The Effects of Male Seminal Fluid Proteins on Gut/Gonad Interactions in Drosophila
Abstract
:Simple Summary
Abstract
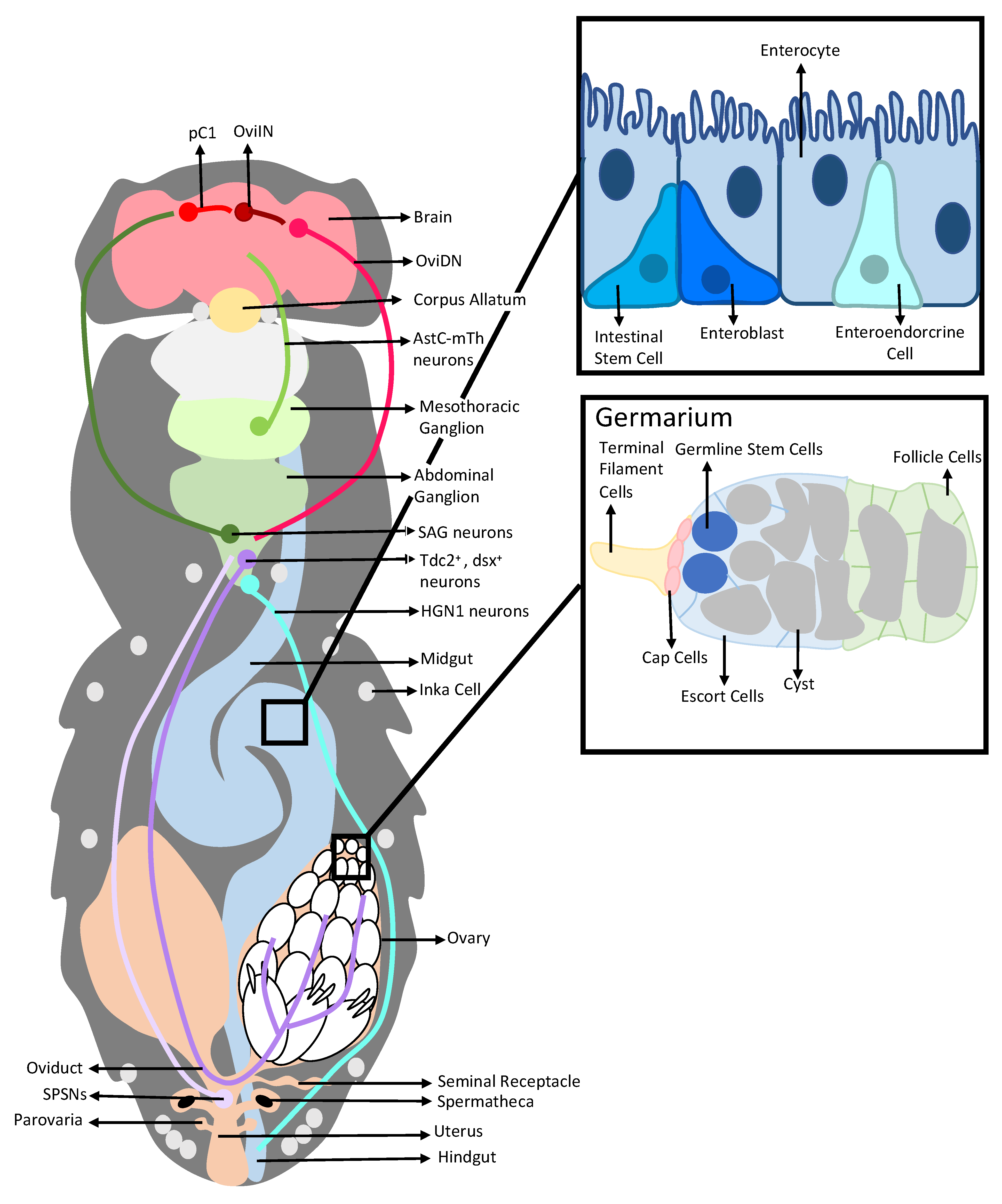
1. Introduction
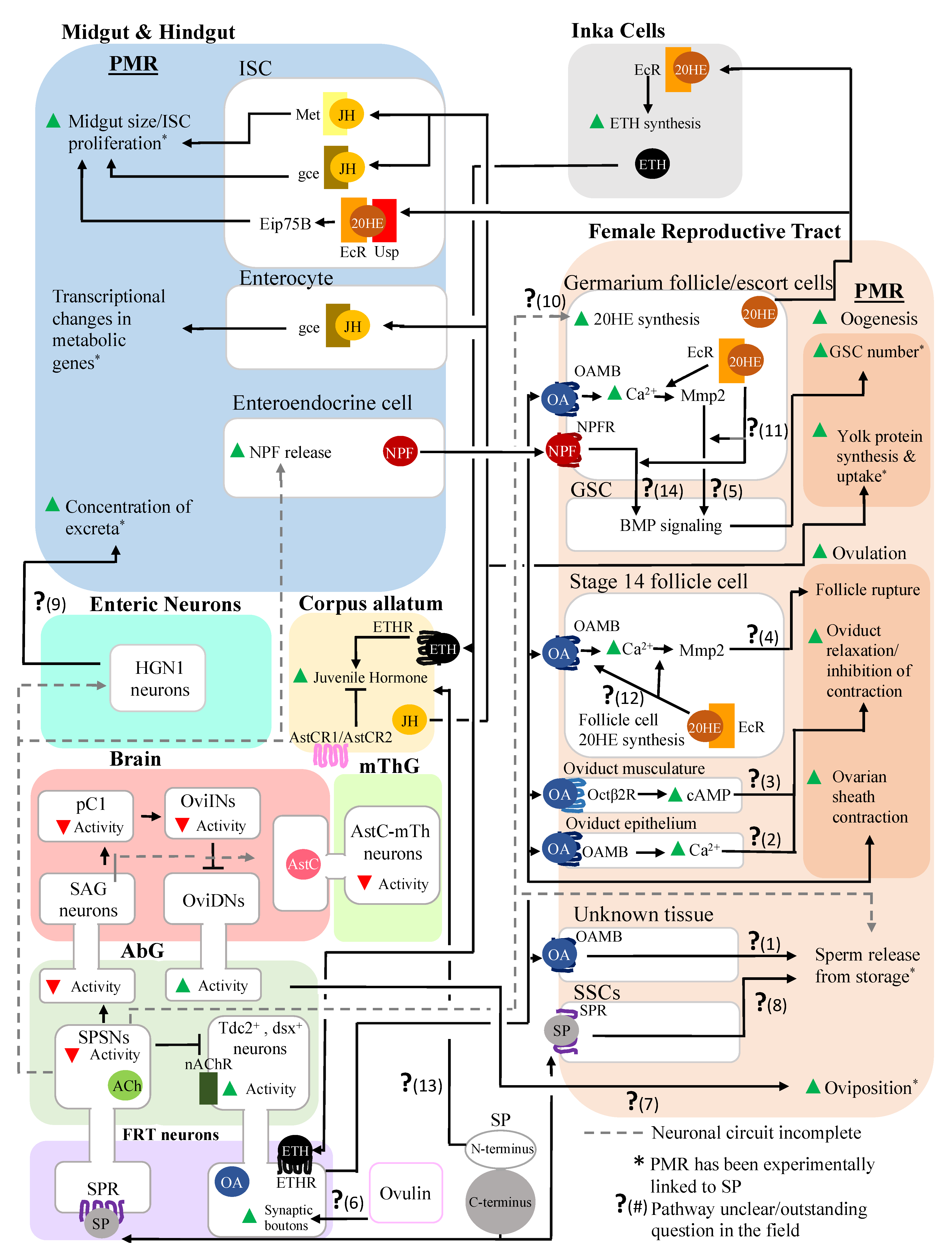
2. Interactions between a Seminal Protein and the Nervous System Affect the Reproductive and Digestive Systems
2.1. Sex Peptide, SP-SPSNs, and the Female Reproductive Tract
2.2. Sex Peptide’s Interaction with the Nervous System Also Modulates Gut Function and Physiology
3. Post-Mating Hormonal Changes Induced by SP also Affect Both the Reproductive and the Digestive Systems
3.1. Alteration of JH and 20E Levels Mediated by SP Stimulates Female Reproductive Physiology
3.2. Alteration of JH and 20E Levels Mediated by SP Affects the Digestive System
4. Inter-organ Signaling Occurs between the Reproductive and Digestive Systems in Mated Females, Enabling Coordination of the Responses of These Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roa, J.; Tena-Sempere, M. Connecting metabolism and reproduction: Roles of central energy sensors and key molecular mediators. Mol. Cell. Endocrinol. 2014, 397, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Mirth, C.K.; Alves, A.N.; Piper, M.D.W. Turning food into eggs: Insights from nutritional biology and developmental physiology of Drosophila. Curr. Opin. Insect Sci. 2019, 31, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Häsemeyer, M.; Yapici, N.; Heberlein, U.; Dickson, B.J. Sensory Neurons in the Drosophila Genital Tract Regulate Female Reproductive Behavior. Neuron 2009, 61, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Yapici, N.; Kim, Y.-J.; Ribeiro, C.; Dickson, B.J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 2008, 451, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Rumpf, S.; Xiang, Y.; Gordon, M.D.; Song, W.; Jan, L.Y.; Jan, Y.-N. Control of the Postmating Behavioral Switch in Drosophila Females by Internal Sensory Neurons. Neuron 2009, 61, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moshitzky, P.; Fleischmann, I.; Chaimov, N.; Saudan, P.; Klauser, S.; Kubli, E.; Applebaum, S.W. Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Arch. Insect Biochem. Physiol. 1996, 32, 363–374. [Google Scholar] [CrossRef]
- Ameku, T.; Niwa, R. Ovarian Ecdysteroid Biosynthesis and Female Germline Stem Cells. PLoS Genet. 2016, 12, e1006123. [Google Scholar] [CrossRef] [Green Version]
- Meiselman, M.R.; Kingan, T.G.; Adams, M.E. Stress-induced reproductive arrest in Drosophila occurs through ETH deficiency-mediated suppression of oogenesis and ovulation. BMC Biol. 2018, 16, 18. [Google Scholar] [CrossRef] [Green Version]
- Meiselman, M.; Lee, S.S.; Tran, R.-T.; Dai, H.; Ding, Y.; Rivera-Perez, C.; Wijesekera, T.P.; Dauwalder, B.; Noriega, F.G.; Adams, M.E. Endocrine network essential for reproductive success in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2017, 114, E3849–E3858. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Kim, A.J.; Rivera-Perez, C.; Noriega, F.G.; Kim, Y.-J. The insect somatostatin pathway gates vitellogenesis progression during reproductive maturation and the post-mating response. Nat. Commun. 2022, 13, 969. [Google Scholar] [CrossRef]
- Ahmed, S.M.H.; Maldera, J.A.; Krunic, D.; Paiva-Silva, G.O.; Pénalva, C.; Teleman, A.A.; Edgar, B.A. Fitness trade-offs incurred by ovary-to-gut steroid signalling in Drosophila. Nature 2020, 584, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Ameku, T.; Yoshinari, Y.; Texada, M.; Kondo, S.; Amezawa, K.; Yoshizaki, G.; Shimada-Niwa, Y.; Niwa, R. Midgut-derived neuropeptide F controls germline stem cell proliferation in a mating-dependent manner. PLoS Biol. 2018, 16, e2005004. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, Y.; Ameku, T.; Kondo, S.; Tanimoto, H.; Kuraishi, T.; Shimada-Niwa, Y.; Niwa, R. Neuronal octopamine signaling regulates mating-induced germline stem cell increase in female Drosophila melanogaster. eLife 2020, 9, e57101. [Google Scholar] [CrossRef]
- Reiff, T.; Jacobson, J.; Cognigni, P.; Antonello, Z.; Ballesta, E.; Tan, K.J.; Yew, J.Y.; Dominguez, M.; Miguel-Aliaga, I. Endocrine remodelling of the adult intestine sustains reproduction in Drosophila. eLife 2015, 4, e06930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soller, M.; Bownes, M.; Kubli, E. Control of Oocyte Maturation in Sexually Mature Drosophila Females. Dev. Biol. 1999, 208, 337–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soller, M.; Bownes, M.; Kubli, E. Mating and Sex Peptide Stimulate the Accumulation of Yolk in Oocytes of Droosophila melanogaster. Eur. J. Biochem. 1997, 243, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Ameku, T.; Niwa, R. Mating-Induced Increase in Germline Stem Cells via the Neuroendocrine System in Female Drosophila. PLoS Genet. 2016, 12, e1006123. [Google Scholar] [CrossRef] [Green Version]
- Heifetz, Y.; Lung, O.; Frongillo, E.A.; Wolfner, M.F. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 2000, 10, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, C.D.; Wolfner, M.F. Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 17420–17425. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, K.; Forknall, N.; Patrick, C.; Yang, T.; Parekh, R.; Bock, D.; Dickson, B.J. Neural circuitry linking mating and egg laying in Drosophila females. Nature 2020, 579, 101–105. [Google Scholar] [CrossRef]
- Avila, F.W.; Mattei, A.L.; Wolfner, M.F. Sex peptide receptor is required for the release of stored sperm by mated Drosophila melanogaster females. J. Insect Physiol. 2015, 76, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, F.W.; Qazi, M.C.B.; Rubinstein, C.D.; Wolfner, M.F. A requirement for the neuromodulators octopamine and tyramine in Drosophila melanogaster female sperm storage. Proc. Natl. Acad. Sci. USA 2012, 109, 4562–4567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, E.M.; Wolfner, M.F. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J. Insect Physiol. 2007, 53, 319–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, F.W.; Ram, K.R.; Qazi, M.C.B.; Wolfner, M.F. Sex Peptide Is Required for the Efficient Release of Stored Sperm in Mated Drosophila Females. Genetics 2010, 186, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Cognigni, P.; Bailey, A.P.; Miguel-Aliaga, I. Enteric Neurons and Systemic Signals Couple Nutritional and Reproductive Status with Intestinal Homeostasis. Cell Metab. 2011, 13, 92–104. [Google Scholar] [CrossRef] [Green Version]
- White, M.A.; Bonfini, A.; Wolfner, M.F.; Buchon, N. Drosophila melanogaster Sex Peptide Regulates Mated Female Midgut Morphology and Physiology. Proc. Natl. Acad. Sci. USA 2021, 118, e2018112118. [Google Scholar] [CrossRef]
- Avila, F.W.; Sirot, L.K.; LaFlamme, B.A.; Rubinstein, C.D.; Wolfner, M.F. Insect Seminal Fluid Proteins: Identification and Function. Annu. Rev. Entomol. 2011, 56, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Monastirioti, M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev. Biol. 2003, 264, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-G.; Seong, C.-S.; Kim, Y.; Davis, R.; Han, K.-A. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev. Biol. 2003, 264, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Sabandal, P.R.; Fernandez, A.; Sabandal, J.M.; Lee, H.-G.; Evans, P.; Han, K.-A. The Octopamine Receptor Octβ2R Regulates Ovulation in Drosophila melanogaster. PLoS ONE 2014, 9, e104441. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fink, C.; El-Kholy, S.; Roeder, T. The Octopamine Receptor Octß2r Is Essential for Ovulation and Fertilization in the Fruit Fly Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2014, 88, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Palfreyman, M.T.; Häsemeyer, M.; Talsma, A.; Dickson, B.J. Ascending SAG Neurons Control Sexual Receptivity of Drosophila Females. Neuron 2014, 83, 135–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Navascués, J.; Perdigoto, C.N.; Bian, Y.; Schneider, M.H.; Bardin, A.J.; Martínez-Arias, A.; Simons, B.D. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J. 2012, 31, 2473–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshino, R.; Niwa, R. Regulation of Mating-Induced Increase in Female Germline Stem Cells in the Fruit Fly Drosophila melanogaster. Front. Physiol. 2021, 12, 785435. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.X.; Spradling, A.C. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development 2011, 138, 2207–2215. [Google Scholar] [CrossRef] [Green Version]
- Abdou, M.A.; He, Q.; Wen, D.; Zyaan, O.; Wang, J.; Xu, J.; Baumann, A.A.; Joseph, J.; Wilson, T.G.; Li, S.; et al. Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 2011, 41, 938–945. [Google Scholar] [CrossRef]
- Charles, J.-P.; Iwema, T.; Epa, V.C.; Takaki, K.; Rynes, J.; Jindra, M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. USA 2011, 108, 21128–21133. [Google Scholar] [CrossRef] [Green Version]
- Yao, T.; Forman, B.M.; Jiangi, Z.; Cherbas, L.; Chen, J.; Mckeown, M.; Cherbas, P.; Evans, R.M. Functional Ecdysone Receptor Is the Product of EcR and Ultraspiracle Genes. Nature 1993, 366, 476–478. [Google Scholar] [CrossRef]
- Chen, P.; Stumm-Zollinger, E.; Aigaki, T.; Balmer, J.; Bienz, M.; Böhlen, P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell 1988, 54, 291–298. [Google Scholar] [CrossRef]
- Liu, H.; Kubli, E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2003, 100, 9929–9933. [Google Scholar] [CrossRef] [Green Version]
- Chapman, T.; Bangham, J.; Vinti, G.; Seifried, B.; Lung, O.; Wolfner, M.F.; Smith, H.K.; Partridge, L. The sex peptide of Drosophila melanogaster: Female post-mating responses analyzed by using RNA interference. Proc. Natl. Acad. Sci. USA 2003, 100, 9923–9928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Chen, S.; Büsser, S.; Liu, H.; Honegger, T.; Kubli, E. Gradual Release of Sperm Bound Sex-Peptide Controls Female Postmating Behavior in Drosophila. Curr. Biol. 2005, 15, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haussmann, I.U.; Hemani, Y.; Wijesekera, T.; Dauwalder, B.; Soller, M. Multiple pathways mediate the sex-peptide-regulated switch in female Drosophila reproductive behaviours. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-J.; Bartalska, K.; Audsley, N.; Yamanaka, N.; Yapici, N.; Lee, J.-Y.; Kim, Y.-C.; Markovic, M.; Isaac, E.; Tanaka, Y.; et al. MIPs are ancestral ligands for the sex peptide receptor. Proc. Natl. Acad. Sci. USA 2010, 107, 6520–6525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezával, C.; Pavlou, H.J.; Dornan, A.J.; Chan, Y.-B.; Kravitz, E.A.; Goodwin, S.F. Neural Circuitry Underlying Drosophila Female Postmating Behavioral Responses. Curr. Biol. 2012, 22, 1155–1165. [Google Scholar] [CrossRef] [Green Version]
- Pauls, D.; Blechschmidt, C.; Frantzmann, F.; el Jundi, B.; Selcho, M. A comprehensive anatomical map of the peripheral octopaminergic/tyraminergic system of Drosophila melanogaster. Sci. Rep. 2018, 8, 15314. [Google Scholar] [CrossRef] [Green Version]
- Rezával, C.; Nojima, T.; Neville, M.C.; Lin, A.C.; Goodwin, S.F. Sexually Dimorphic Octopaminergic Neurons Modulate Female Postmating Behaviors in Drosophila. Curr. Biol. 2014, 24, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Deady, L.D.; Sun, J. A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Drosophila Ovulation. PLoS Genet. 2015, 11, e1005604. [Google Scholar] [CrossRef]
- Middleton, C.A.; Nongthomba, U.; Parry, K.; Sweeney, S.T.; Sparrow, J.C.; Elliott, C.J. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Valentín, R.; López-González, I.; Jorquera, R.; Labarca, P.; Zurita, M.; Reynaud, E. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J. Cell. Physiol. 2006, 209, 183–198. [Google Scholar] [CrossRef]
- White, M.A.; Chen, D.S.; Wolfner, M.F. She’s got nerve: Roles of octopamine in insect female reproduction. J. Neurogenet. 2021, 35, 132–153. [Google Scholar] [CrossRef] [PubMed]
- Herndon, L.A.; Wolfner, M.F. A Drosophila Seminal Fluid Protein, Acp26Aa, Stimulates Egg Laying in Females for 1 Day after Mating. Proc. Natl. Acad. Sci. USA 1995, 92, 10114–10118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apger-McGlaughon, J.; Wolfner, M.F. Post-mating change in excretion by mated Drosophila melanogaster females is a long-term response that depends on sex peptide and sperm. J. Insect Physiol. 2013, 59, 1024–1030. [Google Scholar] [CrossRef] [Green Version]
- Knapp, E.; Sun, J. Steroid signaling in mature follicles is important for Drosophila ovulation. Proc. Natl. Acad. Sci. USA 2017, 114, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Rafaeli, A.; Gileadi, C.; Kubli, E.; Applebaum, S.W. Drosophila melanogaster Sex Peptide Stimulates Juvenile Hormone Synthesis and Depresses Sex Pheromone Production in Helicoverpa Armigera. J. Insect Physiol. 1999, 45, 127–133. [Google Scholar] [CrossRef]
- Fan, Y.; Rafaeli, A.; Moshitzky, P.; Kubli, E.; Choffat, Y.; Applebaum, S.W. Common functional elements of Drosophila melanogaster seminal peptides involved in reproduction of Drosophila melanogaster and Helicoverpa armigera females. Insect Biochem. Mol. Biol. 2000, 30, 805–812. [Google Scholar] [CrossRef]
- Jindra, M.; Bellés, X.; Shinoda, T. Molecular Basis of Juvenile Hormone Signaling. Curr. Opin. Insect Sci. 2015, 11, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jindra, M.; Uhlirova, M.; Charles, J.-P.; Smykal, V.; Hill, R.J. Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor. PLoS Genet. 2015, 11, e1005394. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, M.A.; Wolfner, M.F. The Effects of Male Seminal Fluid Proteins on Gut/Gonad Interactions in Drosophila. Insects 2022, 13, 623. https://doi.org/10.3390/insects13070623
White MA, Wolfner MF. The Effects of Male Seminal Fluid Proteins on Gut/Gonad Interactions in Drosophila. Insects. 2022; 13(7):623. https://doi.org/10.3390/insects13070623
Chicago/Turabian StyleWhite, Melissa A., and Mariana F. Wolfner. 2022. "The Effects of Male Seminal Fluid Proteins on Gut/Gonad Interactions in Drosophila" Insects 13, no. 7: 623. https://doi.org/10.3390/insects13070623
APA StyleWhite, M. A., & Wolfner, M. F. (2022). The Effects of Male Seminal Fluid Proteins on Gut/Gonad Interactions in Drosophila. Insects, 13(7), 623. https://doi.org/10.3390/insects13070623






