Simple Summary
Diapause is a dormant period typically controlled by daylength that ensures an insect’s survival through harsh environmental conditions. The convergent lady beetle, Hippodamia convergens, undergoes a reproductive diapause in winter, where female ovaries remain immature and no eggs are laid. This species is an important biological control agent, but during diapause, beetles are less likely to eat pest insects. Thus, knowledge of diapause mechanisms may facilitate manipulation thereof to improve biological control. Further, molecular studies of adult diapause and diapause in Coleoptera are relatively lacking. Here, we assembled and annotated a transcriptome for this species and quantified transcript expression changes during diapause. Female beetles were sampled at three times in diapause (early, mid, and late diapause), which allowed us to characterize the molecular processes occurring at distinct transitions throughout diapause. We found that transcripts involved in flight were consistently upregulated during diapause, which is consistent with dispersal flights at this stage, while transcripts involved in ovarian development were downregulated, which is consistent with the shutdown of reproduction in diapausing females. These findings identify key regulators of diapause in H. convergens and contribute to a growing body of literature on the molecular mechanisms of diapause across the insect phylogeny.
Abstract
Diapause is an alternate development program that synchronizes an insect’s life cycle with seasonally abundant resources and ensures survival in unfavorable conditions. The physiological basis of diapause has been well characterized, but the molecular mechanisms regulating it are still being elucidated. Here, we present a de novo transcriptome and quantify transcript expression during diapause in the convergent lady beetle Hippodamia convergens. H. convergens is used as an augmentative biocontrol agent, and adult females undergo reproductive diapause that is regulated by photoperiod. We sampled females at three stages (early, mid, and late diapause) and compared transcript expression to non-diapausing individuals. Based on principle component analysis, the transcriptomes of diapausing beetles were distinct from non-diapausing beetles, and the three diapausing points tended to cluster together. However, there were still classes of transcripts that differed in expression across distinct phases of diapause. In general, transcripts involved in muscle function and flight were upregulated during diapause, likely to support dispersal flights that occur during diapause, while transcripts involved in ovarian development were downregulated. This information could be used to improve biological control by manipulating diapause. Additionally, our data contribute to a growing understanding of the genetic regulation of diapause across diverse insects.
1. Introduction
Diapause is an alternate development program that synchronizes an insect’s life cycle and improves survival during harsh winter conditions [1,2,3]. Two common characteristics of diapause are arrested development and a suppression of metabolism, and diapause typically happens at a single life stage for a given species [2]. While originally considered a static period with little change, diapause is a dynamic trait that includes distinct phases, with initiation occurring during early diapause (ED), maintenance during mid-diapause (MD), and termination during late diapause (LD) as insects transition to normal development [4]. These distinct eco-physiological phases are supported by recent suborganismal studies, with gene expression studies indicating distinct molecular transitions that occur as diapause progresses [5,6,7,8,9,10]. The ED period generally involves processes for regulating energy storage, upregulating stress response, regulating hormone production, and seeking protection in overwintering sites [11,12,13,14]. During MD, developmental arrest and metabolic suppression are maintained, so processes involved in cell cycle arrest, transcriptional suppression, and energy conservation are often active at this time [15,16,17]. In LD, insects begin progressing towards normal development, and this phase is characterized by an increase in transcription, cellular respiration, metabolism, and hormone signaling [18,19,20]. These physiological hallmarks are typically consistent with changes in gene expression [8], although the molecular regulation of diapause has only been assessed in a select few insect species.
While molecular studies of diapause have increased in recent years [21,22,23,24,25], there is still a lack of consensus on whether conserved elements regulate diapause. For some processes involved in diapause regulation, there appears to be a conserved “toolkit” of genes and pathways across insect orders [26]. For example, insulin and Wnt signaling seem to regulate several aspects of diapause, including developmental and reproductive arrest, extending lifespan, suppressing metabolism, fat hypertrophy and enhancing stress tolerance [27,28]. Canonical circadian rhythm feedback loops are also commonly involved in diapause, likely as a mechanism to sense daylength during diapause induction [29]. Molecular pathways involved in endocrine regulation of diapause, such as ecdysone and/or juvenile hormone signaling, are also commonly observed across diverse diapause programs [1]. Aside from these commonalities, many of the specific molecular processes regulating diapause seem to be unique across species. However, in-depth analysis of the evolutionary physiology of diapause has been hampered by a lack of taxonomic breadth in previous work. The literature has historically been heavily skewed toward Dipterans in pupal diapause; for example, a meta-analysis of diapause transcriptomes included nine Dipteran, one Lepidopteran, and one Hymenopteran species [26]. The accessibility of next generation sequencing has led to a recent increase in diapause transcriptomes in non-Dipteran species, including Hymenoptera, Coleoptera, and Orthoptera [8,30], but several taxa still remain under-sampled.
Here, we assess transcriptomic changes during female overwintering diapause in the convergent lady beetle, Hippodamia convergens (Guérin-Méneville) (Coleoptera, Coccinellidae). Diapause in H. convergens is associated with arrested ovarian development and lipid accumulation in the fat body [31]. Based on studies of five North American populations, photoperiod was found to be the primary cue for triggering hibernal diapause [32]. Females typically complete a single spring generation while feeding heavily on pest insects, mate with males, and then disperse en masse to undergo a winter diapause. Reproductive diapause in winter allows females to conserve energy reserves by avoiding egg-laying. During this overwintering diapause, females need to be mobile enough to find sufficient prey and water resources to ensure their survival until spring [33]. Our study will specifically address the molecular mechanisms of the overwintering diapause that is primarily regulated by photoperiod.
The diapause status of H. convergens also influences its efficacy in biological control programs. H. convergens is commonly released throughout the US to control pest aphids [34,35,36], and in commercial operations diapausing adults are typically collected en masse from overwintering aggregations. When released outside, beetles often show one of the following behaviors: dispersal from the intended field, or limited feeding that prevents effective biological control [36]. Which behavior is observed depends on the diapause state of the aggregations [33], with beetles collected in early-diapause aggregations remaining near the release site but not feeding, while beetles collected in the spring from late-diapause aggregations disperse from the release site prior to feeding. This phenomenon has been a source of frustration for growers, so the ability to manipulate diapause could lead to significant improvements in augmentative biological control. The primary goal of our study is to thoroughly characterize transcriptional changes during diapause in H. convergens to identify the regulatory processes that control diapause in this species. Based on previous molecular studies of adult diapause [2,5,8,9], we predicted a downregulation of transcripts involved in ovarian maturation and egg development, an upregulation of transcripts involved in flight and locomotion due to the dispersal flight of the beetles, and differential regulation of insulin signaling transcripts, a common metabolic regulator during diapause. Together, these results may contribute to efforts for improving biological control by providing molecular biomarkers for distinct diapause phases and/or targets for disrupting diapause.
2. Materials and Methods
2.1. Study Animals
Adult H. convergens, collected in Riley County, Kansas 39.19° N, 96.57° W in June 2016, were paired in 0.24 L (8 oz.) paper containers (Choice® brand, Webstaurant Store, Lancaster, PA, USA) covered with organdy mesh. Beetles were maintained at 22 ± 1 °C with a photoperiod of 16:8 L:D and provided water, a Wheast-honey mixture (Wheast is from GreenMethods.com, Beneficial Insectary, Redding, CA, USA), and a daily supply of pea aphids, Acyrthosiphon pisum (Harris) (Hemiptera, Aphididae). Egg masses were collected daily from one female and systematically placed in L:D 16:8 or 10:14, at 22 °C ± 1.0 °C. These F1 offspring were individually reared in glass vials at each photoperiod on A. pisum and frozen Ephestia kuehniella (Zeller) (Lepidoptera, Pyralidae) eggs (Beneficial Insectary, Redding, CA, USA).
After eclosion, F1 adults were mated and maintained in 0.24 L paper containers at the same photoperiods, and provided water, Wheast-honey mixture, and a daily supply of pea aphids. The date of first oviposition at L:D 16:8 was recorded, and ovipositing females at L:D 16:8 were flash frozen in liquid nitrogen between 1 and 7 days after the first oviposition and comprised the non-diapause (ND) group. Females at L:D 10:14 that did not oviposit (presumably because they were in reproductive diapause) were frozen at three time periods after female eclosion. Early diapause (ED) females were frozen 7–10 days post-eclosion. This time period is when ND females began laying eggs. Mid-diapause (MD) females were frozen 16–20 days post-eclosion. Late diapause (LD) females were frozen 27–28 days post-eclosion. One female in the late-diapause group (LD-4) was ovipositing at the time of sampling and this sample was removed from subsequent analyses (see below).
2.2. RNA Extraction, cDNA Library Preparation, and Sequencing
Each replicate consisted of total body mRNA from a single female, and four independent replicates were sequenced per group (N = 16 RNA-seq libraries in total). RNA was extracted from female beetles using the Direct-Zol RNA Miniprep Kit (Zymo Research, Irvine, CA, USA) according to manufacturer’s instructions. RNA purity and quantity were assessed with UV-VIS spectroscopy, and RNA integrity was measured on a Bioanalyzer (Agilent, Santa Clara, CA, USA). RNA samples were sent to Macrogen (currently Psomagen, Rockville, MD, USA) for mRNA sequencing, and mRNA-seq libraries were sequenced on an Illumina HiSeq 2500 (software version HCS v2.2.38, Illumina, San Diego, CA, USA) to produce 100 bp paired end reads. The number of reads per sample ranged from 33 million to 49 million for a total of 636,203,876 million paired end reads. Each sample was split into left and right read libraries for a total of 32 sequence files. Raw data and the assembled transcriptome from Trinity are available through NCBI, Bioproject # PRJNA753921.
2.3. De novo Transcriptome Assembly and Annotation
Before assembly, we analyzed sequence quality with FastQC on Galaxy (Galaxy Version 0.72+galaxy1) [37]. Adapter sequences were removed by the sequencing company prior to transcriptome assembly, and FastQC analysis indicated that there were no issues with per sequence quality scores, sequence duplication scores, or overrepresented sequences. These results indicated that no further quality trimming was necessary. De novo assembly was conducted with the default settings of Trinity v2.4.0 [38,39] on the University of Kentucky Lipscomb (DLX) supercomputer (accession number PRJNA753921). We compared the completeness of the assembled transcriptome to a database of arthropod Benchmark Universal Single Copy Orthologs (BUSCO) using BUSCO v3.0.2 [40]. Transcripts were annotated with Trinotate using BLASTx and BLASTp with an e-value threshold of 1 × 10−3 [41]. We also used Trinotate to annotate Gene Ontology (GO) terms [42], Kyoto Encyclopedia of Genes and Genomes (KEGG) terms [43], and protein family (Pfam) domains [44].
2.4. Differential Transcipt Expression Analysis
To filter assembly artifacts and poorly supported transcripts, original sequences were mapped back onto the Trinity assembly with Bowtie 2 v2.4.2 [45,46] and reassembled with Cufflinks v2.2.1 [47]. The Cufflinks pipeline reduces the number of transcriptional artifacts, misassembled transcripts, and poorly supported transcripts and increases the statistical support for each remaining transcript [48]. We then quantified transcript abundance for all 16 samples with HTSeq v0.12.4 [49]. Using principal component analysis (PCA) to cluster samples, we identified two outlying samples: MD-2 and LD-4. LD-4 was ovipositing at the time of sampling, which resulted in LD-4 clustering with the nondiapause samples, so we elected to remove it from downstream analyses (see Section 3). A PCA score plot indicated that MD-2 was clearly an outlying sample with likely technical issues, so this sample was also removed from the analyses.
To test for differential transcript expression, we used DESeq2 v1.28.1 on R v.4.0.2 [50,51]. We compared transcript expression between distinct stages of diapause, and the primary comparisons we focused on were ED vs. ND, MD vs. ND, and LD vs. ND. To confirm the results found in these pair-wise comparisons, we also compared All Diapause vs. ND, MD+LD+ND vs. ED, ED+LD+ND vs. MD, and ED+MD+ND vs. LD (Supplementary Table S1–S3). Two additional comparisons were made between MD vs. ED and MD vs. LD, to determine how mid- and late diapause differed from the previous diapausing stage. All comparisons were done with alpha = 0.05 after Benjamini-Hochberg correction. To identify biological processes involved in diapause, we performed GO enrichment analysis on differentially expressed transcripts using goseq [52], assuming a Wallenius distribution. A Benjamini-Hochberg correction was applied to raw p-values to determine GO terms that were significantly enriched. We used REVIGO with the stringency set to “Medium” to generate a list of nonredundant GO terms [53] and visualize the results. KEGG pathway enrichment was performed with the Generally Applicable Gene-set Enrichment (GAGE) and Pathview packages [54,55]. We accepted pathways as significant if the FDR-adjusted p-value was < 0.1.
3. Results
3.1. Transcriptome Assembly and Annotation
We assembled 636 million paired end, 100-bp reads from 16 libraries into a de novo reference transcriptome (see Table 1 for details).

Table 1.
De novo assembly and annotation statistics. 636 million paired-end reads were assembled into 238,614 contigs with Trinity. BUSCOs were compared with all other available Arthropod transcriptomes. BLAST, GO, KEGG, and Pfam domains were annotated using Trinotate.
Our assembly contained 96.2% complete BUSCOs when compared to the arthropod database, which is a high degree of completeness compared to other recent de novo insect transcriptomes [48,56,57]. The assembly produced 238,614 contigs with a mean contig length of 836 bp, a median contig length of 441 bp, an N50 of 1442 bp, and a GC% of 37.1%. The Trinotate annotation rates were as follows: 31.8% of contigs contained BLAST hits, 29.38% with GO descriptions, 23.9% with KEGG IDs, and 21.93% with Pfam domain(s) (Table 1).
3.2. Transcript Expression Changes in Early Diapause
The PCA showed that the ED, MD, and LD samples cluster together with the ND samples forming their own cluster (Figure 1).
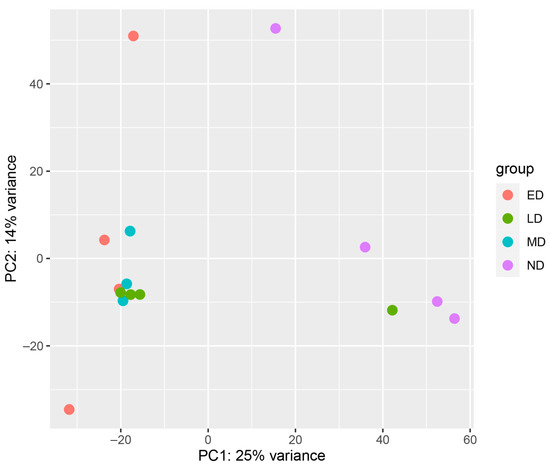
Figure 1.
Principle Component Analysis (PCA) score plots of samples. This PCA was used to determine the relationship between transcript expression profiles from various stages of diapause. One of the mid-diapause samples was removed from the dataset prior to this analysis due to technical problems with the sample, so there are only three replicates for this group. One LD sample clustered with the ND samples, and that female had already broken diapause and started ovipositing at the time of sample collection. Thus, this sample was removed from further analyses. ED = early diapause, LD = late diapause, MD = mid-diapause, ND = non-diapause.
To identify transcript expression changes associated with early diapause, we compared the ND and ED groups. In this comparison, there were 1620 upregulated and 2409 downregulated transcripts at FDR < 0.05 (Figure 2a, Supplementary Table S1).
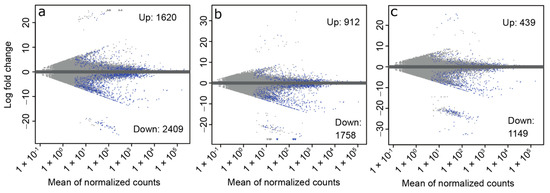
Figure 2.
MA plots showing the number of differentially expressed transcripts between each diapausing stage and non-diapause beetles. For each comparison ((a): ED vs. ND; (b): MD vs. ND; (c): LD vs. ND), transcripts with FDR < 0.05 are shown in blue. In each panel the total number of up- and downregulated transcripts are indicated. For the full lists of differentially expressed transcripts, see Table S1. ED = early diapause, MD = mid-diapause, LD = late diapause, ND = non-diapause.
GO enrichment analysis indicated a total of 106 enriched GO terms among upregulated transcripts and 116 enriched terms at p-value < 0.1 among downregulated transcripts. After clustering semantically redundant terms with REVIGO, most of the enriched BP GO terms in the upregulated transcripts were involved in locomotion (Table 2). For downregulated transcripts, several of the enriched BP GO terms reflect a reduction in protein synthesis and cell division (Table 2). We also ran a contrast to compare gene expression between ED and all the other groups (ND, MD, and LD). Among the genes downregulated in ED relative to all other groups, GO terms related to cell cycle and cell division were similarly enriched (Supplementary Table S2).

Table 2.
Summary of the GO biological processes that are involved in ED. The table shows GO terms that are significantly enriched among transcripts that are either up- or downregulated during ED relative to ND. The top five up and down enriched GO terms for this comparison are shown. Redundant GO terms were removed using semantic clustering with REVIGO. GO terms that are enriched in the MD vs. ED comparison are shown in Table 3, and a complete list of enriched GO terms can be found in Supplementary Table S2.
Between ED and ND, there were 32 significantly upregulated and 19 significantly downregulated KEGG terms at p-value < 0.1, and the significantly upregulated and downregulated KEGG term results were qualitatively similar to the enriched GO terms. The top five upregulated KEGG pathways (based on p-value) in ED vs. ND were oxidative phosphorylation, cardiac muscle contraction, retrograde endocannabinoid signaling, microbial metabolism in diverse environments, and cGMP-PKG signaling pathway (Table 3). The top five downregulated KEGG pathways in ED vs. ND were RNA transport, cell cycle, DNA replication, ubiquitin mediated proteolysis, and RNA degradation (Table 3). Similar KEGG terms were found when we compared MD+LD+ND vs. ED (Supplementary Table S3).

Table 3.
Summary of the top five (when available) KEGG pathways involved in ED. The table shows KEGG pathways that were up- and downregulated relative to ED. Enriched KEGG pathways relative to ND were identified with the GAGE and Pathview packages in R. Terms were sorted by the Benjamini-Hochberg adjusted p-value. KEGG pathways differentially expressed relative to MD are shown in Table 5, and a complete list of up- and downregulated KEGG terms can be found in Supplementary Table S3.
3.3. Transcript Expression Changes in Mid-Diapause
Relative to ND samples, there were 912 significantly upregulated and 1758 significantly downregulated transcripts in the MD group relative to ND at FDR < 0.05 (Figure 2b). GO enrichment analysis indicated 83 enriched terms among the upregulated transcripts and 73 enriched terms among the downregulated transcripts. Prominent terms present after REVIGO reduction of GO terms among the upregulated transcripts include muscle contraction, flight, and locomotion (Table 4). Terms of interest among the downregulated transcripts after REVIGO reduction included cell division and cell cycle (Table 4). The list of GO terms was expanded when we compared MD to all other groups simultaneously (ND, ED, and LD) (Supplementary Table S2). Protein refolding and regulation of translational initiation by eIF2 alpha phosphorylation were upregulated in MD vs. All Diapause. Negative regulation of stress-activated MAPK cascade, glutaminyl-tRNA aminoacylation, and receptor-mediated endocytosis were downregulated in MD vs. All Diapause.

Table 4.
Summary of the GO biological processes that are involved in MD. The table shows GO terms that are significantly enriched in differentially expressed transcripts from the MD vs. ND, MD vs. ED, and MD vs. LD comparisons. The top five enriched GO terms among up- and downregulated transcripts for these comparisons are shown. Redundant GO terms were removed using semantic clustering with REVIGO. A complete list of enriched GO terms can be found in Supplementary Table S2.
There were 10 enriched KEGG pathways at p-value < 0.1 in the upregulated transcripts. The top five were cardiac muscle contraction, cGMP-PKG signaling pathway, calcium signaling pathway, retrograde endocannabinoid signaling, and phototransduction—fly (Table 5). There were 5 enriched KEGG pathways at p-value < 0.1 in the downregulated transcripts: cell cycle, ubiquitin mediated proteolysis, homologous recombination, DNA replication and RNA transport (Table 5). The list of significantly downregulated KEGG terms was expanded when we compared MD to the three other groups simultaneously in a planned contrast (Supplementary Table S3, All vs. MD Up OR MD vs. All Down tab). The top five downregulated KEGG terms in this comparison were carbon metabolism, starch and sucrose metabolism, cysteine and methionine metabolism, valine, leucine, and isoleucine degradation, and sphingolipid metabolism.

Table 5.
Summary of the top five (when available) KEGG pathways involved in MD. The table shows KEGG pathways that were up- or downregulated relative to ND, ED, and LD. Enriched KEGG pathways were identified with the GAGE and Pathview packages in R. Terms were sorted by the Benjamini-Hochberg adjusted p-value. A complete list of up- and downregulated KEGG terms can be found in Supplementary Table S3.
To further characterize processes involved in mid-diapause, we also compared ED vs. MD (Figure S1). Relative to ED, there were 556 upregulated and 210 downregulated transcripts in the MD group at FDR < 0.05 (Supplementary Table S1). GO enrichment analysis indicated 53 enriched terms among the upregulated transcripts and 2 enriched terms among downregulated transcripts (Supplementary Table S2, Table 4). A few examples of the upregulated BP terms after REVIGO reduction include polysaccharide catabolic process, lipid transport, and lipid biosynthetic process (Table 4). Only flavin adenine dinucleotide binding and structural constituent of ribosome, which are both MF terms, were significantly enriched in the downregulated transcripts (Supplementary Table S2). There were 18 significantly upregulated and 3 significantly downregulated KEGG terms at p-value < 0.1. The top 5 upregulated KEGG pathways include biosynthesis of secondary metabolites, starch and sucrose metabolism, oxidative phosphorylation, ABC transporters, and galactose metabolism (Table 5). The three downregulated KEGG pathways are ribosome, RNA transport and cell cycle (Table 5).
3.4. Transcript Expression Changes Occurring in Late Diapause
Relative to the ND group, 439 significantly upregulated and 1149 significantly downregulated differentially expressed transcripts at FDR < 0.05 (Figure 2c, Supplementary Table S1). There were 36 significantly enriched GO terms among the upregulated transcripts. Terms of interest remaining after REVIGO reduction included locomotion, protein refolding, protein folding chaperone, and misfolded protein binding (Table 6). There were 18 significantly enriched GO terms among the downregulated transcripts (Table 6). Terms of interest remaining after REVIGO reduction included chitin catabolic process, lipid transporter activity, and nutrient reservoir activity. Chitin catabolic process, among others, were also found when we compared LD against all other groups with a planned contrast (Supplementary Table S2).

Table 6.
Summary of the GO biological processes that are involved in LD. The table shows GO terms that are significantly enriched among transcripts that are either up- or downregulated during LD relative to ND. The top five up and down enriched GO terms for this comparison are shown. Redundant GO terms were removed using semantic clustering with REVIGO. GO terms that are enriched in the MD vs. LD comparison are shown in Table 4, and a complete list of enriched GO terms can be found in Supplementary Table S2.
There were no significantly enriched KEGG pathways at p-value < 0.1 in the upregulated transcripts. There were ten significantly enriched KEGG pathways in the upregulated transcripts when we compared LD vs. all other groups at p-value < 0.1. The top five were ribosome, ribosome biogenesis in eukaryotes, spliceosome, DNA replication, and RNA transport (Supplementary Table S3). In the transcripts downregulated in LD relative to ND, there was one significantly enriched KEGG pathway at p-value < 0.1, protein processing in endoplasmic reticulum (Table 7). There were 14 significantly enriched KEGG pathways in the downregulated transcripts when we compared LD vs. all other groups (Supplementary Table S3). The top five were oxidative phosphorylation, lysosome, phagosome, protein processing in endoplasmic reticulum, and AMPK signaling pathway.

Table 7.
Summary of the top five (when available) KEGG pathways involved in LD. The table shows pathways up- and downregulated relative to ND. Enriched KEGG pathways were identified with the GAGE and Pathview packages in R. Terms were sorted by the Benjamini-Hochberg adjusted p-value. Pathways differentially expressed relative to MD are shown in Table 5, and a complete list of up- and downregulated KEGG terms can be found in Supplementary Table S3.
Relative to the MD group, there were 451 upregulated and 411 downregulated differentially expressed transcripts in LD at FDR < 0.05 (Figure S2). There were no significantly enriched GO terms among the up- or downregulated transcripts, and thus no REVIGO reduction was performed. There was one significantly upregulated KEGG term, oxidative phosphorylation, and zero significantly downregulated KEGG terms at p-value < 0.1 (Table 5).
4. Discussion
Insects enter diapause in advance of unfavorable environmental conditions, often under control of seasonal cues such as changes in photoperiod, humidity, and temperature [4]. Diapause allows insects to synchronize their life cycle with seasonally available resources and survive stressful winter environments [4]. The precise physiological and molecular processes during diapause depend on the life stage in which diapause occurs and the species of interest. Here, we assessed transcript expression changes during diapause in the convergent lady beetle, H. convergens, an important biological control agent with an adult reproductive diapause. Our results will be discussed in relation to the phases of diapause, and we will address both conserved processes and novel diapause-associated genes that have not been previously implicated. Identifying putative regulatory transcripts that are expressed at specific points in diapause can generate potential targets for diapause manipulation in future studies.
4.1. Changes Occurring in Early and Mid-Diapause
The transcripts upregulated in ED vs. ND and MD vs. ED were enriched for GO terms involved in the cytoskeleton, as well as related terms involved in sarcomere organization, muscle contraction, flight, and locomotion. Upregulation of these transcripts may be connected to two physiological hallmarks of diapause in H. convergens: flight dispersal during early diapause [36] and cytoskeletal reorganization to protect against cold-induced disruption of cell structure. Hippodamia convergens disperse from feeding fields at the end of the spring to overwinter in the Sierra Nevada Mountains of California [36]. Upregulation of cytoskeletal genes has been documented in another species, Tetranychus urticae (Trombidiformes, Tetranychidae), that has similar migratory behavior during its reproductive diapause [24]. Specific transcripts upregulated relative to ND in H. convergens included components of muscle filaments such as titin (GJNK01217174, log2FC = 1.18, p = 2.84 × 10−4, where the accession number is for the NCBI nucleotide database, “log2FC” is the log2 fold change, and the p-value is the FDR-adjusted p-value), myosin (both heavy [GJNK01010876, log2FC = 1.18, p = 5.59 × 10−3] and light chains [GJNK01120126, log2FC = 1.67, p = 1.46 × 10−3]), troponin (GJNK01183047, log2FC = 1.32, p = 1.08 × 10−2) and tropomyosin (GJNK01150561, log2FC = 1.27, p = 4.81 × 10−3). Additional muscle-related transcripts upregulated during diapause include muscle LIM protein (GJNK01162820, log2FC = 1.42, p = 4.78 × 10−3), which includes a zinc-binding motif that regulates cytoarchitecture and signal transduction for gene expression, and muscle-specific protein 20 mlp20 (GJNK01192324, log2FC = 1.23, p = 1.19 × 10−2), a calponin-like actin binding domain that cross-links actin filaments and titin molecules in the Z-disc [58,59,60]. In addition, nearly all of these genes were also upregulated in ED relative to all other groups in a planned contrast (Supplementary Table S1), indicating that they are specific to ED. In addition to supporting increased flight activity during diapause, cytoskeletal genes are also linked to increased cold tolerance during diapause by increasing the structural stability of cells to enhance survival at low temperatures [24,48,61,62,63,64,65,66].
While metabolic suppression is often a feature of diapause, we observed upregulation of oxidative phosphorylation transcripts during ED and MD (seen after KEGG enrichment analysis, Table 3 and Table 5). Diapause is typically accompanied by a decrease in metabolism and therefore a downregulation of oxidative phosphorylation to conserve energy for post-diapause processes such as metamorphosis or migratory flight [17]. However, H. convergens actively flies during its reproductive diapause and therefore has high energy demands. Thus, increased expression of oxidative phosphorylation transcripts may be required to meet the metabolic demands of the dispersal flight. However, it is worth noting that a similar pattern of oxidative phosphorylation gene expression was observed in the Colorado potato beetle which spends diapause underground [67]. Therefore, these changes in transcript expression may be unrelated to flight and instead a general feature of adult reproductive diapause in Coleoptera.
GO and KEGG terms related to the cell cycle were significantly downregulated in both the ED vs. ND and MD vs. ED comparisons. This downregulation of cell cycle transcripts is consistent with previous observations that cell division is reduced or stopped during diapause [15,16,68,69,70]. A recent study by Shimizu et al. (2018) showed clear evidence that pupal diapause in Nasonia vitripennis (Hymenoptera, Pteromalidae) caused the disappearance of the S fraction of the cell cycle and a high proportion (80%) of cells arrested in the G0/G1 phase. In H. convergens, the cell cycle arrest likely reflects the halt in ovarian development during reproductive diapause to prevent egg maturation. We also observed many core ribosomal protein transcripts downregulated in ED relative to ND, including 60S ribosomal protein L32 (GJNK01022121, log2FC = −23.21, p = 2.58 × 10−12) and 60S ribosomal protein L21 (GJNK01130154, log2FC = −22.24, p = 2.57 × 10−11), and both of these were also downregulated in ED relative to all other stages in a planned contrast. When comparing ED to all other stages, 40S ribosomal protein S2 (GJNK01061680, log2FC = −0.43, p = 6.12 × 10−3), was also downregulated, and this gene has previously been linked to slowed and halted cell growth [71]. Diapause in the lady beetle Coccinella septempunctata (Coleoptera, Coccinellidae) also appears to be accompanied by cell cycle arrest, as several genes involved in cell cycle are downregulated during diapause termination [22]. In particular, DNA replication licensing factor MCM6 was downregulated in C. septempunctata, and while this specific gene was not downregulated in H. convergens, the licensing factor MCM3 (GJNK01201780, log2FC = −0.70, p = 3.57 × 10−2), which is a part of the MCM2-7 complex along with MCM6, was downregulated in MD relative to ND. This complex is essential for DNA replication and the cell cycle [72], so downregulation of members of this complex likely prevents cell division. Cyclin A/B, which activates cyclin-dependent kinases at various points in the cell cycle to progress cell division [73,74] is also downregulated during diapause in C. septempunctata [22]. While this specific gene wasn’t downregulated in our study, Cyclin-A1, another A-type cyclin that contributes to the G1 to S cell progression [75], was downregulated at all three diapause time points relative to ND.
During normal development in many insect species, juvenile hormone (JH) induces vitellogenesis and ovarian maturation [76,77]. Indeed, several transcripts encoding vitellogenin-1, one of the main yolk proteins, were strongly downregulated during ED and MD, which is consistent of a shutdown in ovarian maturation. Transcript expression data also indicated an involvement of juvenile hormone in this ovarian arrest. Juvenile hormone esterases can control the JH titer by hydrolyzing JH and leading to its degradation [78]. In H. convergens, juvenile hormone esterase (JHE) had lower expression in LD compared to MD (GJNK01138836, log2FC = −24.70, p = 4.57 × 10−16), which is consistent with JH signaling being activated during LD as the beetles resume reproduction. Thus, low expression of JHE during late diapause likely facilitates termination of ovarian arrest, and this pattern of JHE was also observed during the reproductive diapause of Galeruca daurica (Coleoptera, Chrysomelidae) [30].
In many insects, JH mediated vitellogenesis is regulated upstream by insulin signaling [79,80]. Culex pipiens (Diptera, Culicidae), which has perhaps the best characterized adult diapause, shuts down insulin signaling during diapause which in turn suppresses JH synthesis and leads to the activation of FOXO, a transcription factor involved in fat hypertrophy and stress tolerance [77]. The suppression of JH synthesis in turn prevents ovarian maturation. Because diapause in H. convergens is accompanied by arrested ovarian development, we predicted that diapause would result in downregulation of transcripts involved in both insulin and JH signaling [27]. Indeed, insulin-like growth factor-binding protein (IGFBP) complex acid labile subunit is downregulated during MD relative to both ND (GJNK01122314, log2FC = −7.61, p = 2.42 × 10−2) and all other groups (log2FC = −8.06, p = 5.2 × 10−3). IGFBPs bind to members of the insulin superfamily, which include insulin-related peptides (IRPs) and insulin-like peptides (ILPs), and they positively regulate ecdysteroidogenesis, JH synthesis, and vitellogenesis [79,80]. Thus, downregulation of this transcript in MD is consistent with suppression of ovarian development and vitellogenesis in H. convergens. We did observe downregulation of another insulin-related transcript, insulin-like peptide receptor, during ED and LD relative to ND (GJNK01203393, log2FC = −0.83, p = 5.10 × 10−3 for ED; log2FC = −0.73, p = 1.57 × 10−2 for LD). In mosquitoes, suppression of insulin signaling leads to an upregulation of FOXO to promote fat hypertrophy. In the mosquito C. pipiens, forkhead box protein O3 (FOXO3) is upregulated in diapause [77], and consistent with this model FOXO3 is upregulated during diapause in the lady beetle C. semptempunctata, which likely results in fat hypertrophy observed in this species [22]. However, we did not see any evidence of FOXO being involved in diapause in H. convergens.
While the above results indicate a possible role for insulin and JH in H. convergens diapause, many transcripts involved in insulin and JH signaling did not change expression during diapause. One possible reason is that we only sampled adults. Although we do not have direct evidence for H. convergens, a study on the closely related Hippodamia variegata (Coleoptera, Coccinellidae) determined that the photosensitive stage is the pupal stage [81], so if insulin and/or JH are involved in diapause induction, expression may have changed prior to our sampling interval. Further, we also sampled whole bodies, instead of tissues directly related to insulin-signaling such as the fat body and the brain, which could have prevented us from observing expression changes in these transcripts [67,82]. Likely, for these same reasons, we didn’t detect any evidence of circadian clock genes being involved in H. convergens diapause, despite their prominent role in other species [29].
Our results point to the cGMP-Protein Kinase G (PKG) pathway as a potential novel regulator of diapause (Table 3 and Table 5). This KEGG pathway for cGMP-PKG was upregulated in both ED and MD relative to ND, and this pathway has established roles in increasing survival during anoxic stress [83], as well as being a key pathway for associative learning and memory [84] and food search behavior [85]. A proteomics analysis linked cGMP-PKG signaling to pupal diapause termination in the fly Bactrocera minax (Diptera, Tephritidae), although there was no evidence of transcriptional regulation of this pathway [86]. Because signaling pathways like cGMP-PKG are often primarily regulated at the post-translational level through reversible protein modifications, it’s difficult to conclude a definitive role for this pathway in reproductive diapause in H. convergens. However, coordinated changes in transcript expression across this pathway suggest this pathway are consistent with cGMP-PKG being involved in diapause regulation.
4.2. Changes Occurring in Late Diapause
In contrast to the other comparisons, many of the transcripts involved in LD were not annotated and thus have unclear function. This result could suggest that processes occurring in LD are more species-specific and less conserved than other diapause time points. As discussed above, we did see evidence of juvenile hormone involvement in LD, as juvenile hormone esterase (GJNK01138836) was downregulated in late diapause, presumably to facilitate accumulation of JH as oogenesis resumes. However, for the most part, transcripts involved in LD were sparsely annotated, which was also reflected by lack of enriched GO and KEGG terms at this time point.
One somewhat surprising result is that we observed strong upregulation of hsp68 in LD relative to ND (GJNK01155541, log2FC = 1.22, p = 2.48 × 10−9), and this transcript was also upregulated in LD relative to all other time points. Heat shock proteins (HSPs) are often upregulated in direct response to abiotic stress, but during diapause there is often anticipatory upregulation of HSPs that occurs before any environmental stress occurs [87]. However, while a role for HSPs in diapause is well-established, the specific HSP genes involved in diapause varies considerably across species [88,89,90,91]. While we expected HSP expression to return to baseline in LD, at this time H. convergens leave their aggregations and may still experience periodic low temperatures. Thus, we speculate that upregulation in hsp68 may be needed to withstand environmental stress as beetles disperse from aggregations, and indeed, this gene is known to be important for surviving low temperatures [92]. It is worth noting that different HSP transcripts were upregulated in ED (e.g., hsc70 [GJNK01113255, log2FC = 8.32, p = 1.10 × 10−2], hsp70 A2 [GJNK01189679, log2FC = 2.12, p = 3.88 × 10−2] and small heat shock protein C1 [GJNK01016550, log2FC = 1.17, p = 9.69 × 10−4]), but taken together, our results are consistent with previous observations that HSP expression patterns during diapause tend to be species- and stage-specific.
4.3. Practical Applications and Future Directions
Diapause complicates the use of H. convergens as a biocontrol agent because females have different feeding behaviors based on their diapause phase. Further, the diapause trajectory can vary across populations, across individual aggregations, and even across beetles within a single aggregation [32]. The information provided in this study will permit in-depth characterizations of diapause variation in future work and may also be used to manipulate diapause for applied purposes.
We envision two possible benefits of diapause manipulation in H. convergens: extending the shelf life of stored beetles and breaking diapause so that beetles continue to eat pest insects. In C. septempunctata, diapausing females have a lifespan of more than 220 days, compared to approximately 60 days in non-diapausing beetles. Thus, the ability to artificially promote diapause and/or prevent diapause termination could improve the long-term storage capacity of beetles. Conversely, the ability to break diapause could lead to increased predation at times of year when beetles are normally dormant, and our results could be used to develop transcriptional biomarkers for distinct phases of diapause to determine the developmental stage of beetles collected in the field. For example, there are several genes with distinct expression patterns in MD that could be used to assess whether a population of beetles is in the maintenance phase of diapause. Some of these genes include nucleosome assembly protein 1-like 4, which is downregulated in MD relative to ND and all other groups (GJNK01191961, log2FC = −1.86, p = 8.68 × 10−8 vs. ND; log2FC = −1.27, p = 4.35 × 10−5 vs. all other groups) and divergent protein kinase domain 1C, which is upregulated in MD relative to ND and all other groups (GJNK01027417, log2FC = 2.80, p = 5.04 × 10−4 vs. ND; log2FC = 1.94, p = 7.42 × 10−3 vs. all other groups). This idea has been previously proposed for C. septempunctata [22], which has similar reproductive diapause as H. convergens, although to our knowledge, these ideas have not been put into practice. Diapause manipulation using molecular means has been successfully achieved for some pest insects, including the red palm weevil [93] and corn earworm [94], and similar strategies could be applied to beneficial insects. However, these strategies require a thorough understanding of the molecular regulation of diapause, and our study provides essential information on the processes regulating distinct phases of diapause in H. convergens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13040343/s1, Table S1: all differentially expressed genes for all comparisons; Table S2: all GO terms for all comparisons; Table S3: all KEGG terms for all comparisons, Figure S1: early diapause versus mid-diapause, Figure S2: mid-diapause versus late diapause, Table S1–S3: compared All Diapause vs. ND, MD+LD+ND vs. ED, ED+LD+ND vs. MD, and ED+MD+ND vs. LD.
Author Contributions
Conceptualization, J.J.O. and N.M.T.; methodology, J.J.O., N.M.T. and E.A.W.N.; software, E.A.W.N. and M.C.L.; validation, E.A.W.N.; formal analysis, E.A.W.N. and M.C.L.; investigation, J.J.O. and N.M.T.; resources, J.J.O. and N.M.T.; data curation, E.A.W.N.; writing—original draft preparation, E.A.W.N.; writing—review and editing, E.A.W.N. and N.M.T.; visualization, E.A.W.N. and M.C.L.; supervision, N.M.T.; project administration, N.M.T.; funding acquisition, J.J.O. and N.M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hatch Project 1010996 from the USDA National Institute of Food and Agriculture to N.M.T and a University of Kentucky College of Food, Agriculture and Environment Research Activity Award to J.J.O.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available at https://www.ncbi.nlm.nih.gov/bioproject/753921 under accession number PRJNA753921. Data were published on 11 August 2021.
Acknowledgments
The authors would like to acknowledge Dave Awde, for his support in R and J.R. Nechols, Kansas State University, for assistance collecting beetles in Riley County, KS, USA.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ragland, G.J.; Armbruster, P.A.; Meuti, M.E. Evolutionary and Functional Genetics of Insect Diapause: A Call for Greater Integration. Curr. Opin. Insect Sci. 2019, 36, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L. Regulation of Diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L.; Joplin, K.H.; Flannagan, R.D.; Tammariello, S.P.; Zhang, M.-L.; Yocum, G.D.; Lee, K.-Y. Diapause-Specific Gene Expression. In Molecular Mechanisms of Insect Metamorphosis and Diapause; Suzuki, A., Kataoka, H., Matsumoto, S., Eds.; Industrial Publishing: Tokyo, Japan, 1995; pp. 289–297. [Google Scholar]
- Koštál, V. Eco-Physiological Phases of Insect Diapause. J. Insect Physiol. 2006, 52, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cui, D.N.; Ullah, H.; Li, S.; Pan, F.; Xu, C.M.; Tu, X.B.; Zhang, Z.H. The Function of Lmprx6 in Diapause Regulation in Locusta migratoria through the Insulin Signaling Pathway. Insects 2020, 11, 763. [Google Scholar] [CrossRef] [PubMed]
- Jarwar, A.R.; Hao, K.; Bitume, E.V.; Ullah, H.; Cui, D.N.; Nong, X.Q.; Wang, G.J.; Tu, X.B.; Zhang, Z.H. Comparative Transcriptomic Analysis Reveals Molecular Profiles of Central Nervous System in Maternal Diapause Induction of Locusta migratoria. G3 Genes Genom. Genet. 2019, 9, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, P.; Zhu, L.B.; You, L.L.; Cao, H.H.; Wang, J.; Zhang, S.Z.; Liu, M.H.; Toufeeq, S.; Huang, S.J.; et al. DNA Methylation Is Correlated with Gene Expression During Diapause Termination of Early Embryonic Development in the Silkworm (Bombyx mori). Int. J. Mol. Sci. 2020, 21, 671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, W.M.; Zhang, J.J.; Zou, Z.; Ruan, C.C. High-Throughput Profiling of Diapause Regulated Genes from Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae). BMC Genom. 2020, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ragland, G.J.; Denlinger, D.L.; Hahn, D.A. Mechanisms of Suspended Animation Are Revealed by Transcript Profiling of Diapause in the Flesh Fly. Proc. Natl. Acad. Sci. USA 2010, 107, 14909–14914. [Google Scholar] [CrossRef] [PubMed]
- Ragland, G.J.; Egan, S.P.; Feder, J.L.; Berlocher, S.H.; Hahn, D.A. Developmental Trajectories of Gene Expression Reveal Candidates for Diapause Termination: A Key Life-History Transition in the Apple Maggot Fly Rhagoletis pomonella. J. Exp. Biol. 2011, 214, 3948–3959. [Google Scholar] [CrossRef] [PubMed]
- Connett, R.J. Analysis of Metabolic Control—New Insights Using Scaled Creatine-Kinase Model. Am. J. Physiol. 1988, 254, R949–R959. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Metabolic-Rate Depression and Biochemical Adaptation in Anaerobiosis, Hibernation and Estivation. Q. Rev. Biol. 1990, 65, 145–174. [Google Scholar] [CrossRef]
- Xue, F.S.; Spieth, H.R.; Aiqing, L.; Ai, H. The Role of Photoperiod and Temperature in Determination of Summer and Winter Diapause in the Cabbage Beetle, Colaphellus bowringi (Coleoptera: Chrysomelidae). J. Insect Physiol. 2002, 48, 279–286. [Google Scholar] [CrossRef]
- Hodkova, M.; Berková, P.; Zahradníčková, H. Photoperiodic Regulation of the Phospholipid Molecular Species Composition in Thoracic Muscles and Fat Body of Pyrrhocoris apterus (Heteroptera) Via an Endocrine Gland, Corpus Allatum. J. Insect Physiol. 2002, 48, 1009–1019. [Google Scholar] [CrossRef]
- Nakagaki, M.; Takei, R.; Nagashima, E.; Yaginuma, T. Cell-Cycles in Embryos of the Silkworm, Bombyx mori—G2-Arrest at Diapause Stage. Roux Arch. Dev. Biol. 1991, 200, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Tammariello, S.P.; Denlinger, D.L. G0/G1 Cell Cycle Arrest in the Brain of Sarcophaga crassipalpis During Pupal Diapause and the Expression Pattern of the Cell Cycle Regulator, Proliferating Cell Nuclear Antigen. Insect Biochem. Mol. Biol. 1998, 28, 83–89. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Energetics of Insect Diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef]
- Hodek, I. Diapause Development, Diapause Termination and the End of Diapause. Eur. J. Entomol. 1996, 93, 475–487. [Google Scholar]
- Masaki, S. Summer Diapause. Annu. Rev. Entomol. 1980, 25, 1–25. [Google Scholar] [CrossRef]
- Clegg, J.S.; Drinkwater, L.; Sorgeloos, P. The Metabolic Status of Diapause Embryos of Artemia franciscana (Sfb). Physiol. Zool. 1996, 69, 49–66. [Google Scholar] [CrossRef]
- Kučerová, L.; Kubrak, O.I.; Bengtsson, J.M.; Strnad, H.; Nylin, S.; Theopold, U.; Nässel, D.R. Slowed Aging During Reproductive Dormancy Is Reflected in Genome-Wide Transcriptome Changes in Drosophila melanogaster. BMC Genom. 2016, 17, 50. [Google Scholar] [CrossRef]
- Qi, X.Y.; Zhang, L.S.; Han, Y.H.; Ren, X.Y.; Huang, J.; Chen, H.Y. De Novo Transcriptome Sequencing and Analysis of Coccinella septempunctata L. In Non-Diapause, Diapause and Diapause-Terminated States to Identify Diapause-Associated Genes. BMC Genom. 2015, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.K.F.; Araujo, N.D.S.; Françoso, E.; Zuntini, A.R.; Arias, M.C. Diapause in a Tropical Oil-Collecting Bee: Molecular Basis Unveiled by Rna-Seq. BMC Genom. 2018, 19, 305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-Y.; Zhao, X.-T.; Sun, J.-T.; Zou, L.-F.; Yang, S.-X.; Han, X.; Zhu, W.-C.; Yin, Q.; Hong, X.-Y. Transcriptome and Proteome Analyses Reveal Complex Mechanisms of Reproductive Diapause in the Two-Spotted Spider Mite, Tetranychus urticae. Insect Mol. Biol. 2017, 26, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Hase, H.; Koukai, M.; Hamanaka, Y.; Goto, S.G.; Tachibana, S.; Shiga, S. Transcriptome Analysis of the Brain under Diapause and Nondiapause Conditions in the Blowfly Protophormia terraenovae. Physiol. Èntomol. 2017, 42, 282–289. [Google Scholar] [CrossRef]
- Ragland, G.J.; Keep, E. Comparative Transcriptomics Support Evolutionary Convergence of Diapause Responses across Insecta. Physiol. Èntomol. 2017, 42, 246–256. [Google Scholar] [CrossRef]
- Sim, C.; Denlinger, D.L. Insulin Signaling and the Regulation of Insect Diapause. Front. Physiol. 2013, 4, 189. [Google Scholar] [CrossRef]
- Chen, W.; Xu, W.-H. Wnt/Beta-Catenin Signaling Regulates Helicoverpa armigera Pupal Development by up-Regulating C-Myc and Ap-4. Insect Biochem. Mol. Biol. 2014, 53, 44–53. [Google Scholar] [CrossRef]
- Denlinger, D.L.; Hahn, D.A.; Merlin, C.; Holzapfel, C.M.; Bradshaw, W.E. Keeping Time without a Spine: What Can the Insect Clock Teach Us About Seasonal Adaptation? Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160257. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Li, Y.-Y.; Li, L.; Tan, Y.; Pang, B.-P. Regulation of Juvenile Hormone on Summer Diapause of Geleruca daurica and Its Pathway Analysis. Insects 2021, 12, 14. [Google Scholar] [CrossRef]
- Michaud, J.; Qureshi, J.A. Induction of Reproductive Diapause in Hippodamia convergens (Coleoptera: Coccinellidae) Hinges on Prey Quality and Availability. Eur. J. Èntomol. 2005, 102, 483–487. [Google Scholar] [CrossRef]
- Obrycki, J.J.; Mccord, J.S.; Mercer, N.H.; White, J.A. Photoperiodic Induction of Adult Diapause in North American Populations of the Convergent Lady Beetle (Coleoptera: Coccinellidae). Environ. Èntomol. 2018, 47, 1596–1600. [Google Scholar] [CrossRef]
- Hagen, K.S. Biology and Ecology of Predaceous Coccinellidae. Annu. Rev. Èntomol. 1962, 7, 289–326. [Google Scholar] [CrossRef]
- Flint, M.L.; Dreistadt, S.H. Interactions among Convergent Lady Beetle (Hippodamia convergens) Releases, Aphid Populations, and Rose Cultivar. Biol. Control 2005, 34, 38–46. [Google Scholar] [CrossRef]
- Dreistadt, S.H.; Flint, M.L. Melon Aphid (Homoptera: Aphididae) Control by Inundative Convergent Lady Beetle (Coleoptera: Coccinellidae) Release on Chrysanthemum. Environ. Èntomol. 1996, 25, 688–697. [Google Scholar] [CrossRef][Green Version]
- Davis, J.R.; Kirkland, R.L. Physiological and Environmental-Factors Related to the Dispersal Flight of the Convergent Lady Beetle, Hippodamia convergens (Guerin-Meneville). J. Kansas Entomol. Soc. 1982, 55, 187–196. [Google Scholar]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-Length Transcriptome Assembly from Rna-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De Novo Transcript Sequence Reconstruction from Rna-Seq Using the Trinity Platform for Reference Generation and Analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. Busco: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. Kegg for Integration and Interpretation of Large-Scale Molecular Data Sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Proc, G.P.D. The Sequence Alignment/Map Format and Samtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential Gene and Transcript Expression Analysis of Rna-Seq Experiments with Tophat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Marteaux, L.E.D.; McKinnon, A.H.; Udaka, H.; Toxopeus, J.; Sinclair, B.J. Effects of Cold-Acclimation on Gene Expression in Fall Field Cricket (Gryllus pennsylvanicus) Ionoregulatory Tissues. BMC Genom. 2017, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. Htseq-a Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for Rna-Seq Data with Deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- RC Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for Rna-Seq: Accounting for Selection Bias. Genome Biol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Smuc, T. Revigo Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Luo, W.; Friedman, M.S.; Shedden, K.; Hankenson, K.D.; Woolf, P.J. Gage: Generally Applicable Gene Set Enrichment for Pathway Analysis. BMC Bioinform. 2009, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor Package for Pathway-Based Data Integration and Visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [PubMed]
- Toxopeus, J.; Marteaux, L.D.; Sinclair, B.J. How Crickets Become Freeze Tolerant: The Transcriptomic Underpinnings of Acclimation in Gryllus veletis. Comp. Biochem. Phys. D 2019, 29, 55–66. [Google Scholar] [CrossRef]
- Hou, Z.; Wei, C. De Novo Comparative Transcriptome Analysis of a Rare Cicada, with Identification of Candidate Genes Related to Adaptation to a Novel Host Plant and Drier Habitats. BMC Genom. 2019, 20, 182. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, Y.; Zhao, F.-Q.; Yu, J.; Craig, R.; Hu, S. Analysis of Tarantula Skeletal Muscle Protein Sequences and Identification of Transcriptional Isoforms. BMC Genom. 2009, 10, 15. [Google Scholar] [CrossRef]
- Ayme-Southgate, A.; Lasko, P.; French, C.; Pardue, M.L. Characterization of the Gene for Mp20—A Drosophila Muscle Protein That Is Not Found in Asynchronous Oscillatory Flight-Muscle. J. Cell Biol. 1989, 108, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Kadrmas, J.L.; Beckerle, M.C. The Lim Domain: From the Cytoskeleton to the Nucleus. Nat. Rev. Mol. Cell Biol. 2004, 5, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Renault, D.; Charoy-Guével, B.; Com, E. Metabolic and Proteomic Profiling of Diapause in the Aphid Parasitoid Praon volucre. PLoS ONE 2012, 7, e32606. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Lin, J.; Zhong, Y.; Liu, J. Shotgun Proteomic Analysis on the Diapause and Non-Diapause Eggs of Domesticated Silkworm Bombyx mori. PLoS ONE 2013, 8, e60386. [Google Scholar] [CrossRef]
- Teets, N.M.; Denlinger, D.L. Quantitative Phosphoproteomics Reveals Signaling Mechanisms Associated with Rapid Cold Hardening in a Chill-Tolerant Fly. J. Proteome Res. 2016, 15, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Koštál, V. Cell Structural Modifications in Insects at Low Temperatures. In Low Temperature Biology of Insects; Denlinger, D.L., Lee, R.E., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 116–140. [Google Scholar]
- Kim, M.; Robich, R.M.; Rinehart, J.P.; Denlinger, D.L. Upregulation of Two Actin Genes and Redistribution of Actin During Diapause and Cold Stress in the Northern House Mosquito, Culex pipiens. J. Insect Physiol. 2006, 52, 1226–1233. [Google Scholar] [CrossRef]
- Stuckas, H.; Mende, M.B.; Hundsdoerfer, A. Response to Cold Acclimation in Diapause Pupae of Hyles euphorbiae (Lepidoptera: Sphingidae): Candidate Biomarker Identification Using Proteomics. Insect Mol. Biol. 2014, 23, 444–456. [Google Scholar] [CrossRef]
- Lebenzon, J.E.; Torson, A.S.; Sinclair, B.J. Diapause Differentially Modulates the Transcriptomes of Fat Body and Flight Muscle in the Colorado Potato Beetle. Comp. Biochem. Phys. D 2021, 40, 100906. [Google Scholar] [CrossRef] [PubMed]
- Koštál, V.; Šimůnková, P.; Kobelková, A.; Shimada, K. Cell Cycle Arrest as a Hallmark of Insect Diapause: Changes in Gene Transcription During Diapause Induction in the Drosophilid Fly, Chymomyza costata. Insect Biochem. Mol. Biol. 2009, 39, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Mukai, A.; Goto, S.G. Cell Cycle Arrest in the Jewel Wasp Nasonia vitripennis in Larval Diapause. J. Insect Physiol. 2018, 106, 147–152. [Google Scholar] [CrossRef]
- Shimizu, Y.; Tamai, T.; Goto, S.G. Cell Cycle Regulator, Small Silencing Rna, and Segmentation Patterning Gene Expression in Relation to Embryonic Diapause in the Band-Legged Ground Cricket. Insect Biochem. Mol. Biol. 2018, 102, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.; Kos-Braun, I.C.; Henras, A.K.; Dez, C.; Rueda, M.P.; Zhang, X.; Gadal, O.; Kos, M.; Shore, D. A Ribosome Assembly Stress Response Regulates Transcription to Maintain Proteome Homeostasis. eLife 2019, 8, e45002. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Merchant, A.M.; Tye, B.K. Cell-Cycle-Regulated Nuclear-Localization of Mcm2 and Mcm3, Which Are Required for the Initiation of DNA-Synthesis at Chromosomal Replication Origins in Yeast. Genes Dev. 1993, 7, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Osanai, K.; Ohyoshi, T.; Wang, H.-B.; Iwanaga, M.; Kawasaki, H. Ecdysteroid Promotes Cell Cycle Progression in the Bombyx Wing Disc through Activation of C-Myc. Insect Biochem. Mol. Biol. 2016, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bendris, N.; Lemmers, B.; Blanchard, J.-M.; Arsic, N. Cyclin A2 Mutagenesis Analysis: A New Insight into Cdk Activation and Cellular Localization Requirements. PLoS ONE 2011, 6, e22879. [Google Scholar] [CrossRef]
- Ji, P.; Agrawal, S.; Diederichs, S.; Bäumer, N.; Becker, A.; Cauvet, T.; Kowski, S.; Beger, C.; Welte, K.; Berdel, W.E.; et al. Cyclin A1, the Alternative a-Type Cyclin, Contributes to G1/S Cell Cycle Progression in Somatic Cells. Oncogene 2005, 24, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, H.H.; Kunkel, J.G. Vitellogenin and Vitellin in Insects. Annu. Rev. Èntomol. 1979, 24, 475–505. [Google Scholar] [CrossRef]
- Sim, C.; Denlinger, D.L. Insulin Signaling and Foxo Regulate the Overwintering Diapause of the Mosquito Culex pipiens. Proc. Natl. Acad. Sci. USA 2008, 105, 6777–6781. [Google Scholar] [CrossRef]
- Zhu, L.; Yin, T.-Y.; Sun, D.; Liu, W.; Zhu, F.; Lei, C.-L.; Wang, X.-P. Juvenile Hormone Regulates the Differential Expression of Putative Juvenile Hormone Esterases Via Methoprene-Tolerant in Non-Diapause-Destined and Diapause-Destined Adult Female Beetle. Gene 2017, 627, 373–378. [Google Scholar] [CrossRef]
- Badisco, L.; Marchal, E.; Van Wielendaele, P.; Verlinden, H.; Vleugels, R.; Broeck, J.V. RNA Interference of Insulin-Related Peptide and Neuroparsins Affects Vitellogenesis in the Desert Locust Schistocerca gregaria. Peptides 2011, 32, 573–580. [Google Scholar] [CrossRef]
- Brown, M.R.; Clark, K.D.; Gulia, M.; Zhao, Z.; Garczynski, S.F.; Crim, J.W.; Suderman, R.J.; Strand, M.R. An Insulin-Like Peptide Regulates Egg Maturation and Metabolism in the Mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2008, 105, 5716–5721. [Google Scholar] [CrossRef]
- Obrycki, J.J. Reproductive Diapause in North American Populations of the Introduced Lady Beetle Hippodamia variegata (Coleoptera: Coccinellidae). Environ. Èntomol. 2018, 47, 1337–1343. [Google Scholar] [CrossRef]
- Lin, X.; Smagghe, G. Roles of the Insulin Signaling Pathway in Insect Development and Organ Growth. Peptides 2019, 122, 169923. [Google Scholar] [CrossRef]
- Mahneva, O.; Caplan, S.L.; Ivko, P.; Dawson-Scully, K.; Milton, S.L. No/Cgmp/Pkg Activation Protects Drosophila Cells Subjected to Hypoxic Stress. Comp. Biochem. Phys. C 2019, 223, 106–114. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Y.; Li, W.; Jiang, H.; Chatzimanolis, L.; Chang, J.; Gong, Z.; Liu, L. Visual Pattern Memory Requires Foraging Function in the Central Complex of Drosophila. Learn. Mem. 2008, 15, 133–142. [Google Scholar] [CrossRef]
- Osborne, K.A.; Robichon, A.; Burgess, E.; Butland, S.; Shaw, R.A.; Coulthard, A.; Pereira, H.S.; Greenspan, R.J.; Sokolowski, M.B. Natural Behavior Polymorphism Due to a Cgmp-Dependent Protein Kinase of Drosophila. Science 1997, 277, 834–836. [Google Scholar] [CrossRef]
- Dong, Y.-C.; Chen, Z.-Z.; Clarke, A.R.; Niu, C.-Y. Changes in Energy Metabolism Trigger Pupal Diapause Transition of Bactrocera minax after 20-Hydroxyecdysone Application. Front. Physiol. 2019, 10, 1288. [Google Scholar] [CrossRef]
- King, A.M.; MacRae, T.H. Insect Heat Shock Proteins During Stress and Diapause. Annu. Rev. Èntomol. 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Goto, S.; Yoshida, K.M.; Kimura, M.T. Accumulation of Hsp70 Mrna under Environmental Stresses in Diapausing and Nondiapausing Adults of Drosophila triauraria. J. Insect Physiol. 1998, 44, 1009–1015. [Google Scholar] [CrossRef]
- Rinehart, J.P.; Robich, R.M.; Denlinger, D.L. Enhanced Cold and Desiccation Tolerance in Diapausing Adults of Culex pipiens, and a Role for Hsp70 in Response to Cold Shock but Not as a Component of the Diapause Program. J. Med. Entomol. 2006, 43, 713–722. [Google Scholar] [CrossRef]
- Robich, R.M.; Rinehart, J.P.; Kitchen, L.J.; Denlinger, D.L. Diapause-Specific Gene Expression in the Northern House Mosquito, Culex pipiens L., Identified by Suppressive Subtractive Hybridization. J. Insect Physiol. 2007, 53, 235–245. [Google Scholar] [CrossRef]
- Yocum, G.D. Differential Expression of Two Hsp70 Transcripts in Response to Cold Shock, Thermoperiod, and Adult Diapause in the Colorado Potato Beetle. J. Insect Physiol. 2001, 47, 1139–1145. [Google Scholar] [CrossRef]
- Colinet, H.; Lee, S.F.; Hoffmann, A. Temporal Expression of Heat Shock Genes During Cold Stress and Recovery from Chill Coma in Adult Drosophila melanogaster. FEBS J. 2009, 277, 174–185. [Google Scholar] [CrossRef]
- Rasool, K.G.; Mehmood, K.; Tufail, M.; Husain, M.; Alwaneen, W.S.; Aldawood, A.S. Silencing of Vitellogenin Gene Contributes to the Promise of Controlling Red Palm Weevil, Rhynchophorus ferrugineus (Olivier). Sci. Rep. 2021, 11, 12. [Google Scholar] [CrossRef]
- Zhang, Q.; Nachman, R.J.; Kaczmarek, K.; Kierus, K.; Zabrocki, J.; Denlinger, D.L. Development of Neuropeptide Analogs Capable of Traversing the Integument: A Case Study Using Diapause Hormone Analogs in Helicoverpa zea. Insect Biochem. Mol. Biol. 2015, 67, 87–93. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).