The Differential Effects of Tuta absoluta Infestations on the Physiological Processes and Growth of Tomato, Potato, and Eggplant
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
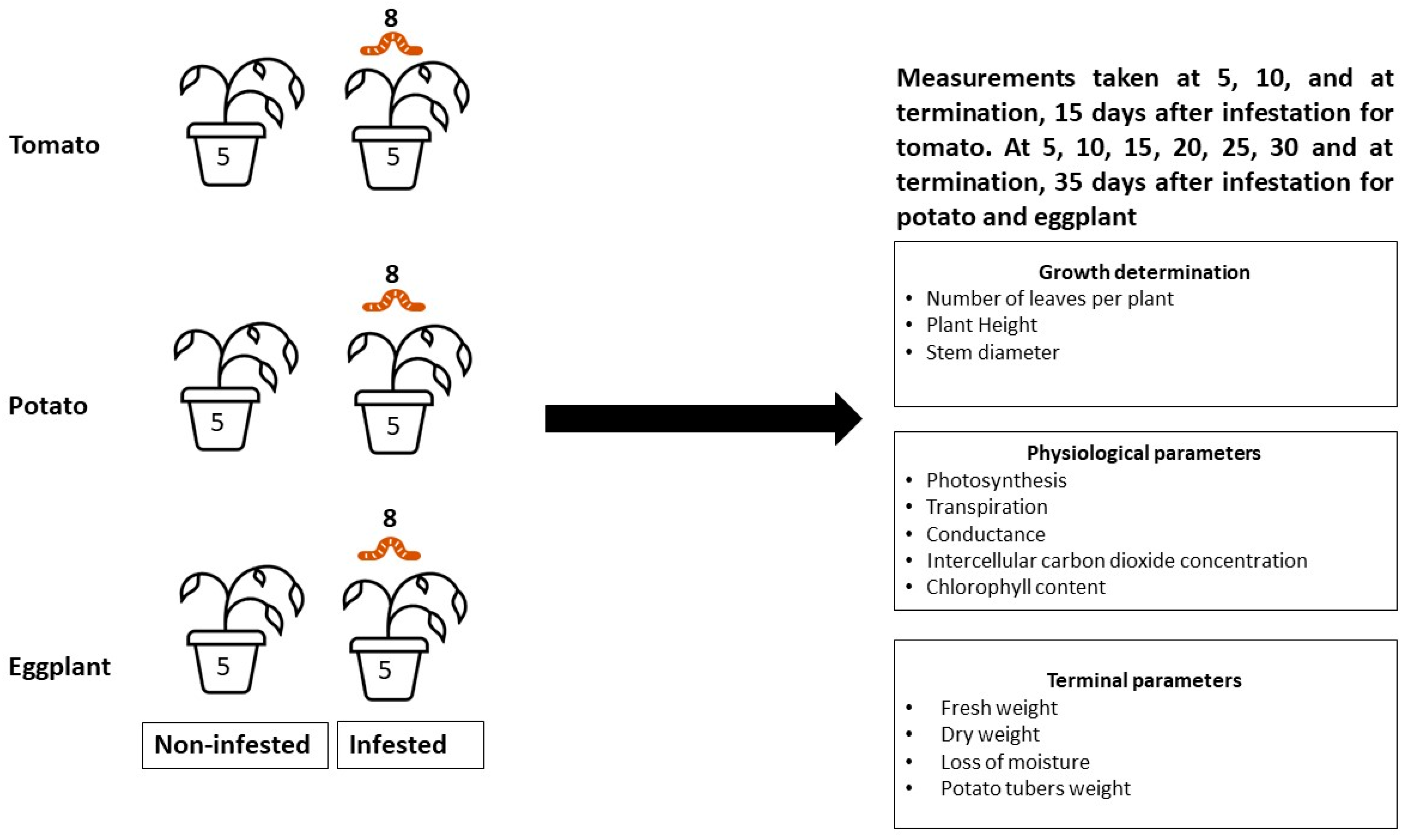
2.1. Experimental Site
2.2. Research Design
2.2.1. Host Plants
2.2.2. Insect Infestation
2.3. Data Collection
2.3.1. Plant Morphological (Growth) Parameters
2.3.2. Plant Physiological Parameters
2.4. Fresh Weight, Dry Weight, and Loss of Moisture
2.5. Statistical Analysis
3. Results
3.1. Growth Parameters
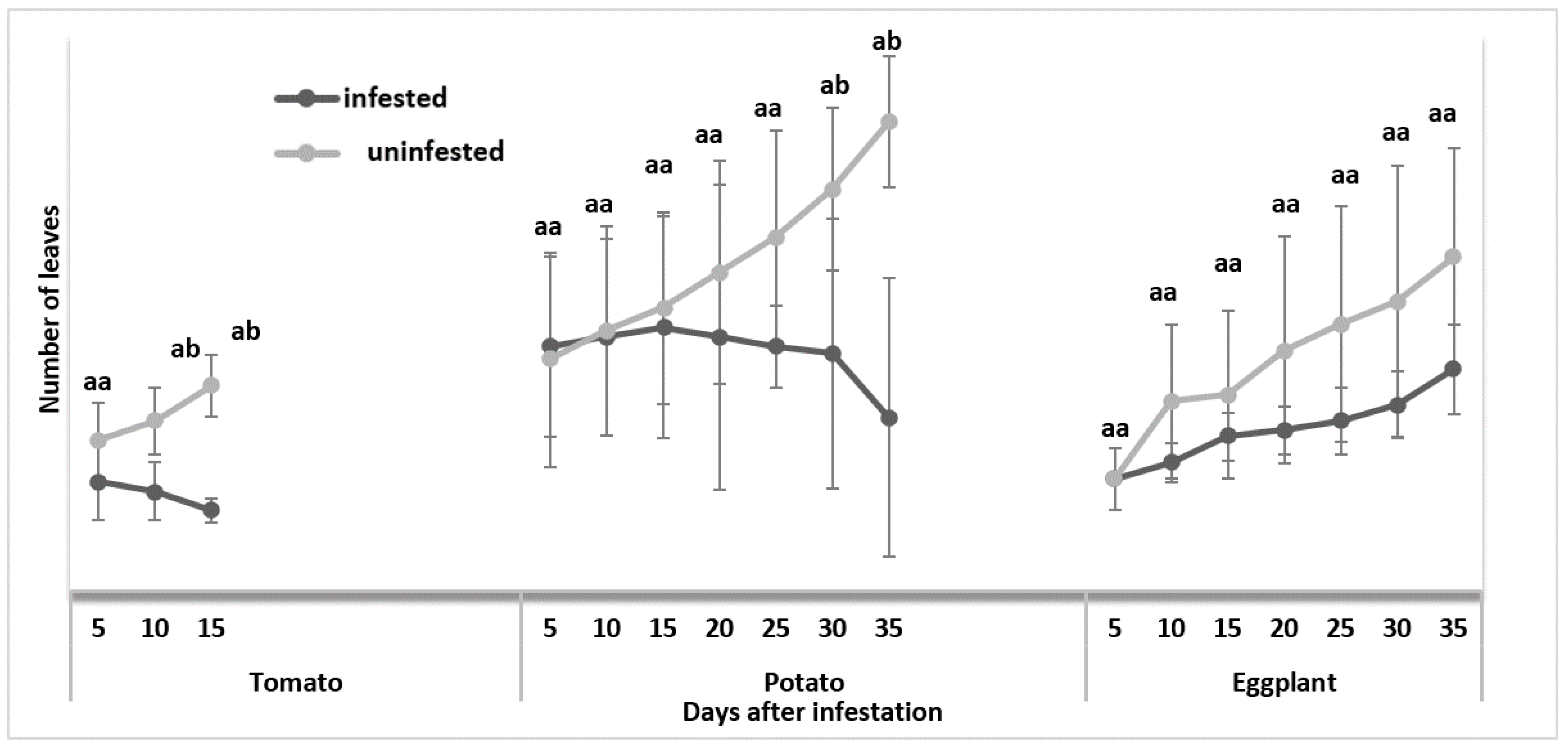
3.1.1. Number of Leaves Per Plant
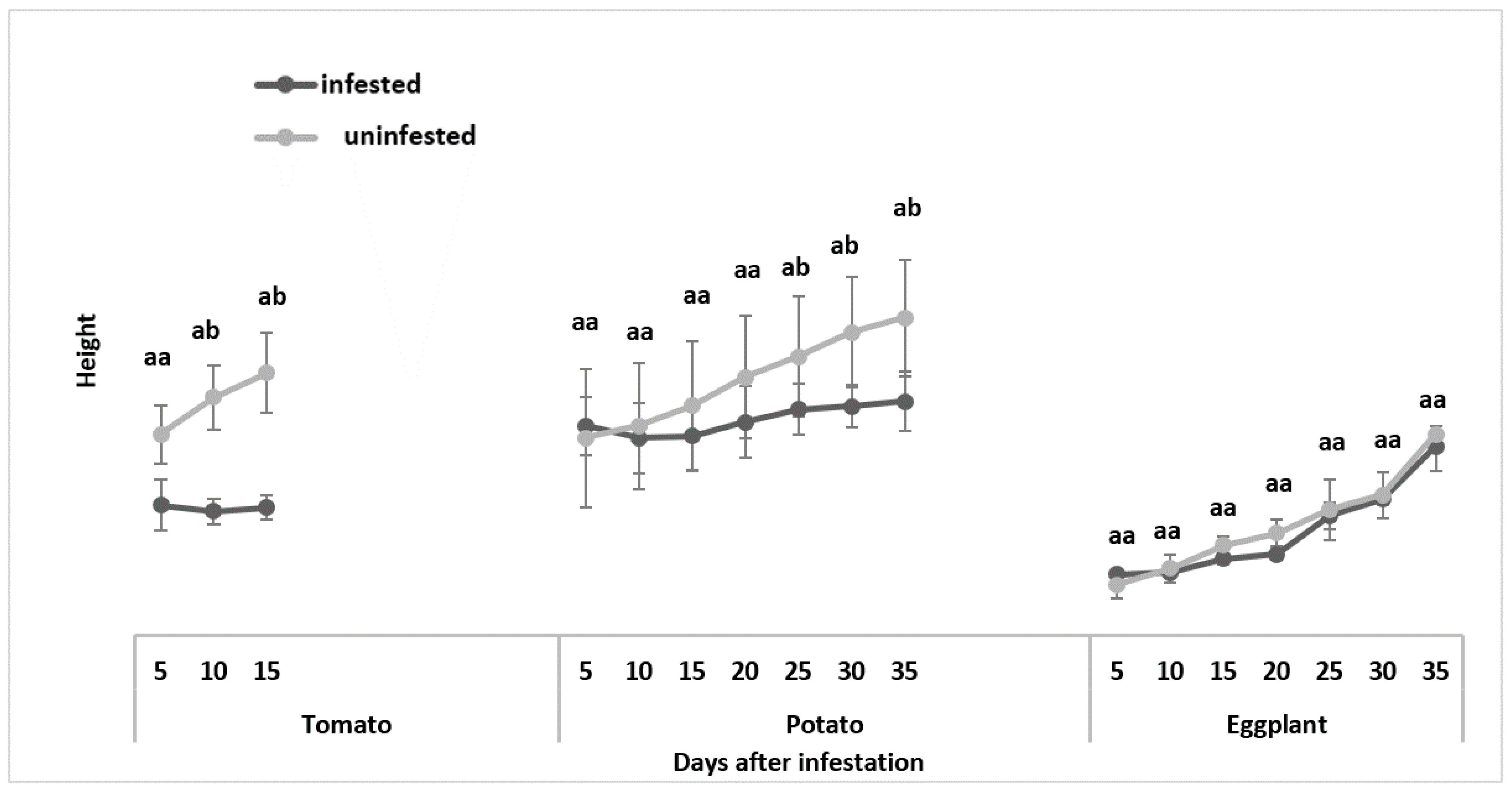
3.1.2. Plant Height
3.1.3. Stem Diameter
3.1.4. Fresh Weight, Dry Weight, and Loss of Moisture
3.1.5. Potato Yields
3.2. Host Plants Physiological Parameters
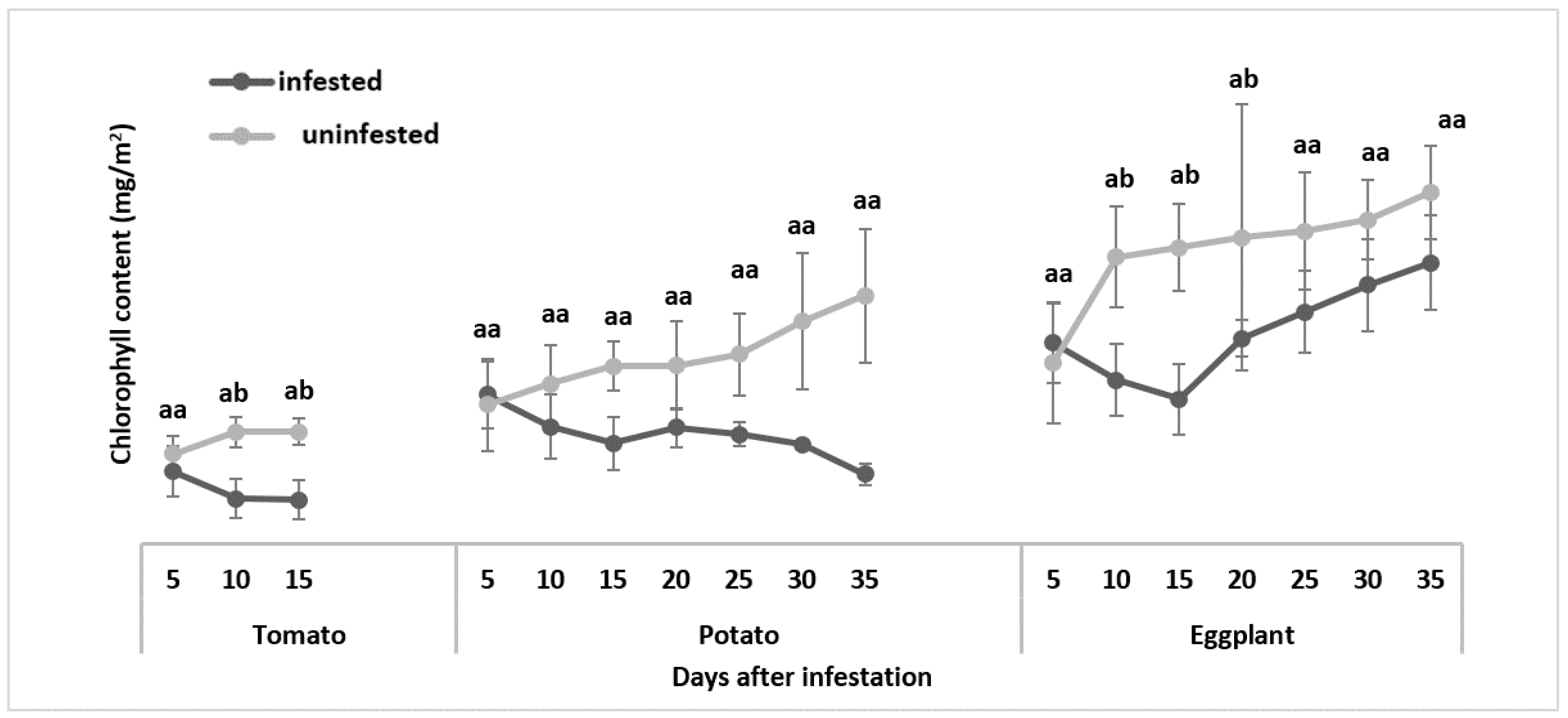
3.2.1. Chlorophyll Content
3.2.2. Photosynthesis Rate
3.2.3. Conductance
3.2.4. Transpiration Rate
3.2.5. Intercellular Carbon Dioxide Concentration
3.3. Correlation of Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs of non-indigenous species in the United States. BioScience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- El-Shafie, H.A. Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae): An Invasive Insect Pest Threatening the World Tomato Production. In Invasive Species-Introduction Pathways, Economic Impact, and Possible Management Options; IntechOpen: London, UK, 2020; pp. 1–16. [Google Scholar]
- Bell, J.C.; McGeoch, M.A. An evaluation of the pest status and research conducted on phytophagous Lepidoptera on cultivated plants in South Africa. Afr. Entomol. 1996, 4, 161–170. [Google Scholar]
- Visser, D.; Uys, V.M.; Nieuwenhuis, R.J.; Pieterse, W. First records of the tomato leaf miner Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) in South Africa. BioInvasions Rec. 2017, 6, 301–305. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest. Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- European Plant Protection Organisation (EPPO). Datasheets on quarantine pests. EPPO 2005, 35, 434–435. [Google Scholar]
- Han, P.; Zhang, Y.N.; Lu, Z.Z.; Wang, S.; Ma, D.Y.; Biondi, A.; Desneux, N. Are we ready for the invasion of Tuta absoluta? Unanswered key questions for elaborating an Integrated Pest Management package in Xinjiang, China. Entomol. Gen. 2018, 38, 113–125. [Google Scholar] [CrossRef]
- Cherif, A.; Verheggen, F. A review of Tuta absoluta (Lepidoptera: Gelechiidae) host plants and their impact on management strategies. Biotechnol. Agron. Soc. Environ. 2019, 23, 270–278. [Google Scholar] [CrossRef]
- Younes, A.A.; Zohdy, Z.M.N.; Abul, F.H.; Fathy, R. Preference and Performance of the Tomato Leafminer, Tuta absoluta (Lepidoptera—Gelechiidae) Towards Three Solanaceous Host Plant Species. CPQ Microbiol. 2018, 1, 1–16. [Google Scholar]
- Smith, J.D.; Dubois, T.; Mallogo, R.; Njau, E.F.; Tua, S.; Srinivasan, R. Host range of the invasive tomato pest Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) on solanaceous crops and weeds in Tanzania. Fla. Entomolo. 2018, 101, 573–579. [Google Scholar] [CrossRef]
- Sylla, S.; Brévault, T.; Monticelli, L.S.; Diarra, K.; Desneux, N. Geographic variation of host preference by the invasive tomato leaf miner Tuta absoluta: Implication for host range expansion. J. Pest Sci. 2019, 92, 1387–1396. [Google Scholar] [CrossRef]
- Abstract of Agricultural Statistics. Available online: http://www.dalrrd.gov.za (accessed on 15 July 2022).
- Guimapi, R.Y.A.; Mohamed, S.A.; Okeyo, G.O.; Ndjomatchoua, F.T.; Ekesi, S.; Tonnang, H.E.Z. Modeling the risk of invasion and spread of Tuta absoluta in Africa. Ecol. Complex. 2016, 28, 77–93. [Google Scholar] [CrossRef]
- Silva, G.A.; Queiroz, E.A.; Arcanjo, L.P.; Lopes, M.C.; Araújo, T.A.; Galdino, T.S.V.; Samuels, R.I.; Rodrigues-Silva, N.; Picanço, M.C. Biological performance and oviposition preference of tomato pinworm Tuta absoluta when offered a range of Solanaceous host plants. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, S.; Fathipour, Y.; Asgari, S. Susceptibility of Seven Selected Tomato Cultivars to Tuta absoluta (Lepidoptera: Gelechiidae): Implications for Its Management. J. Appl. Entomol. 2017, 110, 42–429. [Google Scholar] [CrossRef]
- Pereyra, P.C.; Sánchez, N.E. Effect of two Solanaceous plants on developmental and population parameters of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2006, 35, 671–676. [Google Scholar] [CrossRef]
- Cely, P.L.; Cantor, F.; Rodríguez, D. Determination of levels of damage caused by different densities of Tuta absoluta populations (Lepidoptera: Gelechiidae) under greenhouse conditions. Agron. Colomb. 2010, 28, 392–402. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants; Julius Kühn-Istitut: Quedlinburg, Germany, 2018. [Google Scholar]
- Zangerl, A.R.; Hamilton, J.G.; Miller, T.J.; Crofts, A.R.; Oxborough, K.; Berenbaum, M.R.; De Lucia, E.H. Impact of folivory on photosynthesis is greater than the sum of its holes. Proc. Natl. Acad. Sci. USA 2002, 99, 1088–1091. [Google Scholar] [CrossRef]
- Neves, A.D.; Oliveira, R.F.; Parra, J.R. A new concept for insect damage evaluation based on plant physiological variables. An. Acad. Brasi. Ciênc. 2006, 78, 821–835. [Google Scholar] [CrossRef][Green Version]
- Pincebourde, S.; Ngao, J. The impact of phloem feeding insects on leaf ecophysiology varies with leaf age. Front. Plant Sci. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Soliman, S.A.; Imam, A.I. First documentation of Tuta absoluta Meyrick larval infestation to eggplant fruits at matrouh governorate, Arab republic of Egypt. J. Plant. Prot. Pathol. 2013, 4, 241–244. [Google Scholar] [CrossRef]
- Nabity, P.D.; Zavala, J.A.; DeLucia, E.H. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Ann. Bot. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Pallardy, S.G. Physiology of Woody Plants; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Myers, J.H.; Sarfraz, R.M. Impacts of insect herbivores on plant populations. Annu. Rev. Entomol. 2017, 62, 207–230. [Google Scholar] [CrossRef] [PubMed]
- Arnó, J.; Gabarra, R. Controlling Tuta absoluta, a new invasive pest in Europe. Tra. IPM 2010, 5, 1–8. [Google Scholar]
- Mohamed, E.S.I.; Mahmoud, M.E.; Elhaj, M.A.M.; Mohamed, S.A.; Ekesi, S. Host plants record for tomato leaf miner Tuta absoluta (Meyrick) in Sudan. EPPO Bull. 2015, 45, 108–111. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Hussain, A.; Razaq, M.; Zaka, S.M.; Shahzad, W.; Mahmood, K. Effect of aphid infestation on photosynthesis, growth and yield of Brassica carinata A. Braun. Pak. J. Zool. 2015, 47, 1335–1340. [Google Scholar]
- Sperdouli, I.; Andreadis, S.; Moustaka, J.; Panteris, E.; Tsaballa, A.; Moustakas, M. Changes in light energy utilization in photosystem II and reactive oxygen species generation in potato leaves by the pinworm Tuta absoluta. Molecules 2021, 26, 2984. [Google Scholar] [CrossRef] [PubMed]
- Welter, S.C. Arthropod Impact on Plant Gas Exchange. In Insect-Plant Interaction, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 135–164. [Google Scholar]
- Sterling, T.M. Transpiration: Water movement through plants. J. Nat. Resour. Life Sci. Educ. 2005, 34, 123. [Google Scholar] [CrossRef]
- Wali, A.S.; Kabura, B.H. Correlation studies in tomato (Lycopersicon lycopersicum L. Karst) as affected by mulching and cultivar during the heat period in the Semi-Arid Region of Nigeria. Int. Lett. Nat. Sci. 2014, 10, 1–7. [Google Scholar] [CrossRef]
- Onyia, V.N.; Chukwudi, U.P.; Ezea, A.C.; Atugwu, A.I.; Ene, C.O. Correlation and path coefficient analyses of yield and yield components of eggplant (Solanum melongena) in a coarse-textured Ultisol. Inf. Process. Agric. 2020, 7, 173–181. [Google Scholar] [CrossRef]
- Sattar, M.A.; Sultana, N.; Hossain, M.M.; Rashid, M.H.; Islam, A.A. Genetic variability, correlation and path analysis in potato (Solanum tuberosum L.). Bangladesh J. Plant Breed. Genet. 2007, 20, 33–38. [Google Scholar] [CrossRef]
- Acatrinei, L. Photosynthesis rate, transpiration and stomatal conductance of vegetable species in protected organic crops. Lucr. Ştiinţifice 2010, 53, 32–35. [Google Scholar]
- Buntin, D.G.; Gilbertz, D.A.; Oetting, R.D. Chlorophyll loss and gas exchange in tomato leaves after feeding injury by Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 1993, 86, 517–522. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Z.H.; Yang, B.; Chen, H.; Zhang, H.; Hou, D.B. The contribution of photosynthesis traits and plant height components to plant height in wheat at the individual quantitative trait locus level. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, M.U. Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol. 2011, 155, 117–124. [Google Scholar] [CrossRef]
- Jayanthi, P.D.; Ravindra, M.A.; Kempraj, V.; Roy, T.K.; Shivashankara, K.S.; Singh, T.H. Morphological diversity of trichomes and phytochemicals in wild and cultivated eggplant species. Indian J. Hortic. 2018, 75, 265–272. [Google Scholar] [CrossRef]
- Transpiration—Factors Affecting Rates of Transpiration. Available online: https://passel2.unl.edu/view/lesson/c242ac4fbaaf/6 (accessed on 4 May 2022).
- Hamani, A.K.; Li, S.; Chen, J.; Amin, A.S.; Wang, G.; Xiaojun, S.; Zain, M.; Gao, Y. Linking exogenous foliar application of glycine betaine and stomatal characteristics with salinity stress tolerance in cotton (Gossypium hirsutum L.) seedlings. BMC Plant Biol. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Nulit, R.; Sakimin, S.Z. Influence of drought stress on growth, biochemical changes and leaf gas exchange of strawberry (Fragaria× ananassa Duch.) in Indonesia. AIMS Agric. Food. 2022, 7, 37–60. [Google Scholar]
- Cherif, A.; Attia-Barhoumi, S.; Mansour, R.; Zappalà, L.; Grissa-Lebdi, K. Elucidating key biological parameters of Tuta absoluta on different host plants and under various temperature and relative humidity regimes. Entomol. Gen. 2019, 39, 1–7. [Google Scholar] [CrossRef]
- Caparros Medigo, R.; De Backer, L.; Ettaïb, R.; Brostaux, Y.; Fauconnier, M.-L.; Delaplace, P.; Lognay, G.; Belkadhi, M.S.; Haubruge, E.; Francis, F.; et al. Role of larval host plant experience and solanaceous plant volatile emissions in Tuta absoluta (Lepidoptera: Gelechiidae) host finding behavior. Arthropod-Plant Inte. 2014, 8, 293–304. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Zhang, J.; He, T.; Huang, J.; Zhang, Z.; Wang, Y.; Hafeez, M.; Zhou, S.; Ren, X.; et al. Comprehensive metabolome and volatilome analyses in eggplant and tomato reveal their differential responses to Tuta absoluta infestation. Front. Plant Sci. 2021, 12, 757230. [Google Scholar] [CrossRef]
- Vitta, N.; Estay, P.; Chorbadjian, R.A. Characterization of resistance expression in genotypes of Solanum section Lycopersicon against Tuta absoluta (Lepidoptera: Gelechiidae). Cienc. Investig. Agric. 2016, 43, 366–373. [Google Scholar] [CrossRef]
- Ghaderi, S.; Fathipour, Y.; Asgari, S.; Reddy, G.V.P. Economic injury level and crop loss assessment for Tuta absoluta (Lepidoptera: Gelechiidae) on different tomato cultivars. J. Appl. Entomol. 2019, 143, 493–507. [Google Scholar] [CrossRef]




| Tomato | LPP | PH | SD | CC | P | Tr | C | Ci |
|---|---|---|---|---|---|---|---|---|
| LPP | - | |||||||
| PH | 0.93 0.02 | - | ||||||
| SD | 0.81 0.09 | 0.61 0.27 | - | |||||
| CC | 0.52 0.36 | −0.05 0.93 | 0.69 0.20 | - | ||||
| P | 0.85 0.06 | 0.43 0.47 | 0.85 0.07 | 0.84 0.07 | - | |||
| Tr | −0.13 0.83 | −0.38 0.52 | −0.01 0.99 | 0.14 0.81 | 0.07 0.91 | - | ||
| C | −0.13 0.83 | −0.38 0.52 | 0.00 0.99 | 0.15 0.81 | 0.08 0.91 | 1.00 0.006 | - | |
| Ci | 0.05 0.93 | 0.35 0.56 | −0.26 0.67 | −0.26 0.67 | −0.02 0.96 | −0.73 0.10 | −0.74 0.10 | - |
| Potato | LPP | SD | PH | CC | P | Tr | C | Ci |
|---|---|---|---|---|---|---|---|---|
| LPP | - | |||||||
| SD | −0.15 0.84 | - | ||||||
| PH | 0.15 0.84 | 0.62 0.37 | - | |||||
| CC | 0.68 0.31 | 0.45 0.54 | 0.81 0.18 | - | ||||
| P | −0.78 0.21 | 0.40 0.59 | −0.33 0.66 | 0.74 0.05 | - | |||
| Tr | 0.69 0.30 | 0.48 0.51 | 0.20 0.66 | 0.62 0.37 | −0.16 0.84 | - | ||
| C | 0.70 0.29 | 0.45 0.54 | 0.18 0.81 | 0.61 0.38 | −0.17 0.83 | 1.00 0.004 | - | |
| Ci | 0.01 0.98 | −0.46 0.53 | −0.95 0.40 | −0.66 0.33 | 0.33 0.66 | 0.08 0.92 | 0.11 0.89 | - |
| Eggplant | LPP | PH | SD | CC | P | Tr | C | Ci |
|---|---|---|---|---|---|---|---|---|
| LPP | - | |||||||
| PH | 0.90 0.03 | - | ||||||
| SD | 0.21 0.73 | −0.13 0.83 | - | |||||
| CC | 0.69 0.19 | −0.65 0.23 | −0.10 0.86 | - | ||||
| P | −0.70 0.19 | 0.89 0.04 | 0.28 0.64 | 0.70 0.04 | - | |||
| Tr | 0.79 0.11 | −0.92 0.04 | 0.45 0.44 | 0.41 0.49 | −0.73 0.15 | - | ||
| C | 0.80 0.10 | −0.92 0.02 | 0.44 0.45 | 0.41 0.49 | −0.74 0.15 | 1.00 0.001 | - | |
| Ci | −0.14 0.82 | 0.40 0.50 | −0.44 0.45 | 0.37 0.54 | 0.38 0.50 | −0.67 0.21 | −0.66 0.22 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahlangu, L.; Sibisi, P.; Nofemela, R.S.; Ngmenzuma, T.; Ntushelo, K. The Differential Effects of Tuta absoluta Infestations on the Physiological Processes and Growth of Tomato, Potato, and Eggplant. Insects 2022, 13, 754. https://doi.org/10.3390/insects13080754
Mahlangu L, Sibisi P, Nofemela RS, Ngmenzuma T, Ntushelo K. The Differential Effects of Tuta absoluta Infestations on the Physiological Processes and Growth of Tomato, Potato, and Eggplant. Insects. 2022; 13(8):754. https://doi.org/10.3390/insects13080754
Chicago/Turabian StyleMahlangu, Lindiwe, Phumzile Sibisi, Robert S. Nofemela, Titus Ngmenzuma, and Khayalethu Ntushelo. 2022. "The Differential Effects of Tuta absoluta Infestations on the Physiological Processes and Growth of Tomato, Potato, and Eggplant" Insects 13, no. 8: 754. https://doi.org/10.3390/insects13080754
APA StyleMahlangu, L., Sibisi, P., Nofemela, R. S., Ngmenzuma, T., & Ntushelo, K. (2022). The Differential Effects of Tuta absoluta Infestations on the Physiological Processes and Growth of Tomato, Potato, and Eggplant. Insects, 13(8), 754. https://doi.org/10.3390/insects13080754






