Survivorship-Reducing Effect of Propylene Glycol on Vector Mosquito Populations and Its Potential Use in Attractive Toxic Sugar Baits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
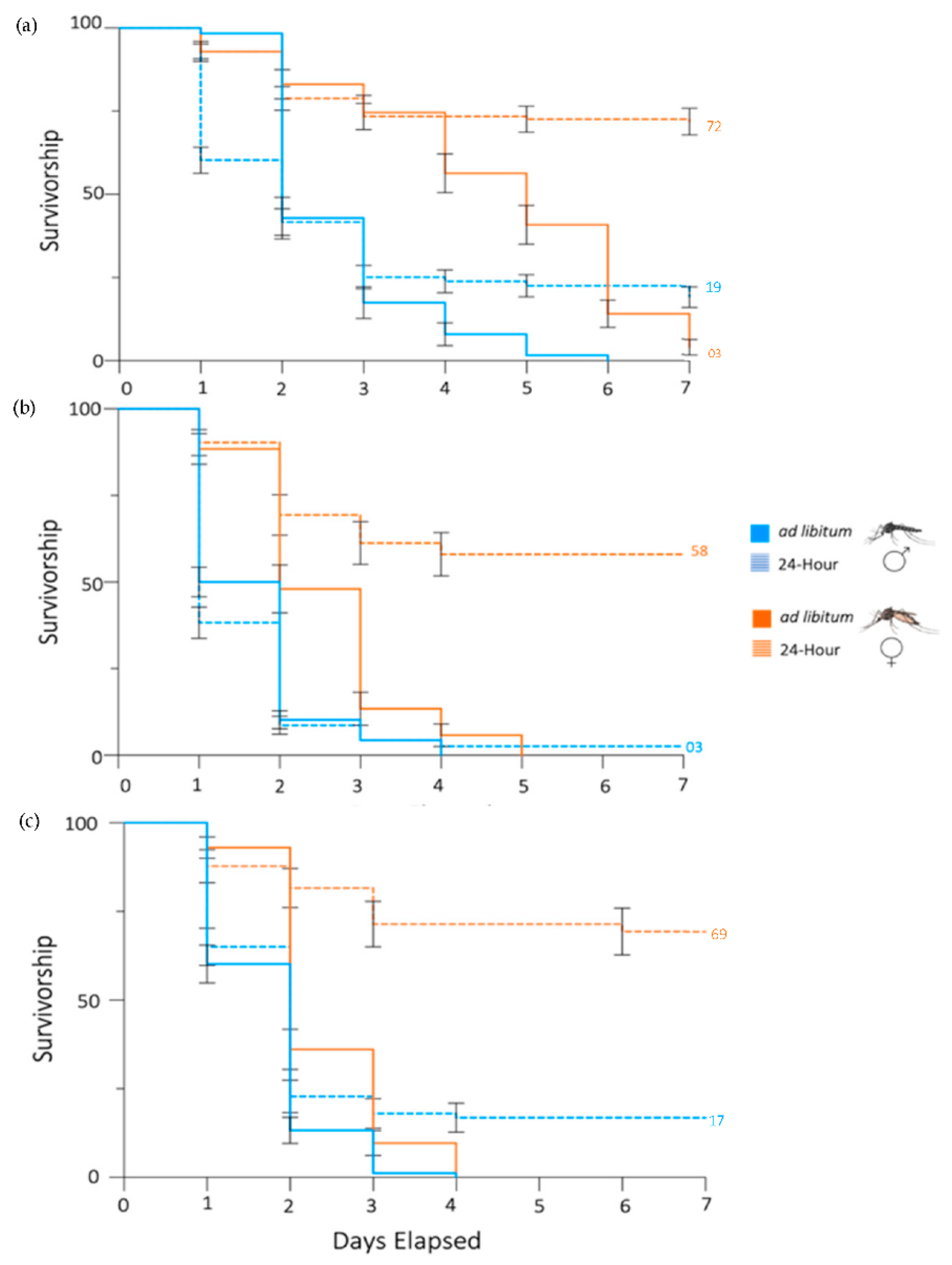
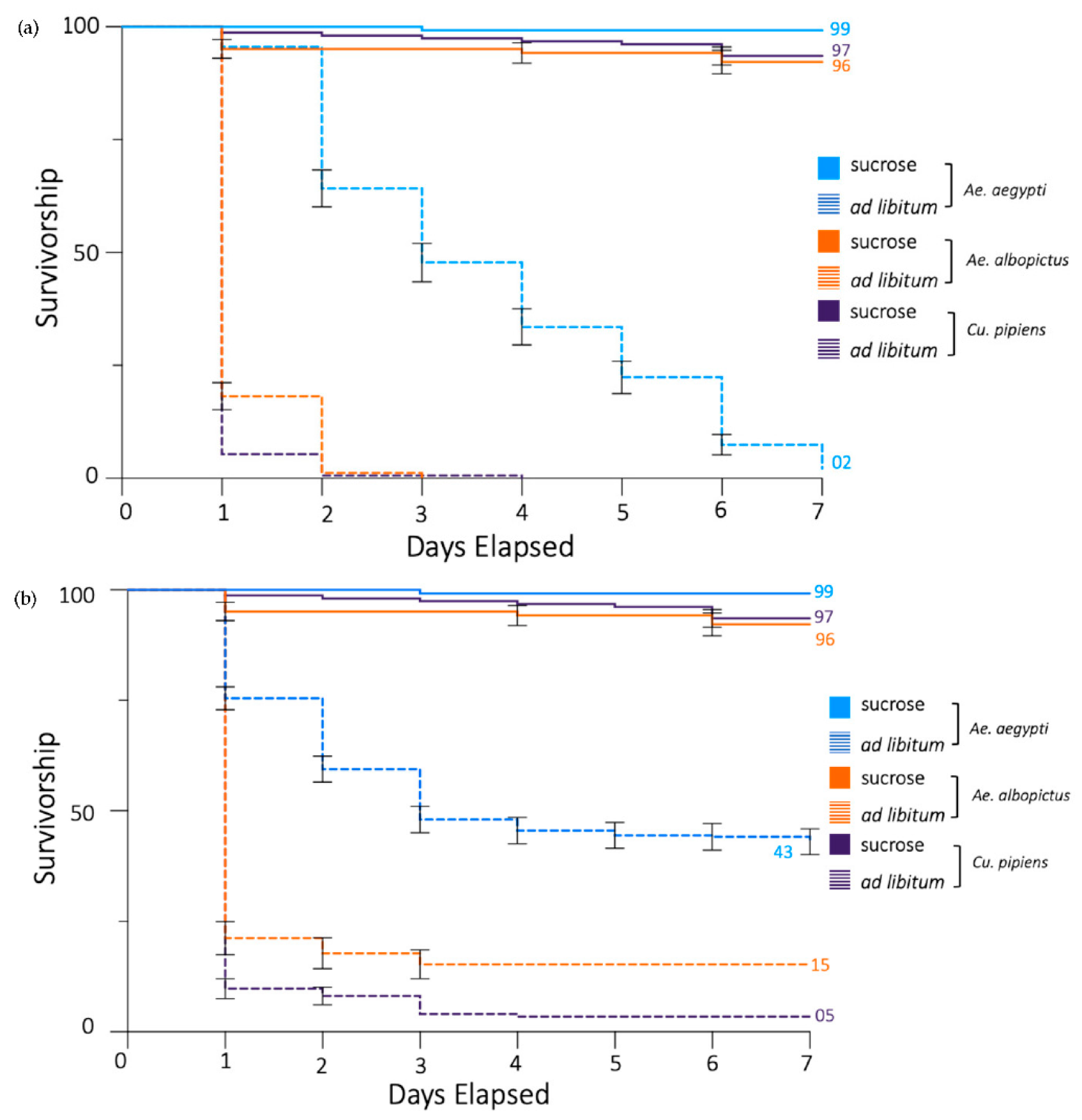
3.1. Propylene Glycol Reduces Survivorship of Ae. aegypti Mosquitoes
3.2. PG Has Sex-Specific Effects on Ae. aegypti Mosquitoes
3.3. Propylene Glycol Decreases the Survivorship of Ae. albopictus and Cx. pipiens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khormi, H.M.; Kumar, L. Climate change and the potential global distribution of Aedes aegypti: Spatial modelling using geographical information system and CLIMEX. Geospat. Health 2014, 8, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Epstein, P.R. Climate change and emerging infectious diseases. Microbes Infect. 2001, 3, 747–754. [Google Scholar] [CrossRef]
- Reinhold, J.; Lazzari, C.; Lahondère, C. Effects of the Environmental Temperature on Aedes aegypti and Aedes albopictus Mosquitoes: A Review. Insects 2018, 9, 158. [Google Scholar] [CrossRef]
- Liu-Helmersson, J.; Rocklöv, J.; Sewe, M.; Brännström, A. Climate change may enable Aedes aegypti infestation in major European cities by 2100. Environ. Res. 2019, 172, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.P.; Luther, C.; Moo-Llanes, D.; Ramsey, J.M.; Danis-Lozano, R.; Peterson, A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140135. [Google Scholar] [CrossRef]
- Christophers, S.R. Aedes aegypti: The Yellow Fever Mosquito; Cambridge University Press: New York, NY, USA, 1960; Available online: http://www.dpi.inpe.br/geocxnets/wiki/lib/exe/fetch.php?media=wiki:christophers_1960.pdf (accessed on 7 February 2022).
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 2018, 67, 191–209. [Google Scholar] [CrossRef]
- Johnson, B.W.; Demanou, M.; Fall, G.; Betoulle, J.-L.; Obiekea, C.; Basile, A.J.; Domingo, C.; Goodman, C.; Mossel, E.; Reusken, C.; et al. Laboratory capacity assessments in 25 African countries at high risk of yellow fever, August-December 2018. Pan Afr. Med. J. 2021, 38, 402. [Google Scholar] [CrossRef]
- Sang, S.; Liu, Q.; Guo, X.; Wu, D.; Ke, C.; Liu-Helmersson, J.; Jiang, J.; Weng, Y.; Wang, Y. The epidemiological characteristics of dengue in high-risk areas of China, 2013–2016. PLoS Neglected Trop. Dis. 2021, 15, e0009970. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An update on Zika virus infection. Lancet 2017, 390, 2099–2109. [Google Scholar] [CrossRef]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-Trotting Aedes aegypti and Aedes albopictus: Risk Factors for Arbovirus Pandemics. Vector-Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, K.; Fotakis, E.A.; Kioulos, I.; Mpellou, S.; Konstantas, S.; Varela, E.; Gewehr, S.; Diamantopoulos, V.; Vontas, J. Detection of West Nile Virus—Lineage 2 in Culex pipiens mosquitoes, associated with disease outbreak in Greece, 2017. Acta Trop. 2018, 182, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Cohen, J.M.; Evans, M.V.; Gudapati, P.; Johnson, L.R.; Lippi, C.A.; Miazgowicz, K.; Murdock, C.C.; Rohr, J.R.; Ryan, S.J.; et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Neglected Trop. Dis. 2017, 11, e0005568. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Schaffner, F.; Versteirt, V.; Hendrickx, G.; Zeller, H.; Van Bortel, W. A Review of the Invasive Mosquitoes in Europe: Ecology, Public Health Risks, and Control Options. Vector-Borne Zoonotic Dis. 2012, 12, 435–447. [Google Scholar] [CrossRef]
- Barbosa, D.S.; Rodrigues, M.M.S.; Silva, A.a.E. Evaluation of Attractive Toxic Sugar Baits (ATSB) against Aedes aegypti (Diptera: Culicidae) in Laboratory. Trop. Biomed. 2019, 36, 578–586. [Google Scholar]
- Fryzlewicz, L.; VanWinkle, A.; Lahondère, C. Development of an Attractive Toxic Sugar Bait for the Control of Aedes j. japonicus (Diptera: Culicidae). J. Med. Èntomol. 2021, 59, 308–313. [Google Scholar] [CrossRef]
- Qualls, W.A.; Müller, G.C.; Traore, S.F.; Traore, M.M.; Arheart, K.L.; Doumbia, S.; Schlein, Y.; Kravchenko, V.D.; Xue, R.-D.; Beier, J.C. Indoor use of attractive toxic sugar bait (ATSB) to effectively control malaria vectors in Mali, West Africa. Malar. J. 2015, 14, 301. [Google Scholar] [CrossRef]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.-D.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef]
- Fikrig, K.; Johnson, B.J.; Fish, D.; Ritchie, S.A. Assessment of synthetic floral-based attractants and sugar baits to capture male and female Aedes aegypti (Diptera: Culicidae). Parasites Vectors 2017, 10, 32. [Google Scholar] [CrossRef]
- Fiorenzano, J.M.; Koehler, P.G.; Xue, R.-D. Attractive Toxic Sugar Bait (ATSB) For Control of Mosquitoes and Its Impact on Non-Target Organisms: A Review. Int. J. Environ. Res. Public Health 2017, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Schlein, Y. Marking of Phlebotomus papatasi (Diptera: Psychodidae) by feeding on sprayed, coloured sugar bait: A possible means for behavioural and control studies. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 599. [Google Scholar] [CrossRef]
- Müller, G.C.; Kravchenko, V.D.; Schlein, Y. Decline of Anopheles sergentii and Aedes caspius Populations Following Presenta-tion of Attractive Toxic (Spinosad) Sugar Bait Stations in an Oasis. J. Am. Mosq. Control. Assoc. 2008, 24, 147–149. [Google Scholar] [CrossRef]
- Mangan, R.L.; Moreno, D.S. Development of Bait Stations for Fruit Fly Population Suppression. J. Econ. Entomol. 2007, 100, 440–450. [Google Scholar] [CrossRef][Green Version]
- Han, W.; Tian, Y.; Shen, X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Stewart, Z.; Oxborough, R.M.; Tungu, P.K.; Kirby, M.J.; Rowland, M.W.; Irish, S.R. Indoor Application of Attractive Toxic Sugar Bait (ATSB) in Combination with Mosquito Nets for Control of Pyrethroid-Resistant Mosquitoes. PLoS ONE 2013, 8, e84168. [Google Scholar] [CrossRef] [PubMed]
- Furnival-Adams, J.E.C.; Camara, S.; Rowland, M.; Koffi, A.A.; Alou, L.P.A.; Oumbouke, W.; N’Guessan, R. Indoor use of attractive toxic sugar bait in combination with long-lasting insecticidal net against pyrethroid-resistant Anopheles gambiae: An experimental hut trial in Mbé, central Côte d’Ivoire. Malar. J. 2020, 19, 11. [Google Scholar] [CrossRef]
- Sippy, R.; Rivera, G.E.; Sanchez, V.; Heras, F.; Morejón, B.; Beltrán, E.; Hikida, R.S.; López-Latorre, M.A.; Aguirre, A.; Stewart-Ibarra, A.M.; et al. Ingested insecticide to control Aedes aegypti: Developing a novel dried attractive toxic sugar bait device for intra-domiciliary control. Parasites Vectors 2020, 13, 78. [Google Scholar] [CrossRef]
- Thompson, B.; Carosso, E.; Griffith, W.; Workman, T.; Hohl, S.; Faustman, E. Disseminating Pesticide Exposure Results to Farmworker and Nonfarmworker Families in an Agricultural Community: A Community-Based Participatory Research Approach. J. Occup. Environ. Med. 2017, 59, 982–987. [Google Scholar] [CrossRef]
- Yang, F.; Schildhauer, S.; Billeter, S.A.; Yoshimizu, M.H.; Payne, R.; Pakingan, M.J.; Metzger, M.E.; Liebman, K.A.; Hu, R.; Kramer, V.; et al. Insecticide Resistance Status of Aedes aegypti (Diptera: Culicidae) in California by Biochemical Assays. J. Med. Èntomol. 2020, 57, 1176–1183. [Google Scholar] [CrossRef]
- Zaim, M.; Guillet, P. Alternative insecticides: An urgent need. Trends Parasitol. 2002, 18, 161–163. [Google Scholar] [CrossRef]
- Yun, X.; Huang, Q.; Rao, W.; Xiao, C.; Zhang, T.; Mao, Z.; Wan, Z. A comparative assessment of cytotoxicity of commonly used agricultural insecticides to human and insect cells. Ecotoxicol. Environ. Saf. 2017, 137, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Èntomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.A. Susceptibility of adult mosquitoes to insecticides in aqueous sucrose baits. J. Vector Ecol. 2011, 36, 59–67. [Google Scholar] [CrossRef]
- Beier, J.C.; Müller, G.C.; Gu, W.; Arheart, K.L.; Schlein, Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar. J. 2012, 11, 31. [Google Scholar] [CrossRef]
- Steele, E.A.; Breen, C.; Campbell, E.; Martin, R. Food Regulations and Enforcement in the USA. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Sharma, A.; Reyes, J.; Borgmeyer, D.; Ayala-Chavez, C.; Snow, K.; Arshad, F.; Nuss, A.; Gulia-Nuss, M. The sugar substitute Stevia shortens the lifespan of Aedes aegypti potentially by N-linked protein glycosylation. Sci. Rep. 2020, 10, 6195. [Google Scholar] [CrossRef]
- Massarsky, A.; Abdel, A.; Glazer, L.; Levin, E.D.; Di Giulio, R.T. Exposure to 1,2-Propanediol Impacts Early Development of Zebrafish (Danio rerio) and Induces Hyperactivity. Zebrafish 2017, 14, 216–222. [Google Scholar] [CrossRef]
- Berthelot-Ricou, A.; Perrin, J.; di Giorgio, C.; de Meo, M.; Botta, A.; Courbiere, B. Assessment of 1,2-propanediol (PrOH) genotoxicity on mouse oocytes by comet assay. Fertil. Steril. 2011, 96, 1002–1007. [Google Scholar] [CrossRef]
- Ruddick, J.A. Toxicology, metabolism, and biochemistry of 1,2-propanediol. Toxicol. Appl. Pharmacol. 1972, 21, 102–111. [Google Scholar] [CrossRef]
- Jacob, S.E.; Scheman, A.; McGowan, M.A. Propylene Glycol®. Dermatitis 2018, 29, 3–5. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Propylene Glycol, Tripropylene Glycol, and PPGs as Used in Cosmetics. Int. J. Toxicol. 2012, 31 (Suppl. 5), 245S–260S. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.A.; Scheman, A.; Jacob, S.E. Propylene Glycol in Contact Dermatitis: A Systematic Review. Dermatitis 2018, 29, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Barakat, L.; Dereure, O.; Raison-Peyron, N. A police case: Finding propylene glycol guilty as culprit allergen. Contact Dermat. 2021, 85, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Zar, T.; Graeber, C.; Perazella, M.A. Reviews: Recognition, Treatment, and Prevention of Propylene Glycol Toxicity. Semin. Dial. 2007, 20, 217–219. [Google Scholar] [CrossRef]
- Murray, E.d.; George, J. Toxicological Report for Propylene Glycol; Agency for Toxic Substances and Disease Registry: Altanta, GA, USA, 1997; pp. 1–176. [Google Scholar]
- Yamashita, W.M.S.; Das, S.S.; Chapiro, G. Numerical modeling of mosquito population dynamics of Aedes aegypti. Parasites Vectors 2018, 11, 245. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Li, Y.; Kamara, F.; Zhou, G.; Puthiyakunnon, S.; Li, C.; Liu, Y.; Zhou, Y.; Yao, L.; Yan, G.; Chen, X.-G. Urbanization Increases Aedes albopictus Larval Habitats and Accelerates Mosquito Development and Survivorship. PLoS Neglected Trop. Dis. 2014, 8, e3301. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Main, A.J.; Delroux, K.; Fikrig, E. Extrinsic Incubation Periods for Horizontal and Vertical Transmission of West Nile Virus by Culex pipiens pipiens (Diptera: Culicidae). J. Med. Entomol. 2008, 45, 445–451. [Google Scholar] [CrossRef]
- Winokur, O.C.; Main, B.J.; Nicholson, J.; Barker, C.M. Impact of temperature on the extrinsic incubation period of Zika virus in Aedes aegypti. PLoS Neglected Trop. Dis. 2020, 14, e0008047. [Google Scholar] [CrossRef]
- Tjaden, N.B.; Thomas, S.M.; Fischer, D.; Beierkuhnlein, C. Extrinsic Incubation Period of Dengue: Knowledge, Backlog, and Applications of Temperature Dependence. PLoS Neglected Trop. Dis. 2013, 7, e2207. [Google Scholar] [CrossRef]
- Dieng, H.; Satho, T.; Arzemi, N.A.B.; Aliasan, N.E.; Abang, F.; Wydiamala, E.; Miake, F.; Zuharah, W.F.; Abu Kassim, N.F.; Vargas, R.E.M.; et al. Exposure of a diurnal mosquito vector to floral mimics: Foraging responses, feeding patterns, and significance for sugar bait technology. Acta Trop. 2018, 185, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Trewin, B.J.; Pagendam, D.E.; Johnson, B.J.; Paton, C.; Snoad, N.; Ritchie, S.A.; Staunton, K.M.; White, B.J.; Mitchell, S.; Beebe, N.W. Mark-release-recapture of male Aedes aegypti (Diptera: Culicidae): Use of rhodamine B to estimate movement, mating and population parameters in preparation for an incompatible male program. PLoS Neglected Trop. Dis. 2021, 15, e0009357. [Google Scholar] [CrossRef] [PubMed]
- Barredo, E.; DeGennaro, M. Not Just from Blood: Mosquito Nutrient Acquisition from Nectar Sources. Trends Parasitol. 2020, 36, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Mužinić, V.; Želježić, D. Non-target toxicity of novel insecticides. Arch. Hyg. Rada Toxicol. 2018, 69, 86–102. [Google Scholar] [CrossRef]
- Schmidt-Jeffris, R.A.; Beers, E.H.; Sater, C. Meta-analysis and review of pesticide non-target effects on phytoseiids, key biological control agents. Pest Manag. Sci. 2021, 77, 4848–4862. [Google Scholar] [CrossRef]
- Dormont, L.; Mulatier, M.; Carrasco, D.; Cohuet, A. Mosquito Attractants. J. Chem. Ecol. 2021, 47, 351–393. [Google Scholar] [CrossRef]
- Baudier, K.M.; Kaschock-Marenda, S.D.; Patel, N.; Diangelus, K.L.; O’Donnell, S.; Marenda, D.R. Erythritol, a Non-Nutritive Sugar Alcohol Sweetener and the Main Component of Truvia®, Is a Palatable Ingested Insecticide. PLoS ONE 2014, 9, e98949. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pullmann Lindsley, H.; Lyons, H.B.; Leon-Noreña, M.; Pitts, R.J. Survivorship-Reducing Effect of Propylene Glycol on Vector Mosquito Populations and Its Potential Use in Attractive Toxic Sugar Baits. Insects 2022, 13, 595. https://doi.org/10.3390/insects13070595
Pullmann Lindsley H, Lyons HB, Leon-Noreña M, Pitts RJ. Survivorship-Reducing Effect of Propylene Glycol on Vector Mosquito Populations and Its Potential Use in Attractive Toxic Sugar Baits. Insects. 2022; 13(7):595. https://doi.org/10.3390/insects13070595
Chicago/Turabian StylePullmann Lindsley, Heidi, Henry B. Lyons, Melissa Leon-Noreña, and Ronald Jason Pitts. 2022. "Survivorship-Reducing Effect of Propylene Glycol on Vector Mosquito Populations and Its Potential Use in Attractive Toxic Sugar Baits" Insects 13, no. 7: 595. https://doi.org/10.3390/insects13070595
APA StylePullmann Lindsley, H., Lyons, H. B., Leon-Noreña, M., & Pitts, R. J. (2022). Survivorship-Reducing Effect of Propylene Glycol on Vector Mosquito Populations and Its Potential Use in Attractive Toxic Sugar Baits. Insects, 13(7), 595. https://doi.org/10.3390/insects13070595




