex Mosquitoes and West Nile Virus Transmission, Northeastern United States. Emerg. Infect. Dis. 2006, 12, 468–474. [Google Scholar] [CrossRef]
- Johnson, B.J.; Robson, M.G.; Fonseca, D.M. Unexpected Spatiotemporal Abundance of Infected Culex Restuans Suggest a Greater Role as a West Nile Virus Vector for This Native Species. Infect. Genet. Evol. 2015, 31, 40–47. [Google Scholar] [CrossRef]
- Moise, I.K.; Riegel, C.; Muturi, E.J. Environmental and Social-Demographic Predictors of the Southern House Mosquito Culex Quinquefasciatus in New Orleans, Louisiana. Parasit. Vectors 2018, 11, 249. [Google Scholar] [CrossRef] [Green Version]
- Reisen, W.K.; Takahashi, R.M.; Carroll, B.D.; Quiring, R. Delinquent Mortgages, Neglected Swimming Pools, and West Nile Virus, California. Emerg. Infect. Dis. 2008, 14, 1747–1749. [Google Scholar] [CrossRef] [PubMed]
- DeGroote, J.P.; Sugumaran, R.; Brend, S.M.; Tucker, B.J.; Bartholomay, L.C. Landscape, Demographic, Entomological, and Climatic Associations with Human Disease Incidence of West Nile Virus in the State of Iowa, USA. Int. J. Health Geogr. 2008, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.M.; Edwards, E.; Hettler, D.L.; Davis, B.S.; Bowen, R.A.; Bunning, M.L. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar]
- Hadfield, J.; Brito, A.F.; Swetnam, D.M.; Vogels, C.B.F.; Tokarz, R.E.; Andersen, K.G.; Smith, R.C.; Bedford, T.; Grubaugh, N.D. Twenty Years of West Nile Virus Spread and Evolution in the Americas Visualized by Nextstrain. PLoS Pathog. 2019, 15, e1008042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swetnam, D.; Widen, S.G.; Wood, T.G.; Reyna, M.; Wilkerson, L.; Debboun, M.; Symonds, D.A.; Mead, D.G.; Beaty, B.J.; Guzman, H.; et al. Terrestrial Bird Migration and West Nile Virus Circulation, United States. Emerg. Infect. Dis. 2018, 24, 2184–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappole, J.H.; Derrickson, S.R.; Hubálek, Z. Migratory Birds and Spread of West Nile Virus in the Western Hemisphere. Emerg. Infect. Dis. 2000, 6, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Ezenwa, V.O.; Godsey, M.S.; King, R.J.; Guptill, S.C. Avian Diversity and West Nile Virus: Testing Associations between Biodiversity and Infectious Disease Risk. Proc. Biol. Sci. 2006, 271, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile Virus Epidemics in North America Are Driven by Shifts in Mosquito Feeding Behavior. PLoS Biol. 2006, 4, 606–610. [Google Scholar] [CrossRef]
- Hamer, G.L.; Kitron, U.D.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Goldberg, T.L.; Walker, E.D. Culex Pipiens (Diptera: Culicidae): A Bridge Vector of West Nile Virus to Humans. J. Med. Entomol. 2008, 45, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Hamer, G.L.; Kitron, U.D.; Goldberg, T.L.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Hayes, D.B.; Walker, E.D. Host Selection by Culex Pipiens Mosquitoes and West Nile Virus Amplification. Am. J. Trop. Med. Hyg. 2009, 80, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Marm Kilpatrick, A.; Daszak, P.; Jones, M.J.; Marra, P.P.; Kramer, L.D. Host Heterogeneity Dominates West Nile Virus Transmission. Proc. R. Soc. B Biol. Sci. 2006, 273, 2327–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokarz, R.E.; Smith, R.C. Crossover Dynamics of Culex (Diptera: Culicidae) Vector Populations Determine WNV Transmission Intensity. J. Med. Entomol. 2020, 57, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Brown, W.M.; Uelmen, J.; Ruiz, M.O.; Smith, R.L. The Drivers of West Nile Virus Human Illness: Fine Scale Dynamic Effects of Weather, Mosquito Infection, Social, and Biological Conditions. PLoS ONE 2020, 15, e0227160. [Google Scholar] [CrossRef] [PubMed]
- Giordano, B.V.; Kaur, S.; Hunter, F.F.; Nasci, R.; Nosal, R.; Drebot, M.; Hayes, D.B.; Walker, E.D. West Nile Virus in Ontario, Canada: A Twelve-Year Analysis of Human Case Prevalence, Mosquito Surveillance, and Climate Data. PLoS ONE 2017, 12, e0183568. [Google Scholar]
- Free Topographic Maps, Elevation, Relief. Available online: https://en-us.topographic-map.com/ (accessed on 15 August 2022).
- Iowa Environmental Mesonet. Available online: https://mesonet.agron.iastate.edu (accessed on 15 August 2022).
- Darsie, R.F.; Ward, R.A. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico; University Press of Florida: Gainesville, FL, USA, 2005; p. 383. [Google Scholar]
- Harrington, L.C.; Poulson, R.L. Considerations for Accurate Identification of Adult Culex Restuans (Diptera: Culicidae) in Field Studies. J. Med. Entomol. 2008, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, B.M.; Rowley, W.A.; Bartholomay, L.C. A Taxonomic Checklist of the Mosquitoes of Iowa. J. Am. Mosq. Control Assoc. 2014, 30, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Sucaet, Y.; Van Hemert, J.; Tucker, B.; Bartholomay, L. A Web-Based Relational Database for Monitoring and Analyzing Mosquito Population Dynamics. J. Med. Entomol. 2008, 45, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kerst, A.J.; Nasci, R.S.; Godsey, M.S.; Mitchell, C.J.; Savage, H.M.; Komar, N.; Panella, N.A.; Allen, B.C.; Volpe, K.E.; et al. Rapid Detection of West Nile Virus from Human Clinical Specimens, Field-Collected Mosquitoes, and Avian Samples by a TaqMan Reverse Transcriptase-PCR Assay. J. Clin. Microbiol. 2000, 38, 4066–4071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggerstaff, B.J. PooledInfRate, Version 3.0: A Microsoft® Excel® Add-In to Compute Prevalence Estimates from Pooled Samples. Available online: https://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html (accessed on 18 July 2022).
- Iowa Department of Natural Resources. Available online: https://www.iowadnr.gov/Conservation/Iowas-Wildlife/Wildlife-Diversity-Program/Diversity-Projects (accessed on 15 August 2022).
- Manley, P.N.; Van Horne, B.; Roth, J.K.; Zielinski, W.J.; McKenzie, M.M.; Weller, T.J.; Weckerly, F.W.; Vojta, C. Multiple Species Inventory and Monitoring Technical Guide 2006. U.S. Department of Agriculture, Forest Service General Technical Report: WO-73. Available online: https://doi.org/10.2737/WO-GTR-73 (accessed on 15 August 2022).
- Hall, D.R.; Tokarz, R.E.; Field, E.N.; Smith, R.C. Surveillance and Genetic Data Support the Introduction and Establishment of Aedes Albopictus in Iowa, USA. Sci. Rep. 2022, 12, 2143. [Google Scholar] [CrossRef]
- R Development Core Team R. A Language and Environment for Statistical Computing (Version 4.1.3); R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: http://CRAN.R-project.org/package=vegan (accessed on 18 July 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; p. 212. ISBN 978-0-387-98140-6. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference (Version R Package Version 1.46.0. 2022). Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 18 July 2022).
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Burnham, K.P.; Anderson, D.R. A Practical Information-Theoretic Approach. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; p. 488. ISBN 0-387-95364-7. [Google Scholar]
- Ruiz, M.O.; Walker, E.D.; Foster, E.S.; Haramis, L.D.; Kitron, U.D. Association of West Nile Virus Illness and Urban Landscapes in Chicago and Detroit. Int. J. Health Geogr. 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowden, S.E.; Magori, K.; Drake, J.M. Regional Differences in the Association between Land Cover and West Nile Virus Disease Incidence in Humans in the United States. Am. J. Trop. Med. Hyg. 2011, 84, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Ronca, S.E.; Murray, K.O.; Nolan, M.S. Cumulative Incidence of West Nile Virus Infection, Continental United States, 1999–2016. Emerg. Infect. Dis. 2019, 25, 325–327. [Google Scholar] [CrossRef]
- DeGroote, J.P.; Sugumaran, R. National and Regional Associations Between Human West Nile Virus Incidence and Demographic, Landscape, and Land Use Conditions in the Coterminous United States. Vector Borne Zoonotic Dis. 2012, 12, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ebel, G.D.; Rochlin, I.; Longacker, J.; Kramer, L.D. Culex Restuans (Diptera: Culicidae) Relative Abundance and Vector Competence for West Nile Virus. J. Med. Entomol. 2005, 42, 838–843. [Google Scholar] [CrossRef]
- Ruiz, M.O.; Chaves, L.F.; Hamer, G.L.; Sun, T.; Brown, W.M.; Walker, E.D.; Haramis, L.; Goldberg, T.L.; Kitron, U.D.; Platonov, A.E.; et al. Local Impact of Temperature and Precipitation on West Nile Virus Infection in Culex Species Mosquitoes in Northeast Illinois, USA. Parasit. Vectors 2010, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Shand, L.; Brown, W.M.; Chaves, L.F.; Goldberg, T.L.; Hamer, G.L.; Haramis, L.; Kitron, U.; Walker, E.D.; Ruiz, M.O. Predicting West Nile Virus Infection Risk from the Synergistic Effects of Rainfall and Temperature. J. Med. Entomol. 2016, 53, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Reiskind, M.H.; Wilson, M.L. Culex Restuans (Diptera: Culicidae) Oviposition Behavior Determined by Larval Habitat Quality and Quantity in Southeastern Michigan. J. Med. Entomol. 2004, 41, 179–186. [Google Scholar] [CrossRef]
- Muturi, E.J.; Shililu, J.I.; Gu, W.; Jacob, B.G.; Githure, J.I.; Novak, R.J. Larval Habitat Dynamics and Diversity of Culex Mosquitoes in Rice Agro-Ecosystem in Mwea, Kenya. Am. J. Trop. Med. Hyg. 2007, 76, 95–102. [Google Scholar] [CrossRef]
- Russell, M.C.; Herzog, C.M.; Gajewski, Z.; Ramsay, C.; El Moustaid, F.; Evans, M.V.; Desai, T.; Gottdenker, N.L.; Hermann, S.L.; Power, A.G.; et al. Both Consumptive and Non-Consumptive Effects of Predators Impact Mosquito Populations and Have Implications for Disease Transmission. Elife 2022, 11, 71503. [Google Scholar] [CrossRef] [PubMed]
- Loss, S.R.; Hamer, G.L.; Walker, E.D.; Ruiz, M.O.; Goldberg, T.L.; Kitron, U.D.; Brawn, J.D. Avian Host Community Structure and Prevalence of West Nile Virus in Chicago, Illinois. Oecologia 2009, 159, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Filion, A.; Eriksson, A.; Jorge, F.; Niebuhr, C.N.; Poulin, R. Large-Scale Disease Patterns Explained by Climatic Seasonality and Host Traits. Oecologia 2020, 194, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Kain, M.P.; Bolker, B.M. Predicting West Nile Virus Transmission in North American Bird Communities Using Phylogenetic Mixed Effects Models and eBird Citizen Science Data. Parasit. Vectors 2019, 12, 395. [Google Scholar] [CrossRef] [Green Version]
- Randall, N.J.; Blitvich, B.J.; Blanchong, J.A. Association between Agricultural Land Use and West Nile Virus Antibody Prevalence in Iowa Birds. J. Wildl. Dis. 2013, 49, 869–878. [Google Scholar] [CrossRef]
- Deichmeister, J.M.; Telang, A. Abundance of West Nile Virus Mosquito Vectors in Relation to Climate and Landscape Variables Abundance of West Nile Virus Mosquito Vectors in Relation to Climate. J. Vector Ecol. 2011, 36, 75–85. [Google Scholar] [CrossRef]
- Larson, S.R.; DeGroote, J.P.; Bartholomay, L.C.; Sugumaran, R. Ecological Niche Modeling of Potential West Nile Virus Vector Mosquito Species in Iowa. J. Insect Sci. 2010, 10, 110. [Google Scholar] [CrossRef]
- Andreadis, T.G.; Anderson, J.F.; Vossbrinck, C.R.; Main, A.J. Epidemiology of West Nile Virus in Connecticut: A Five-Year Analysis of Mosquito Data 1999–2003. Vector Borne Zoonotic Dis. 2004, 4, 360–378. [Google Scholar] [CrossRef]
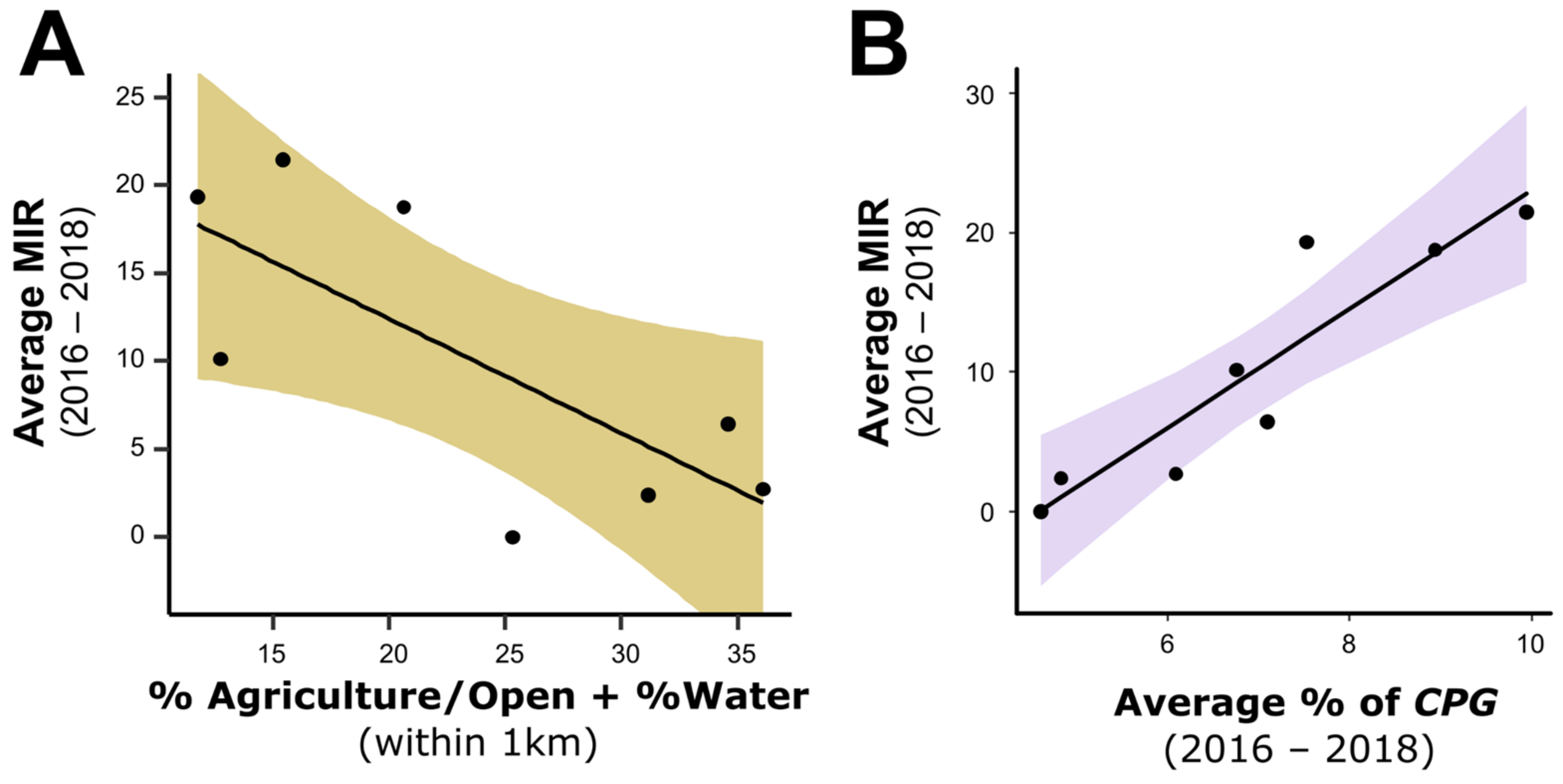
| Model | Predictor Variable | Slope | AICc | ∆AICc | Weight | Adj. r2 |
|---|---|---|---|---|---|---|
| 1 | % Agriculture/Open plus % Water | −0.65 | 61.73 | 0 | 0.22 | 0.47 |
| 2 | % Agriculture/Open plus % Bare | −0.58 | 62.39 | 0.66 | 0.16 | 0.43 |
| 3 | % Tree plus % Built | 0.46 | 62.48 | 0.75 | 0.15 | 0.42 |
| 4 | None (intercept only) | n/a | 62.48 | 0.75 | 0.15 | n/a |
| 5 | % Agriculture/Open | −0.72 | 63.25 | 1.52 | 0.10 | 0.36 |
| Model | Mosquito Variable | Mosquito Slope | Bird Variable | Bird Slope | AICc | ∆AICc | Weight | Adj. r2 |
|---|---|---|---|---|---|---|---|---|
| 1 | % Cx. pipiens group, Late Season | 4.25 | None | n/a | 53.37 | 0 | 0.72 | 0.81 |
| 2 | % Cx. pipiens group, Late Season | 4.51 | Cumulative Amplification Fraction | −2.53 | 57.84 | 4.47 | 0.08 | 0.87 |
| 3 | % Culex spp., Late Season | 3.33 | None | n/a | 58.31 | 4.94 | 0.06 | 0.65 |
| 4 | % Cx. pipiens group, Late Season | 4.44 | PC2 | −0.70 | 58.67 | 5.29 | 0.05 | 0.87 |
| 5 | % Cx. pipiens group, Late Season | 3.98 | Avg. Total Detections per Visit | −0.32 | 60.31 | 6.94 | 0.02 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adelman, J.S.; Tokarz, R.E.; Euken, A.E.; Field, E.N.; Russell, M.C.; Smith, R.C. Relative Influence of Land Use, Mosquito Abundance, and Bird Communities in Defining West Nile Virus Infection Rates in Culex Mosquito Populations. Insects 2022, 13, 758. https://doi.org/10.3390/insects13090758
Adelman JS, Tokarz RE, Euken AE, Field EN, Russell MC, Smith RC. Relative Influence of Land Use, Mosquito Abundance, and Bird Communities in Defining West Nile Virus Infection Rates in Culex Mosquito Populations. Insects. 2022; 13(9):758. https://doi.org/10.3390/insects13090758
Chicago/Turabian StyleAdelman, James S., Ryan E. Tokarz, Alec E. Euken, Eleanor N. Field, Marie C. Russell, and Ryan C. Smith. 2022. "Relative Influence of Land Use, Mosquito Abundance, and Bird Communities in Defining West Nile Virus Infection Rates in Culex Mosquito Populations" Insects 13, no. 9: 758. https://doi.org/10.3390/insects13090758
APA StyleAdelman, J. S., Tokarz, R. E., Euken, A. E., Field, E. N., Russell, M. C., & Smith, R. C. (2022). Relative Influence of Land Use, Mosquito Abundance, and Bird Communities in Defining West Nile Virus Infection Rates in Culex Mosquito Populations. Insects, 13(9), 758. https://doi.org/10.3390/insects13090758






