Evaluation of Entomopathogenic Nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Red Palm Weevil Culture
2.2. Entomopathogenic Nematodes (EPNs)
2.3. Laboratory Studies
2.3.1. Pathogenicity of EPNs against Red Palm Weevil Larvae Based on Dissection
2.3.2. Infective Potential of EPNs against Weevil Larvae Based on Adult Emergence
2.3.3. Infective Potential of EPNs against Red Palm Weevil Pupae Based on Adult Emergence
2.4. Field Studies
2.5. Statistical Analysis
3. Results
3.1. Pathogenicity of EPNs against Red Palm Weevil Larvae Based on Dissection
3.2. Infective Potential of EPNs against Red Palm Weevil Pupae Based on Adult Emergence
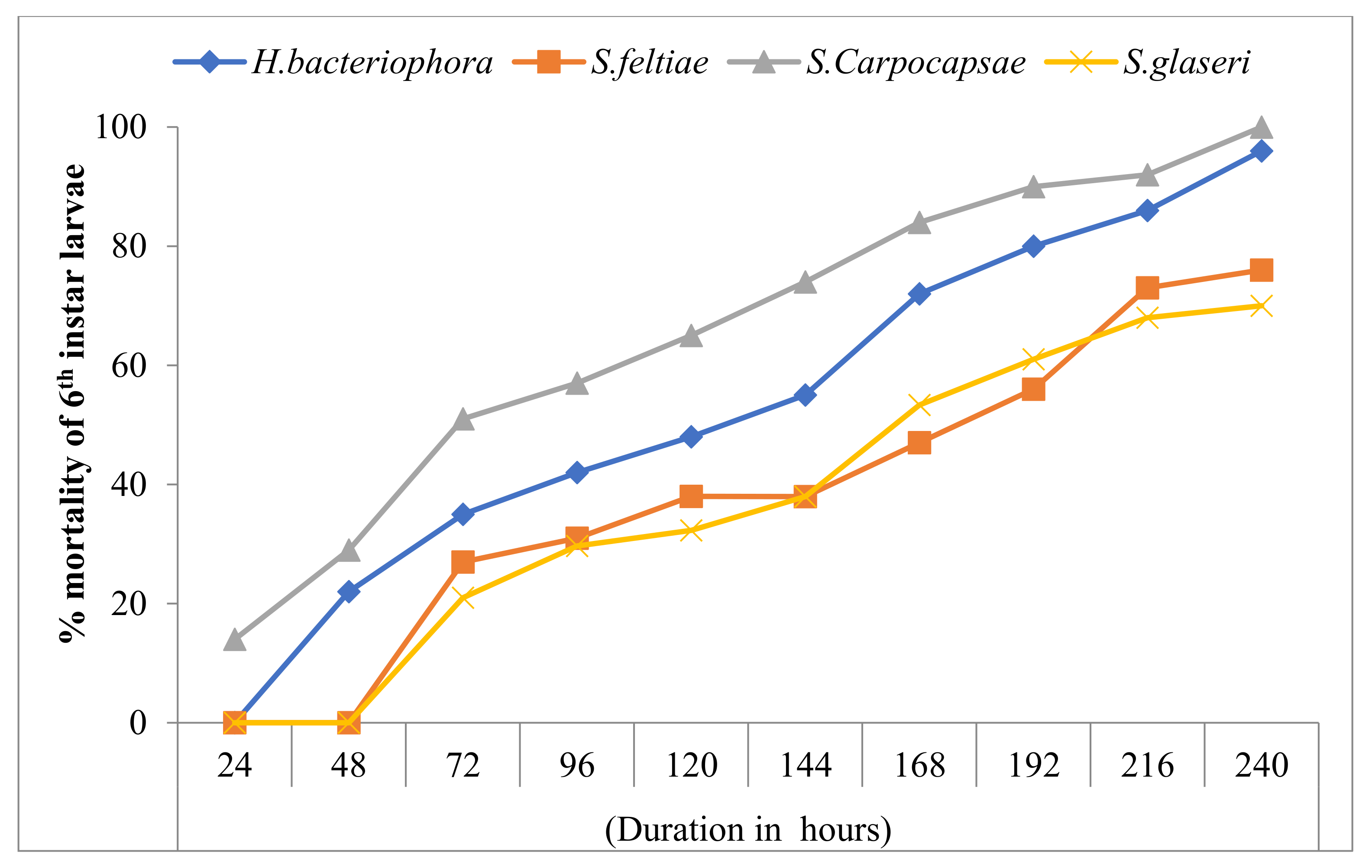
3.3. Effect of EPNs on the Mortality of 6th Instar Larvae
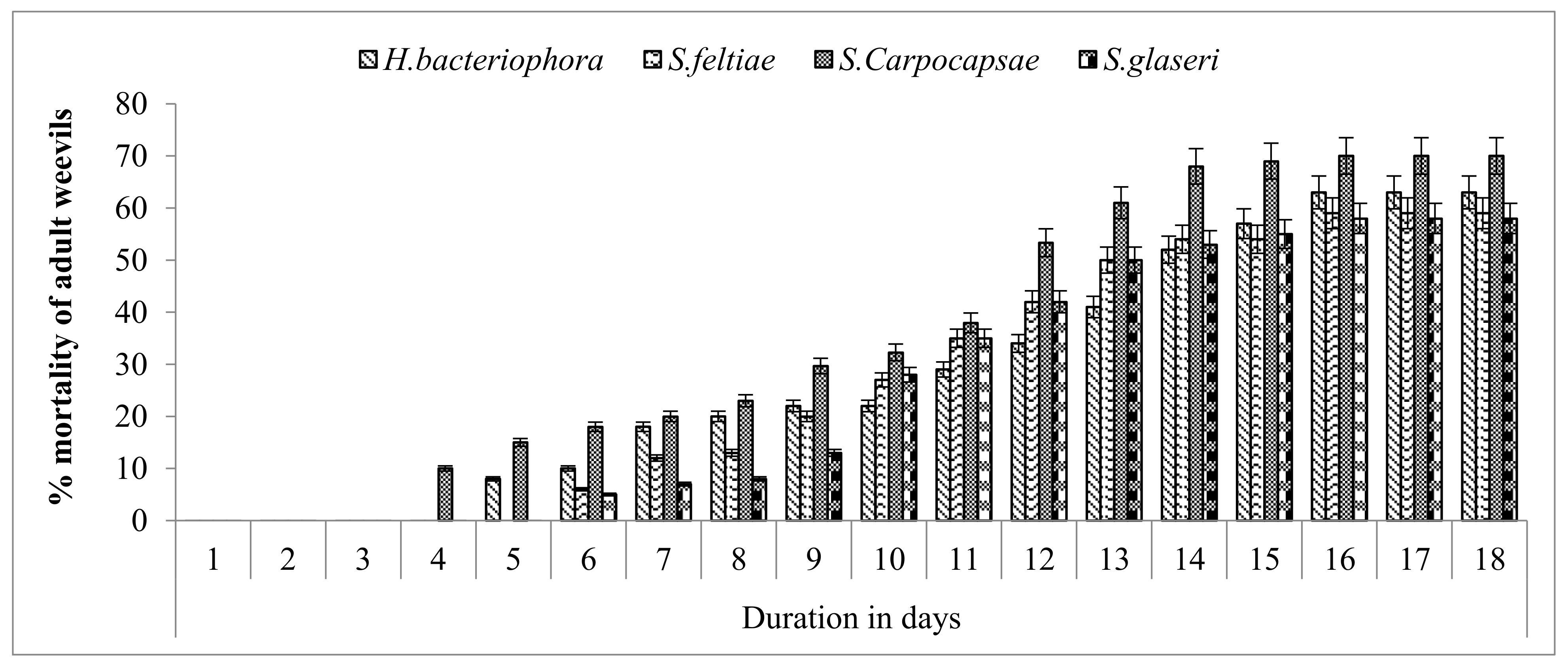
3.4. Infective Potential of EPNs against Red Palm Weevil Adults
3.5. Infective Potential of EPNs against Red Palm Weevil under Field Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abass, M. Microbial contaminants of date palm (Phoenix dactylifera L.) in Iraqi tissue culture laboratories. Emir. J. Food Agric. 2013, 25, 875–882. [Google Scholar] [CrossRef]
- Lee, D.R. Date cultivation in the Coachella valley, California. Ohio J. Sci. 1963, 63, 82–87. [Google Scholar]
- Hadrami, I.E.; Hadrami, A.E. Breeding date palm. In Breeding Plantation Tree Crops: Tropical Species; Springer: Berlin/Heidelberg, Germany, 2009; pp. 191–216. [Google Scholar]
- Johnson, D.V.; Al-Khayri, M.; Jain, S.M. Date production status and prospects in Asia and Europe. In Date Palm Genteic Resources and Utilization; Ch. 1. Africa and the Americas; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2015; Volume 2. [Google Scholar] [CrossRef]
- El Hadrami, A.; Al-Khayri, J.M. Socioeconomic and traditional importance of date palm. Emir. J. Food Agric. 2012, 24, 371. [Google Scholar]
- Maqsood, S.; Adiamo, O.; Ahmad, M.; Mudgil, P. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020, 308, 125522. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.H.; Azizollahi, F.; Moalemi, N. Some quality attributes and biochemical properties of nine Iranian date (Phoenix dactylifera L.) cultivars at different stages of fruit development. Int. J. Hortic. Sci. Technol. 2015, 2, 161–171. [Google Scholar]
- Shabani, F.; Kumar, L.; Nojoumian, A.H.; Esmaeili, A.; Toghyani, M. Projected future distribution of date palm and its potential use in alleviating micronutrient deficiency. J. Sci. Food Agric. 2016, 96, 1132–1140. [Google Scholar] [CrossRef]
- El-Shafie, H. Area-wide Integrated Management of Red Palm Weevil, Rhynchophorus ferrugineus (Olivier 1790) (Coleoptera: Curculionidae) in Date Palm Plantations: A Review. Persian Gulf Crop Prot. 2014, 3, 92–118. [Google Scholar]
- Wakil, W.; Faleiro, J.R.; Miller, T.A. Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges; Springer International Publishing AG: Cham, Switzerland, 2015. [Google Scholar]
- Milosavljević, I.; El-Shafie, H.A.; Faleiro, J.R.; Hoddle, C.D.; Lewis, M.; Hoddle, M.S. Palmageddon: The wasting of ornamental palms by invasive palm weevils Rhynchophorus spp. J. Pest Sci. 2019, 92, 143–156. [Google Scholar] [CrossRef]
- Lefroy, H.M. The More Important Insect Injuries to Indian Agriculture; Government of India Press: Calcutta, India, 1906.
- Abe, F.; Hata, K.; Sone, K. Life history of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Dryophtoridae), in Southern Japan. Fla. Entomol. 2009, 92, 421–425. [Google Scholar]
- Al-Dosary, N.M.; Al-Dobai, S.; Faleiro, J.R. Review on the management of red palm weevil Rhynchophorus ferrugineus Olivier in date palm Phoenix dactylifera L. Emir. J. Food Agric. 2016, 28, 34–44. [Google Scholar] [CrossRef]
- Al-Shawaf, A.M.; Al-Shagag, A.; Al-Bagshi, M.; Al-Saroj, S.; Al-Bather, S.; Al-Dandan, A.M.; Abdallah, A.B.; Faleiro, J.R. A quarantine protocol against red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleptera: Curculiondae) in date palm. J. Plant Prot. Res. 2013, 53, 409–415. [Google Scholar] [CrossRef]
- Ge, X.; He, S.; Wang, T.; Yan, W.; Zong, S. Potential distribution predicted for Rhynchophorus ferrugineus in China under different climate warming scenarios. PLoS ONE 2015, 10, e0141111. [Google Scholar] [CrossRef] [PubMed]
- Giblin-Davis, R.M.; Faleiro, J.R.; Jacas, J.A.; Peña, J.E.; Vidyasagar, P. Biology and management of the red palm weevil, Rhynchophorus ferrugineus. In Potential Invasive Pests of Agricultural Crop Species; Peña, J.E., Ed.; CABI: Wallingford, UK, 2013; pp. 1–34. [Google Scholar]
- Longo, S. The Asian red palm weevil, a serious pest of Canary palm in Sicily. Inf. Fitopatol. 2006, 56, 40–44. [Google Scholar]
- Dembilio, Ó.; Jaques, J.A. Biology and management of red palm weevil. In Sustainable pest Management in Date Palm: Current Status and Emerging Challenges; Springer International Publishing AG: Cham, Switzerland, 2015; pp. 13–36. [Google Scholar]
- Downer, A.J.; Uchida, J.Y.; Hodel, D.R.; Elliott, M.L. Lethal palm diseases common in the United States. HortTechnology 2009, 19, 710–716. [Google Scholar] [CrossRef]
- Kontodimas, D.; Soroker, V.; Pontikakos, C.; Suma, P.; Beaudoin-Ollivier, L.; Karamaouna, F.; Riolo, P. Visual identification and characterization of Rhynchophorus ferrugineus and Paysandisia archon infestation. In Handbook of Major Palm Pests: Biology and Management; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 187–208. [Google Scholar]
- Blumberg, D. Date palm arthropod pests and their management in Israel. Phytoparasitica 2008, 36, 411–448. [Google Scholar] [CrossRef]
- Soroker, V.; Blumberg, D.; Haberman, A.; Hamburger-Rishard, M.; Reneh, S.; Talebaev, S.; Anshelevich, L.; Harari, A. Current status of red palm weevil infestation in date palm plantations in Israel. Phytoparasitica 2005, 33, 97–106. [Google Scholar] [CrossRef]
- Alkhazal, M.; Youssef, L.; Abdel-Wahaed, M.; Kassab, A.; Saleh, M. Factors affecting infestation pattern of the red palm weevil, Rhynchophorus ferrugineus Oliv. in date palm farms in Qatif, Saudi Arabia. Arab Univ. J. Agric. Sci. 2009, 17, 177–183. [Google Scholar]
- Pugliese, M.; Rettori, A.A.; Martinis, R.; Al-Rohily, K.; Velate, S.; Moideen, M.A.; Al-Maashi, A. Evaluation of the efficacy of insecticidal coatings based on teflutrin and chlorpyrifos against Rhynchophorus ferrugineus. Pest Manag. Sci. 2017, 73, 1737–1742. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Jabr, A.M.; Al-Ayied, H.Y. Managing invasive populations of red palm weevil: A worldwide perspective. J. Food Agric. Environ. 2013, 11, 456–463. [Google Scholar]
- Faleiro, J. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci. 2006, 26, 135–154. [Google Scholar]
- Latifian, M. Integrated pest management of date palm fruit pests: A Review. J. Entomol. Res. 2017, 14, 112–121. [Google Scholar] [CrossRef]
- Nakash, J.; Osem, Y.; Kehat, M. A suggestion to use dogs for detecting red palm weevil (Rhynchophorus ferrugineus) in-festation in date palms in Israel. Phytoparasitica 2000, 28, 153–155. [Google Scholar] [CrossRef]
- Soroker, V.; Suma, P.; Pergola, A.L.; Cohen, Y.; Alchanatis, V.; Golomb, O.; Hetzroni, A.; Galazan, L.; Kontodimas, D.C.; Zorovic, M.; et al. Early Detection and Monitoring of Red Palm Weevil: Approaches and Challenges. In Colloque Méditerranéen Sur Les Ravageurs Des Palmiers; Association Française de Protection des Plantes (AFPP): Nice, France, 2013. [Google Scholar]
- Justin, C.; Leelamathi, M.; Thangaselvabai, T.; Johnson, S. Bioecology and management of the Red palm weevil, Rhyn-chophorus ferrugineus Oliv (Coleoptera: Curculionidae) on Coconut—A review. Agric. Rev. 2008, 29, 117–124. [Google Scholar]
- Tapia, G.; Ruiz, M.A.; Téllez, M.M. Recommendations for a preventive strategy to control red palm weevil (Rhynchophorus ferrugineus, Olivier) based on the use of insecticides and entomopathogenic nematodes. EPPO Bulletin. 2011, 41, 136–141. [Google Scholar] [CrossRef]
- Al-Shawaf, A.; Al-Shagagh, A.; Al-Bakshi, M.; Al-Saroj, S.; Al-Badr, S.; Al-Dandan, A.M.; Abdallah, A.B. Toxicity of some insecticides against red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Indian J. Plant Prot. 2010, 38, 13–16. [Google Scholar]
- Milosavljević, I.; Lindberg, E.M.; Anderson, D.; Aguilar, R.; Bruce, B.; Drahn, S.; Hoddle, M.S. Efficacy of selected systemic insecticides against Rhynchophorus palmarum infesting ornamental canary islands Date Palms, 2018–2021. Arthropod Manag. Tests 2022, 47, tsac066. [Google Scholar] [CrossRef]
- El-Sharabasy, H.M. A survey of mite species associated with the red palm weevil, Rhynchophorus ferrugineus (Olivier) in Egypt. Egypt. J. Biol. Pest Control 2010, 20, 67. [Google Scholar]
- Abraham, V.; Shuaibi, M.A.; Faleiro, J.; Abozuhairah, R.; Vidyasagar, P.S. An integrated management approach for red palm weevil Rhynchophorus ferrugineus Oliv. a key pest of date palm in the Middle East. J. Agric. Mar. Sci. 1998, 3, 77–83. [Google Scholar] [CrossRef]
- Griffin, C.T. Perspectives on the behavior of entomopathogenic nematodes from dispersal to reproduction: Traits contributing to nematode fitness and biocontrol efficacy. J. Nematol. 2012, 44, 177. [Google Scholar]
- Wakil, W.; Yasin, M.; Shapiro-Ilan, D. Effects of single and combined applications of entomopathogenic fungi and nematodes against Rhynchophorus ferrugineus (Olivier). Sci. Rep. 2017, 7, 5971. [Google Scholar] [CrossRef] [PubMed]
- Elawad, S.A.; Mousa, S.A.; Shahbad, A.; Alawaash, S.A.; Alamiri, A.M. Efficacy of entomopathogenic nematodes against red palm weevil in UAE. In Proceedings of the IIIrd International Date Palm Conference, Abu Dhabi, United Arab Emirates, 19–21 February 2006; 736, pp. 415–420. [Google Scholar] [CrossRef]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Elawad, S.; Mousa, S.; Shahdad, A.; Alawaash, S.; Alamiri, A. Potential of entomopathogenic nematodes against the Red Palm Weevil in United Arab Emirates. Pak. J. Nematol. 2007, 25, 5–13. [Google Scholar]
- Llácer, E.; Martínez de Altube, M.M.; Jacas, J. Evaluation of the efficacy of Steinernema carpocapsae in a chitosan formulation against the red palm weevil, Rhynchophorus ferrugineus, in Phoenix canariensis. BioControl 2009, 54, 559–565. [Google Scholar] [CrossRef]
- Atwa, A.A.; Hegazi, E.M. Comparative susceptibilities of different life stages of the red palm weevil (Coleoptera: Curculionidae) treated by entomopathogenic nematodes. J. Econ. Entomol. 2014, 107, 1339–1347. [Google Scholar] [CrossRef]
- Kaya, H.K.; Stock, S.P. Technique in insect nematology. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press Ltd.: New York, NY, USA, 1997; pp. 281–324. [Google Scholar]
- Triggiani, O.; Tarasco, E. Evaluation of the effects of autochthonous and commercial isolates of Steinernematidae and Heterorhabditidae on Rhynchophorus ferrugineus. Bull. Insectol. 2011, 64, 175–180. [Google Scholar]
- Gözel, U.; Gözel, Ç.; Yurt, Ç.; İnci, D. Efficacy of entomopathogenic nematodes on the red palm weevil Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Curculionidae) larvae. Int. J. Bioassays 2015, 4, 4436–4439. [Google Scholar]
- Manzoor, M.; Ahmad, J.N.; Sharif, M.Z.; Majeed, D.; Kiran, H.; Jafir, M.; Ali, H. Comparative effectiveness of entomopathogenic nematodes against red palm weevil (Rhynchophorus ferrugineus) in Pakistan. J. Entomol. Zool. Stud. JEZS 2017, 5, 756–760. [Google Scholar]
- Mikaia, N. Management of Entomopathogenic Nematodes on the Red Palm Weevil (Rhynchophorus ferrugineus) Larvae) in Georgia. J. Bioassays 2015, 4, 44364439. [Google Scholar]
- Santhi, V.S.; Salame, L.; Nakache, Y.; Koltai, H.; Soroker, V.; Glazer, I. Attraction of entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora to the red palm weevil (Rhynchophorus ferrugineus). Biol. Control 2015, 83, 75–81. [Google Scholar] [CrossRef]
- El Sadawy, H.A.; Namaky, A.H.; Al Omari, F.; Bahareth, O.M. Susceptibility of Rhynchophorus ferrugineus (Olivier)(Coleoptera: Curculionidae) to entomopathogenic nematodes with regard to its immune response. Biol. Control 2020, 148, 104308. [Google Scholar] [CrossRef]
- Cappa, F.; Torrini, G.; Mazza, G.; Inghilesi, A.F.; Benvenuti, C.; Viliani, L.; Roversi, P.F.; Cervo, R. Assessing immunocompetence in red palm weevil adult and immature stages in response to bacterial challenge and entomopathogenic nematode infection. Insect Sci. 2020, 27, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Dillon, A.B.; Ennis, D.; Hennessy, R.; Griffin, C.T. Differential susceptibility of pine weevil, Hylobius abietis (Coleoptera: Curculionidae), larvae and pupae to entomopathogenic nematodes and death of adults infected as pupae. Biocontrol 2015, 60, 537–546. [Google Scholar] [CrossRef]
- Mikaia, N. EPNs Steinernema carpocapsae and Heterorhabditis bacteriophora for Control of the Brown Marmorated Stink Bug (BMSB) Halyomorpha halys (Hemiptera, Pentatomidae). Bull. Georg. Natl. Acad. Sci 2018, 12, 89–94. [Google Scholar]
- Mannion, C.M.; Jansson, R.K. Comparison of ten entomopathogenic nematodes for control of sweetpotato weevil (Coleoptera: Apionidae). J. Econ. Entomol. 1992, 85, 1642–1650. [Google Scholar] [CrossRef]
- Ramos-Rodríguez, O.; Campbell, J.F.; Ramaswamy, S.B. Pathogenicity of three species of entomopathogenic nematodes to some major stored-product insect pests. J. Stored Prod. Res. 2006, 42, 241–252. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Mizell, R.F., III; Campbell, J.F. Susceptibility of the plum curculio, Conotrachelus nenuphar, to entomopathogenic nematodes. J. Nematol. 2002, 34, 246. [Google Scholar]
| EPN Species | % Infestation | % Adult Emergence |
|---|---|---|
| H. bacteriophora | 92.68 ± 0.41 b | 6.60 ± 0.54 d |
| S. feltiae | 70.88 ± 0.29 c | 28.60 ± 0.54 c |
| S. carpocapsae | 94.68 ± 0.34 a | 4.60 ± 0.54 e |
| S. glaseri | 67.60 ± 0.37 d | 32.40 ± 0.54 b |
| Control | 0.00 ± 0.00 e | 97.80 ± 1.13 a |
| LSD Value | 1.34 | 0.64 |
| EPN Species | % Infestation (Pupae) | % Adults Emerged |
|---|---|---|
| H. bacteriophora | 60.20 ± 0.44 b | 39.56 ± 0.51 c |
| S.feltiae | 44.80 ± 0.83 c | 56.20 ± 0.83 b |
| S.carpocapsae | 63.60 ± 0.54 a | 35.68 ± 0.64 d |
| S. glaseri | 43.60 ± 1.14 d | 56.40 ± 0.54 b |
| Control | 0.20 ± 0.44 e | 98.40 ± 0.51 a |
| LSD | 0.96 | 0.83 |
| EPN Species | % Mortality of Red Palm Weevil |
|---|---|
| H. bacteriophora | 80.20 ± 0.44 b |
| S. feltiae | 64.80 ± 0.83 c |
| S. carpocapsae | 83.60 ± 0.54 a |
| S. glaseri | 60.60 ± 1.14 d |
| Control | 0.20 ± 0.44 e |
| LSD | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, G.; Mamoon-ur-Rashid, M. Evaluation of Entomopathogenic Nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Insects 2022, 13, 733. https://doi.org/10.3390/insects13080733
Rehman G, Mamoon-ur-Rashid M. Evaluation of Entomopathogenic Nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Insects. 2022; 13(8):733. https://doi.org/10.3390/insects13080733
Chicago/Turabian StyleRehman, Gul, and Muhammad Mamoon-ur-Rashid. 2022. "Evaluation of Entomopathogenic Nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae)" Insects 13, no. 8: 733. https://doi.org/10.3390/insects13080733
APA StyleRehman, G., & Mamoon-ur-Rashid, M. (2022). Evaluation of Entomopathogenic Nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Insects, 13(8), 733. https://doi.org/10.3390/insects13080733





