Oviposition Deterrent Activity of Fungicides and Low-Risk Substances for the Integrated Management of the Olive Fruit Fly Bactrocera oleae (Diptera, Tephritidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bactrocera oleae Rearing
2.2. Commercial Formulation Applications
2.3. No-Choice Oviposition Assay
2.4. Dual-Choice Oviposition Assay
2.5. Statistical Analyses
3. Results
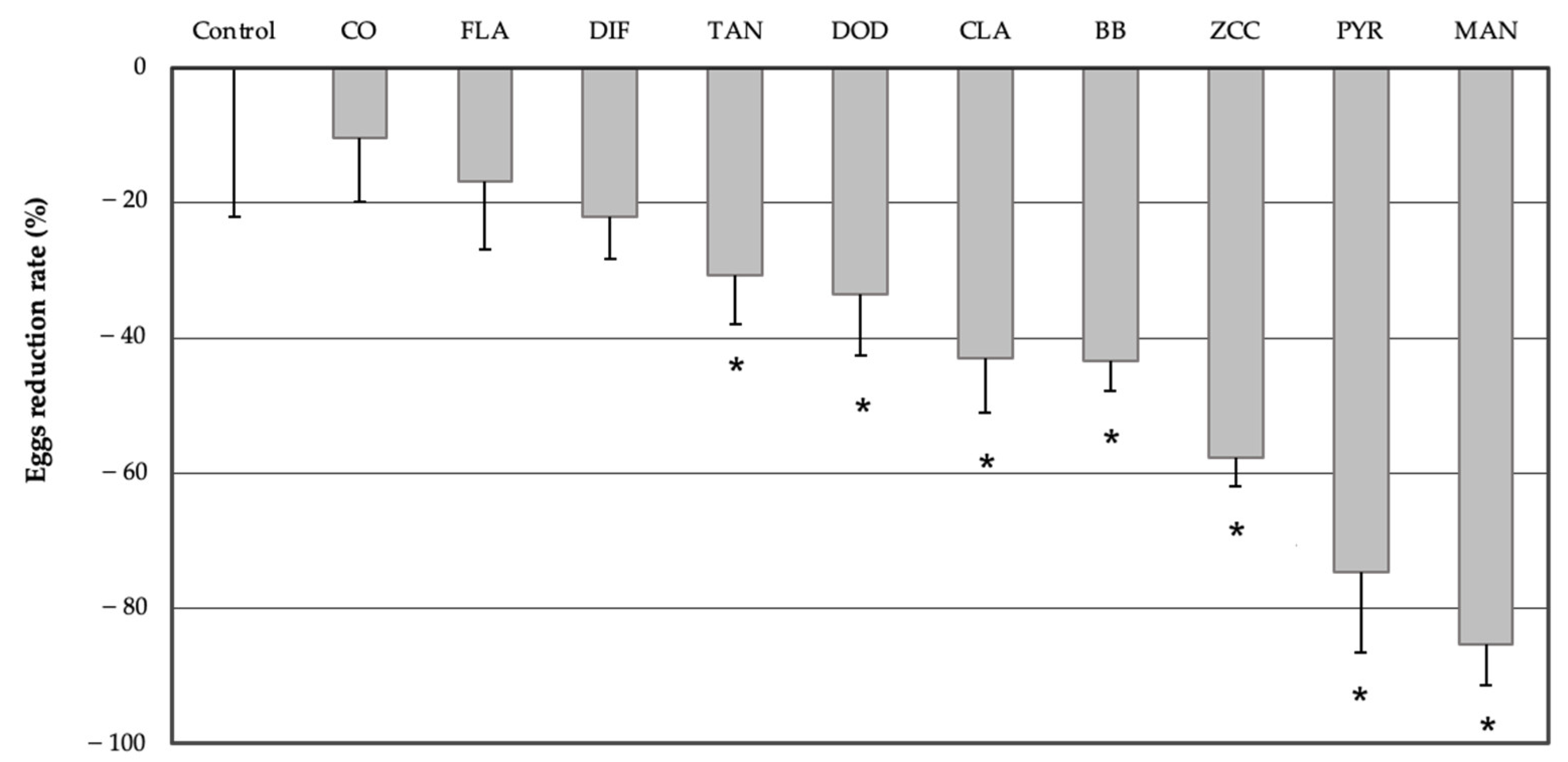
3.1. No-Choice Oviposition Assay
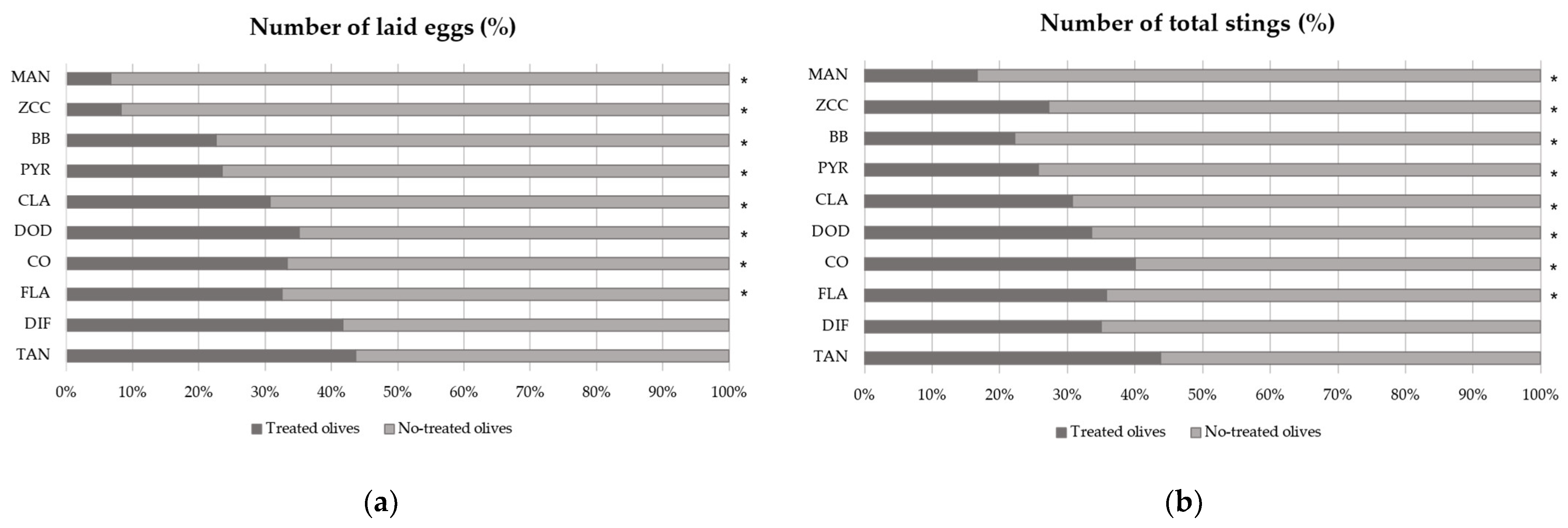
3.2. Dual-Choice Oviposition Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daane, K.M.; Johnson, M.W. Olive fruit fly: Managing an ancient pest in modern times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Medjkouh, L.; Tamendjari, A.; Keciri, S.; Santos, J.; Nunes, M.A.; Oliveira, M.B.P.P. The effect of the olive fruit fly (Bactrocera oleae) on quality parameters, and antioxidant and antibacterial activities of olive oil. Food Funct. 2016, 7, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, S.; Molinari, G.P. Residues of fenthion and trichlorofon in olives and olive oil after olive tree treatments. Food Addit. Contam. 1998, 15, 518–527. [Google Scholar] [CrossRef] [PubMed]
- García-Reyes, J.F.; Ferrer, C.; Gómez-Ramos, M.J.; Fernández-Alba, A.R.; García-Reyes, J.F.; Molina-Díaz, A. Determination of pesticide residues in olive oil and olives. Trends Anal. Chem. 2007, 26, 239–251. [Google Scholar] [CrossRef]
- Kakani, E.G.; Zygouridis, N.E.; Tsoumani, K.T.; Seraphides, N.; Zalom, F.G.; Mathiopoulos, K.D. Spinosad resistance development in wild olive fruit fly Bactrocera oleae (Diptera: Tephritidae) populations in California: Spinosad resistance in California. Pest Manag. Sci. 2010, 66, 447–453. [Google Scholar] [CrossRef]
- Kampouraki, A.; Stavrakaki, M.; Karataraki, A.; Katsikogiannis, G.; Pitika, E.; Varikou, K.; Vlachaki, A.; Chrysargyris, A.; Malandraki, E.; Sidiropoulos, N.; et al. Recent evolution and operational impact of insecticide resistance in olive fruit fly Bactrocera oleae populations from Greece. J. Pest Sci. 2018, 91, 1429–1439. [Google Scholar] [CrossRef]
- Pinheiro, L.A.; Dáder, B.; Wanumen, A.C.; Pereira, J.A.; Santos, S.A.P.; Medina, P. Side effects of pesticides on the olive fruit fly parasitoid Psyttalia concolor (Szépligeti): A Review. Agronomy 2020, 10, 1755. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible plant oil: Global status, health issues, and perspectives. Front. Plant. Sci. 2020, 11, 1315. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Suckling, D.M.; Byers, J.A.; Jang, E.B.; Wearing, C.H. Potential of “lure and kill” in long-term pest management and eradication of invasive species. J. Econ. Entomol. 2009, 102, 815–835. [Google Scholar] [CrossRef]
- Broumas, T.; Haniotakis, G.; Liaropoulos, C.; Tomazou, T.; Ragoussis, N. The efficacy of an improved form of the mass-trapping method, for the control of the olive fruit fly, Bactrocera oleae (Gmelin) (Dipt., Tephritidae): Pilot-scale feasibility studies. J. Appl. Entomol. 2002, 126, 217–223. [Google Scholar] [CrossRef]
- Delrio, G.; Lentini, A.; Satta, A. Biological control of olive fruit fly through inoculative releases of Opius concolor Szèpl. IOBC/WPRS Bull. 2005, 28, 53–58. [Google Scholar]
- Noce, M.E.; Belfiore, T.; Scalercio, S.; Vizzarri, V.; Iannotta, N. Efficacy of new mass-trapping devices against Bactrocera oleae (Diptera Tephritidae) for minimizing pesticide input in agroecosystems. J. Environ. Sci. Health Part B 2009, 44, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Hoelmer, K.A.; Kirk, A.A.; Pickett, C.H.; Daane, K.M.; Johnson, M.W. Prospects for improving biological control of olive fruit fly, Bactrocera oleae (Diptera: Tephritidae), with introduced parasitoids (Hymenoptera). Biocontrol Sci. Technol. 2011, 21, 1005–1025. [Google Scholar] [CrossRef]
- Wang, X.; Levy, K.; Son, Y.; Johnson, M.W.; Daane, K.M. Comparison of the thermal performance between a population of the olive fruit fly and its co-adapted parasitoids. Biol. Control 2012, 60, 247–254. [Google Scholar] [CrossRef]
- Kapranas, A.; Collatz, J.; Michaelakis, A.; Milonas, P. Review of the role of sterile insect technique within biologically-based pest control—An appraisal of existing regulatory frameworks. Entomol. Exp. Appl. 2022. [Google Scholar] [CrossRef]
- Birch, A.N.E.; Begg, G.S.; Squire, G.R. How agro-ecological research helps to address food security issues under new IPM and pesticide reduction policies for global crop production systems. J. Exp. Bot. 2011, 62, 3251–3261. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Birch, A.N.E.; Lindzey, S.; Meadow, R.; Snyder, W.E. REVIEW: A mechanistic framework to improve understanding and applications of push-pull systems in pest management. J. Appl. Ecol. 2016, 53, 202–212. [Google Scholar] [CrossRef]
- Fletcher, B.S.; Prokopy, R.J. Host location and oviposition in tephritid fruit flies. In Reproductive Behaviour of Insects: Individuals and Populations; Bailey, W.J., Ridsdill-Smith, J., Eds.; Chapman & Hall: London, UK, 1991; Chapter 6; pp. 141–171. [Google Scholar]
- Cirio, U.; Vita, G. Fruit fly control by chemical attractants and repellents. Boll. Lab. Entomol. Agrar. 1980, 37, 127–139. [Google Scholar]
- Papanastasiou, S.A.; Ioannou, C.S.; Papadopoulos, N.T. Oviposition-deterrent effect of Linalool—A compound of citrus essential oils on female Mediterranean fruit flies, Ceratitis capitata (Diptera: Thephritidae). Pest Manag. Sci. 2020, 76, 3066–3077. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Ziegler, J.R.; Wong, T.T.Y. Deterrence of repeated oviposition by fruit-marking pheromone in Ceratitis capitata (Diptera: Tephritidae). J. Chem. Ecol. 1978, 4, 55–63. [Google Scholar] [CrossRef]
- Silva, M.A.; Bezerra-Silva, G.C.D.; Mastrangelo, T. The host marking pheromone application on the management of fruit flies—A Review. Braz. Arch. Biol. Technol. 2012, 55, 835–842. [Google Scholar] [CrossRef]
- Thakur, P.; Gupta, D. Oviposition deterrence and egg hatch inhibition of fruit fly, Bactrocera tau (Walker) by some plant products, bio-pesticides and clay. IJBSM 2016, 7, 1161–1164. [Google Scholar] [CrossRef]
- Caleca, V.; Lo Verde, G.; Lo Verde, V.; Palumbo Piccionello, M.; Rizzo, R. Control of Bactrocera oleae and Ceratitis capitata in organic orchards: Use of clays and copper products. Acta Hortic. 2010, 227–234. [Google Scholar] [CrossRef]
- Mojdehi, M.R.A.; Keyhanian, A.A.; Rafiei, B. Application of oviposition deterrent compounds for the control of olive fruit fly, Bactrocera oleae Rossi. (Dip. Tephritidae) control. Int. J. Trop. Insect Sci. 2021, 42, 63–70. [Google Scholar] [CrossRef]
- Anagnou-Veroniki, M.; Kontodimas, D.C.; Adamopoulos, A.D.; Tsimboukis, N.D.; Voulgaropoulou, A. Effects of two fungal based biopesticides on Bactrocera (Dacus) oleae (Gmelin) (Diptera: Tephritidae). In Proceedings of the Meeting Comptes Rendus de la Réunion, Chania, Greece, 29–31 May 2003; Kalaitzaki, A., Alexandrakis, V., Varikou, K., Eds.; The Publication Commission of the IOBC/WPRS, Horst Bathon: Darmstadt, Germany, 2005; Volume 28, pp. 49–51. [Google Scholar]
- Falchi, G.; Marche, M.G.; Mura, M.E.; Ruiu, L. Hydrophobins from aerial conidia of Beauveria bassiana interfere with Ceratitis capitata oviposition behavior. Biol. Control 2015, 81, 37–43. [Google Scholar] [CrossRef]
- R Core Team. European Environment Agency. 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 15 February 2022).
- Pozzebon, A.; Duso, C.; Pavanetto, E. Side effects of some fungicides on Phytoseiid mites (Acari, Phytoseiidae) in North-Italian vineyards. Anz. Schadl. 2002, 75, 132–136. [Google Scholar] [CrossRef]
- Aluja, M.; Boller, E.F. Host marking pheromone of Rhagoletis cerasi: Foraging behavior in response to synthetic pheromonal isomers. J. Chem. Ecol. 1992, 18, 1299–1311. [Google Scholar] [CrossRef]
- Prophetou-Athanasiadou, D.A.; Tzanakakis, M.E.; Myroyannis, D.; Sakas, G. Deterrence of oviposition in Dacus oleae by copper hydroxide. Entomol. Exp. Appl. 1991, 61, 1–5. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A Review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Bigiotti, G.; Pastorelli, R.; Belcari, A.; Sacchetti, P. Symbiosis interruption in the olive fly: Effect of copper and propolis on Candidatus Erwinia Dacicola. J. Appl. Entomol. 2019, 143, 357–364. [Google Scholar] [CrossRef]
- Sinno, M.; Bézier, A.; Vinale, F.; Giron, D.; Laudonia, S.; Garonna, A.P.; Pennacchio, F. Symbiosis disruption in the olive fruit fly, Bactrocera oleae (Rossi), as a potential tool for sustainable control. Pest Manag. Sci. 2020, 76, 3199–3207. [Google Scholar] [CrossRef]
- Scortichini, M.; Chen, J.; De Caroli, M.; Dalessandro, G.; Pucci, N.; Modesti, V.; L’aurora, A.; Petriccione, M.; Zampella, L.; Mastrobuoni, F.; et al. A Zinc, copper and citric acid biocomplex shows promise for control of Xylella fastidios subsp. pauca in olive trees in Apulia region (southern Italy). Phytopathol. Mediterr. 2018, 57, 48–72. [Google Scholar] [CrossRef]
- Gonella, E.; Orrù, B.; Alma, A. Egg Masses treatment with micronutrient fertilizers has a suppressive effect on newly-emerged nymphs of the brown marmorated stink bug Halyomorpha halys. Entomol. Gen 2019, 39, 231–238. [Google Scholar] [CrossRef]
- Ali, E. Effectiveness of particle film technology and copper products in the control of olive fruit fly. J. Plant Prot. Pathol. 2016, 7, 439–444. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J.; Vanderzwet, T.; Byers, R.E.; Feldhake, C. Hydrophobic particle films: A new paradigm for suppression of arthropod pests and plant diseases. J. Econ. Entomol. 1999, 92, 759–771. [Google Scholar] [CrossRef]
- Iannotta, N.; Belfiore, T.; Noce, M.E.; Scalercio, S.; Vizzarri, V. Bactrocera oleae (Gmelin) control in organic olive farming. In Olivebioteq; JA, CAP: Marsala-Mazara del Vallo, Italy, 2006; Volume 2, pp. 323–326. [Google Scholar]
- Puri, S.; Singh, S.; Sohal, S.K. Inhibitory effect of chrysin on growth, development and oviposition behaviour of melon fruit fly, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae). Phytoparasitica 2022, 50, 151–162. [Google Scholar] [CrossRef]
- Sharma, R.; Sohal, S.K. Oviposition response of melon fruit fly, Bactrocera cucurbitae (Coquillett) to different phenolic compounds. J. Biopest. 2016, 9, 46–51. [Google Scholar]
- Benuzzi, M.; Albonetti, E.; Fiorentini, F.; Ladurner, E. A Beauveria bassiana-based bioinsecticide for the microbial control of the olive fly (Bactrocera oleae). In Proceedings of the Insect Pathogens and Insect Parasitic Nematodes: 11th Meeting “From Laboratory to Field—Key Points”, Alès, France, 3–7 June 2007; INRA/Unité de Pathologie Végétale: Avignon, France, 2007; Volume 30, pp. 125–130. [Google Scholar]
- Crespo, R.; Pedrini, N.; Juárez, M.P.; Dal Bello, G.M. Volatile organic compounds released by the entomopathogenic fungus Beauveria bassiana. Microbiol. Res. 2008, 163, 148–151. [Google Scholar] [CrossRef]
- Mohieddine, K.; Serafides, N. Olive GAP Manual: Good Agricultural Practices for the Near East and North Africa Countries. In Pest and Disease Management; Food and agriculture organization (FAO) of the United Nations regional office for the Near East: Cairo, Egypt, 2010; ISBN 978-92-5-106348-4. [Google Scholar]
- Obanor, F.O.; Jaspers, M.V.; Jones, E.E.; Walter, M. Greenhouse and field evaluation of fungicides for control of olive leaf spot in New Zealand. Crop Prot. 2008, 27, 1335–1342. [Google Scholar] [CrossRef]
- Moral, J.; Alsalimiya, M.; Roca, L.F.; Díez, C.M.; León, L.; de la Rosa, R.; Barranco, D.; Rallo, L.; Trapero, A. Relative Susceptibility of new olive cultivars to Spilocaea oleagina, Colletotrichum acutatum, and Pseudocercospora cladosporioides. Plant Dis. 2015, 99, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.; Thuerig, B.; Apostolov, S.; Blogg, H.; Borgo, E.; Corneo, P.E.; Fittje, S.; de Palma, M.; Donko, A.; Experton, C.; et al. Use of copper-based fungicides in organic agriculture in twelve European countries. Agronomy 2022, 12, 673. [Google Scholar] [CrossRef]
- European Commission. Commission Implementig Regulation (EU) 2018/1981 of 13 December 2018 Renewing the Approval of the Active Substances Copper Compounds, as Candidates for Substitution, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. 2018. Available online: http://data.europa.eu/eli/reg_impl/2018/1981/oj (accessed on 23 February 2021).
- Delrio, G.; Deliperi, S.; Lentini, A. Experiments for the control of olive fly using a “push-pull” method. IOBC/WPRS Bull. 2010, 59, 89–92. [Google Scholar]
- Belcari, A.; Sacchetti, P.; Marchi, G.; Surico, G. The olive fly and associated bacteria [Olea europaea L.-Tuscany]. Inf. Fitopatol. 2003, 53, 55–59. [Google Scholar]
- Douglas, A.E. Symbiotic microorganisms: Untapped resources for insect pest control. Trends Biotechnol. 2007, 25, 338–342. [Google Scholar] [CrossRef]
| Active Ingredient (Content) and Formulation | Trade Name | Manufacturer | Olive Production Uses * | Application Rate (g or mL hL−1) |
|---|---|---|---|---|
| Copper oxychloride (3.75%), WG | Neoram® | Isagro S.P.A. | fungicide | 300 |
| Dodine (52.9%), SC | Syllit® 544 SC | ARYSTA LifeScience Italia S.r.l. | fungicide | 165 |
| Mancozeb (75%), WDG | ASPOR WDG | SUMITOMO CHEMICAL S.r.l. | fungicide | 320 |
| Pyraclostrobin (20%), WG | Cabrio® WG | BASF Agricultural Solution Italia | fungicide | 50 |
| Difeconazole (23.6%), EC | Score® 25 EC | Syngenta Italia S.p.A. | fungicide | 50 |
| Tannins (0.13%), SL | Distillato di legno | BioDea | plant biostimulant | 200 |
| Clay; clinoptilolite-heulandite (67.5%) + mordenitis (32.5%), WP | Zeolite CUBANA Bio® | BioAgrotech S.r.l. | plant biostimulant | 400 |
| Flavonoids (2.00%), SL | Propolis serbios | Serbios S.r.l. | plant biostimulant | 300 |
| Cu (2%) + Zn (4%) + citric acid (23.8%), SL | Dentamet® | DIACHEM S.p.A. | plant biostimulant | 547 |
| Beauveria bassiana ATCC 74040 (7.16%), OD | Naturalis® | BIOGARD® | entomopathogenic fungus | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checchia, I.; Perin, C.; Mori, N.; Mazzon, L. Oviposition Deterrent Activity of Fungicides and Low-Risk Substances for the Integrated Management of the Olive Fruit Fly Bactrocera oleae (Diptera, Tephritidae). Insects 2022, 13, 363. https://doi.org/10.3390/insects13040363
Checchia I, Perin C, Mori N, Mazzon L. Oviposition Deterrent Activity of Fungicides and Low-Risk Substances for the Integrated Management of the Olive Fruit Fly Bactrocera oleae (Diptera, Tephritidae). Insects. 2022; 13(4):363. https://doi.org/10.3390/insects13040363
Chicago/Turabian StyleChecchia, Ilaria, Corrado Perin, Nicola Mori, and Luca Mazzon. 2022. "Oviposition Deterrent Activity of Fungicides and Low-Risk Substances for the Integrated Management of the Olive Fruit Fly Bactrocera oleae (Diptera, Tephritidae)" Insects 13, no. 4: 363. https://doi.org/10.3390/insects13040363
APA StyleChecchia, I., Perin, C., Mori, N., & Mazzon, L. (2022). Oviposition Deterrent Activity of Fungicides and Low-Risk Substances for the Integrated Management of the Olive Fruit Fly Bactrocera oleae (Diptera, Tephritidae). Insects, 13(4), 363. https://doi.org/10.3390/insects13040363







