Insecticidal Effect of Four Insecticides for the Control of Different Populations of Three Stored-Product Beetle Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Populations
2.2. Contact Insecticides
2.3. Phosphine
2.4. Statistical Analysis
3. Results
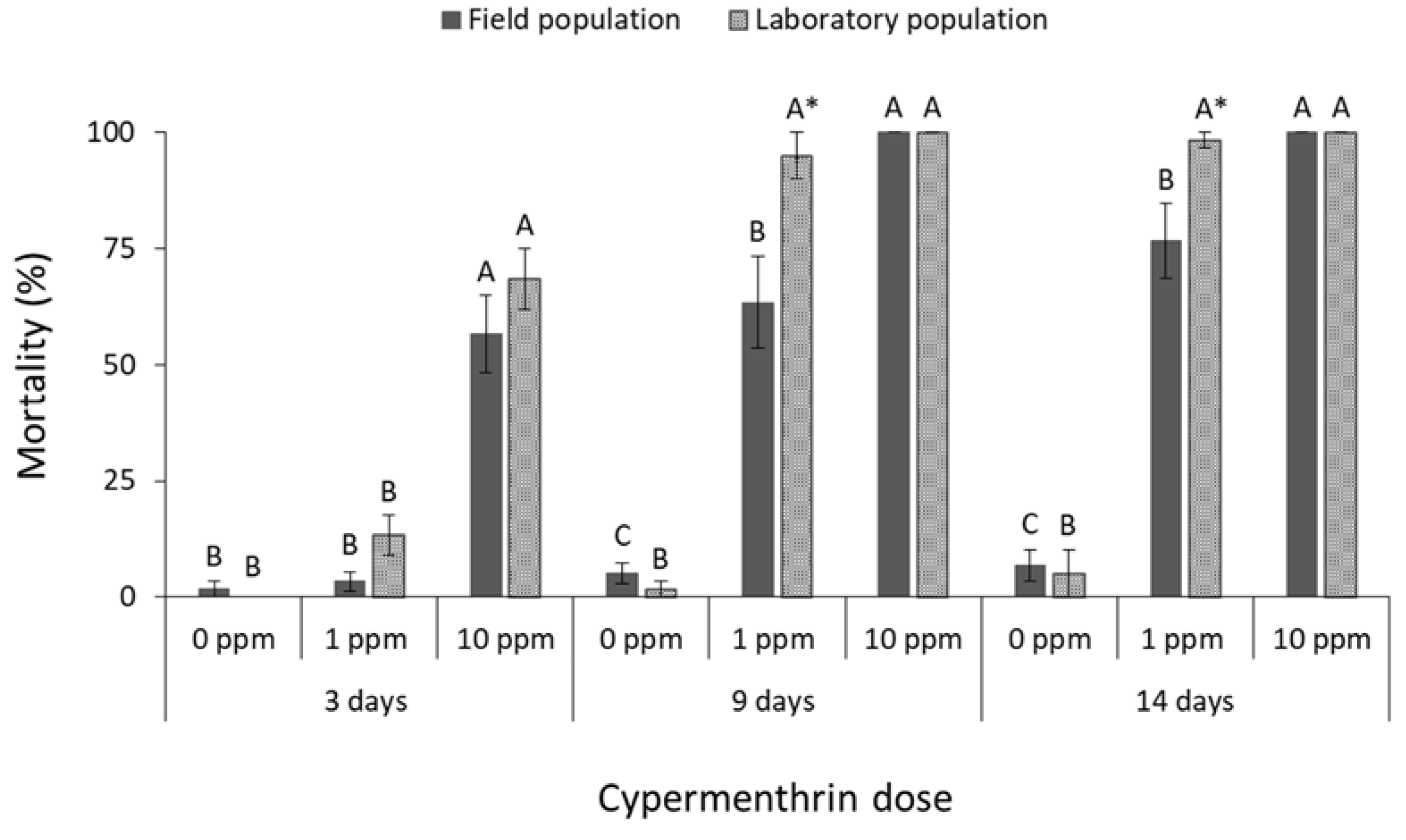
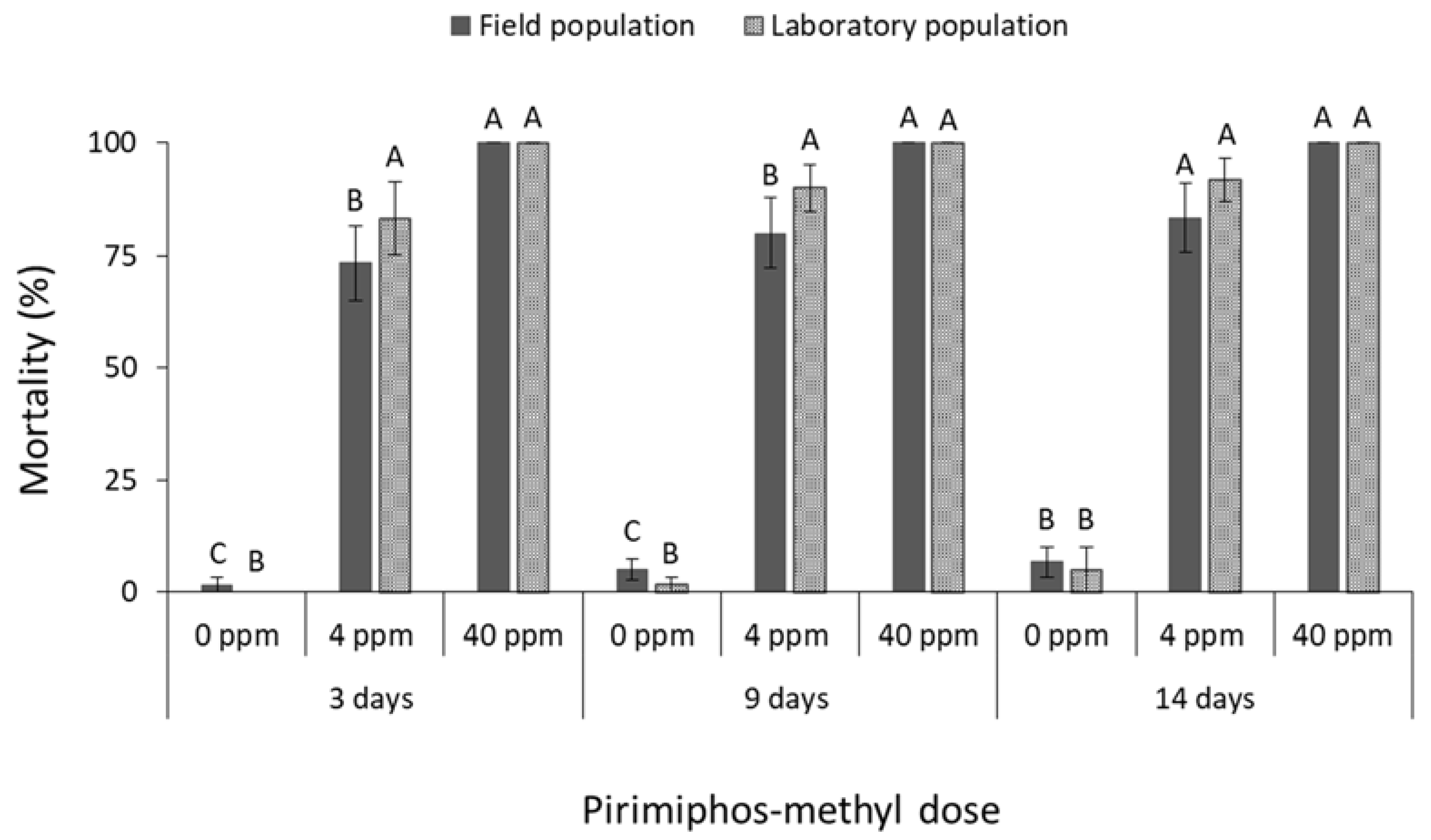
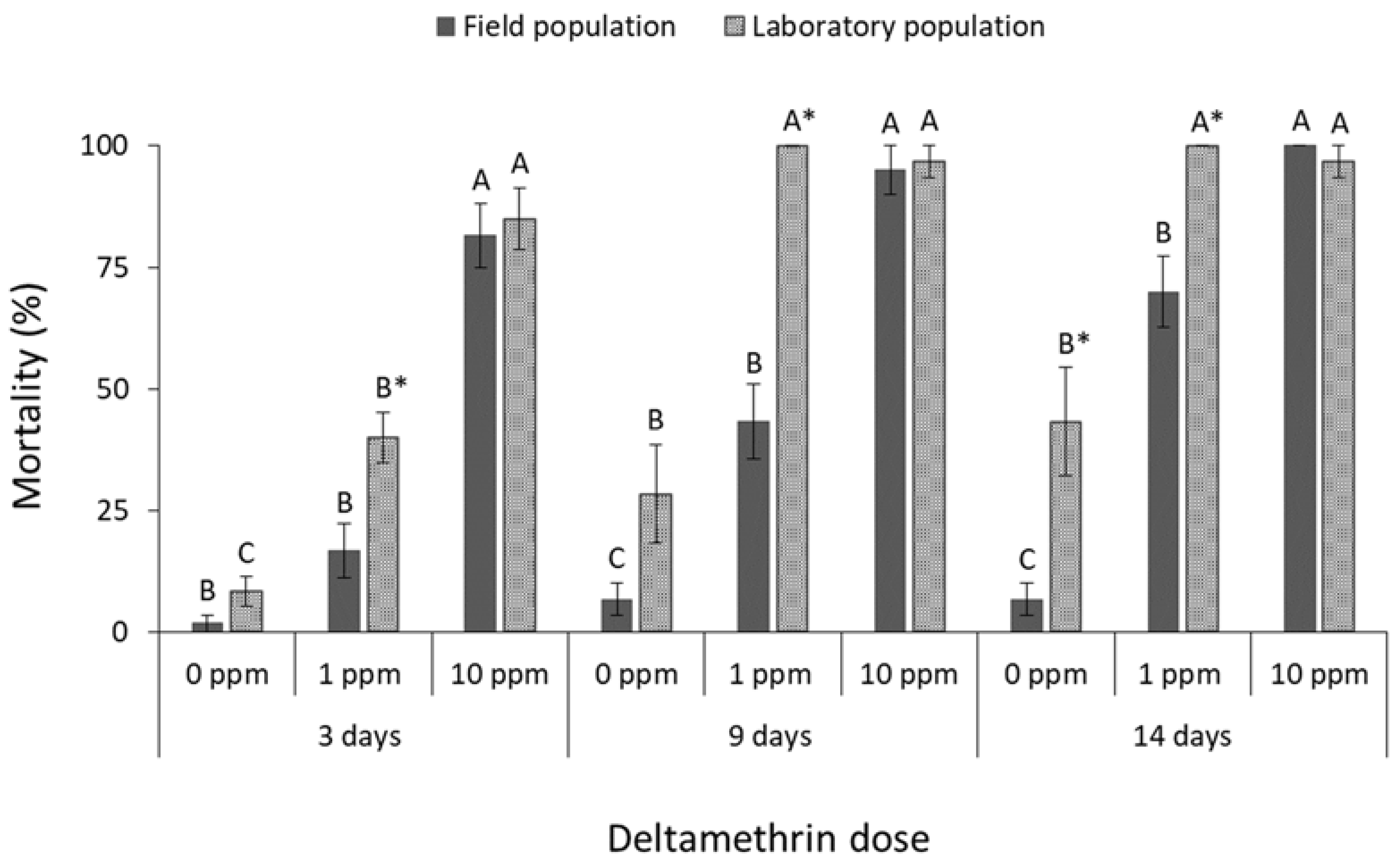
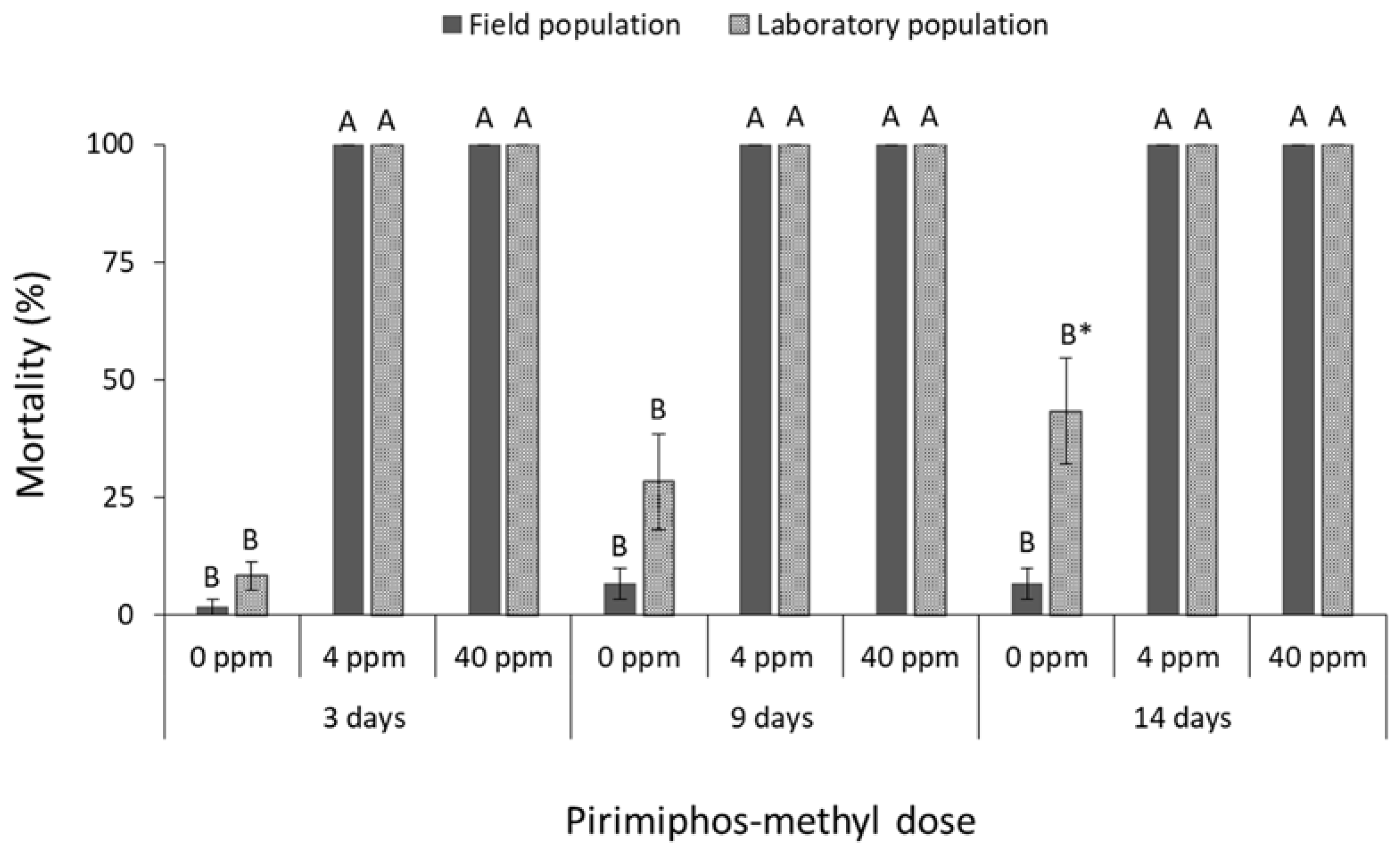
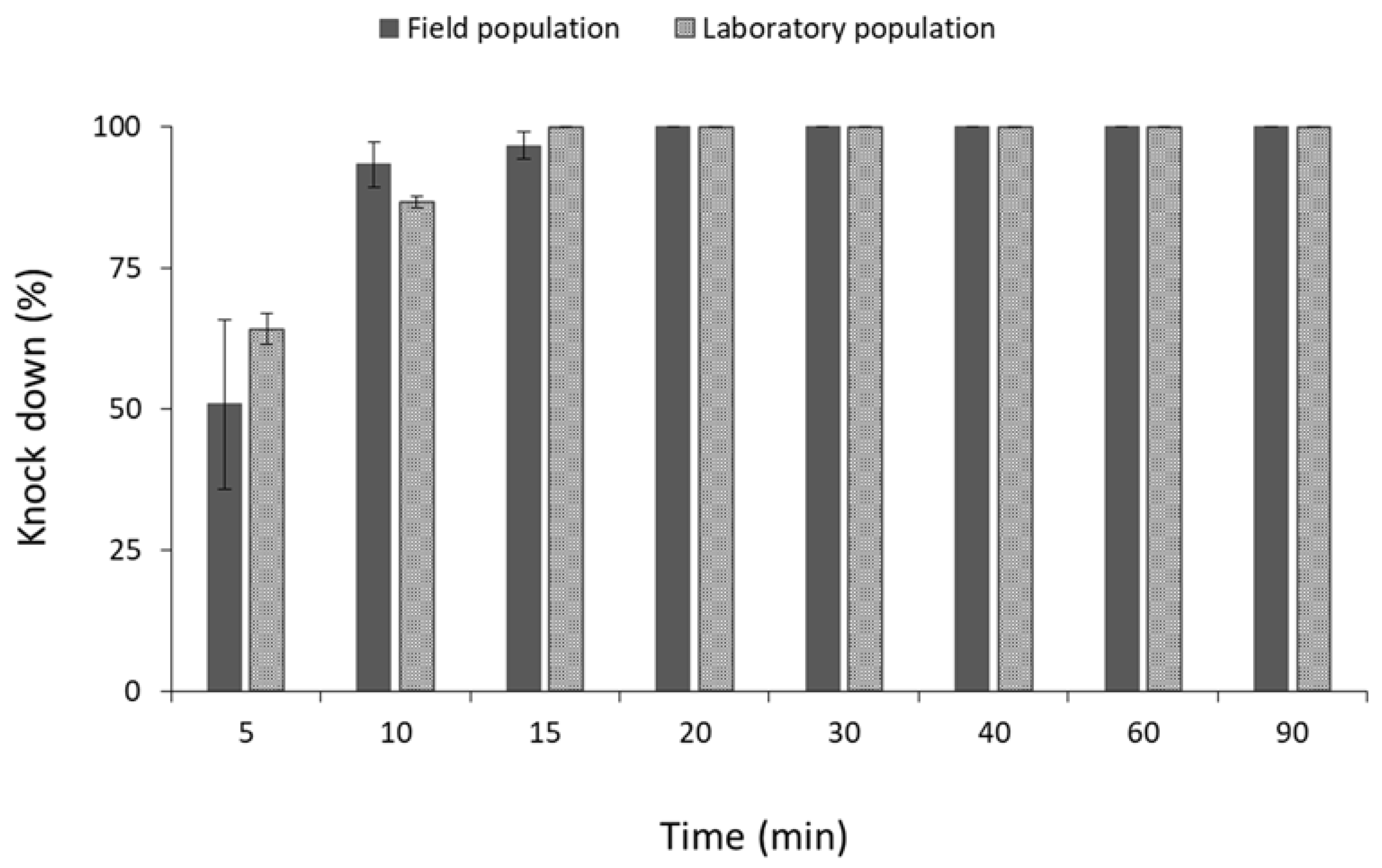
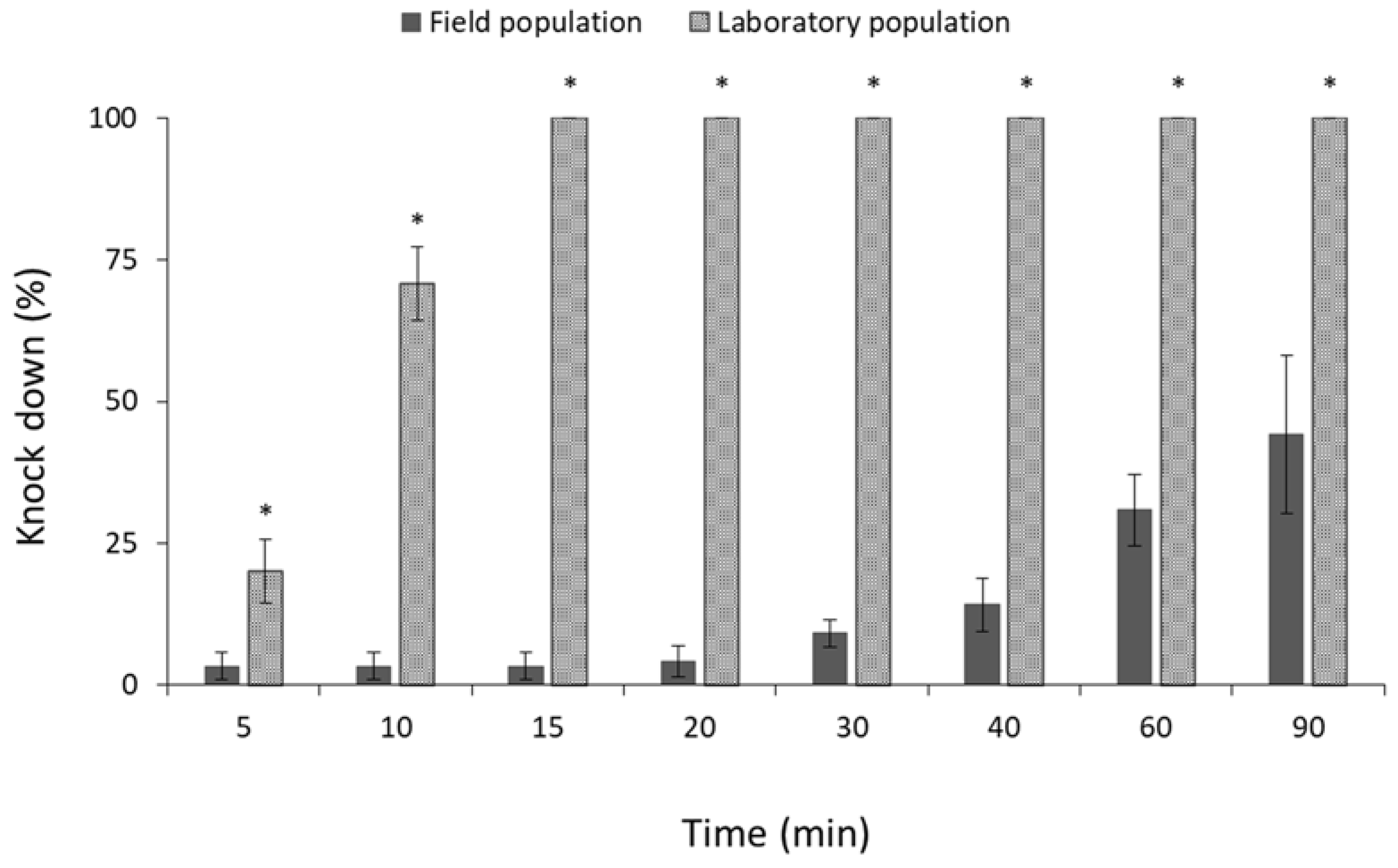
3.1. Contact Insecticides
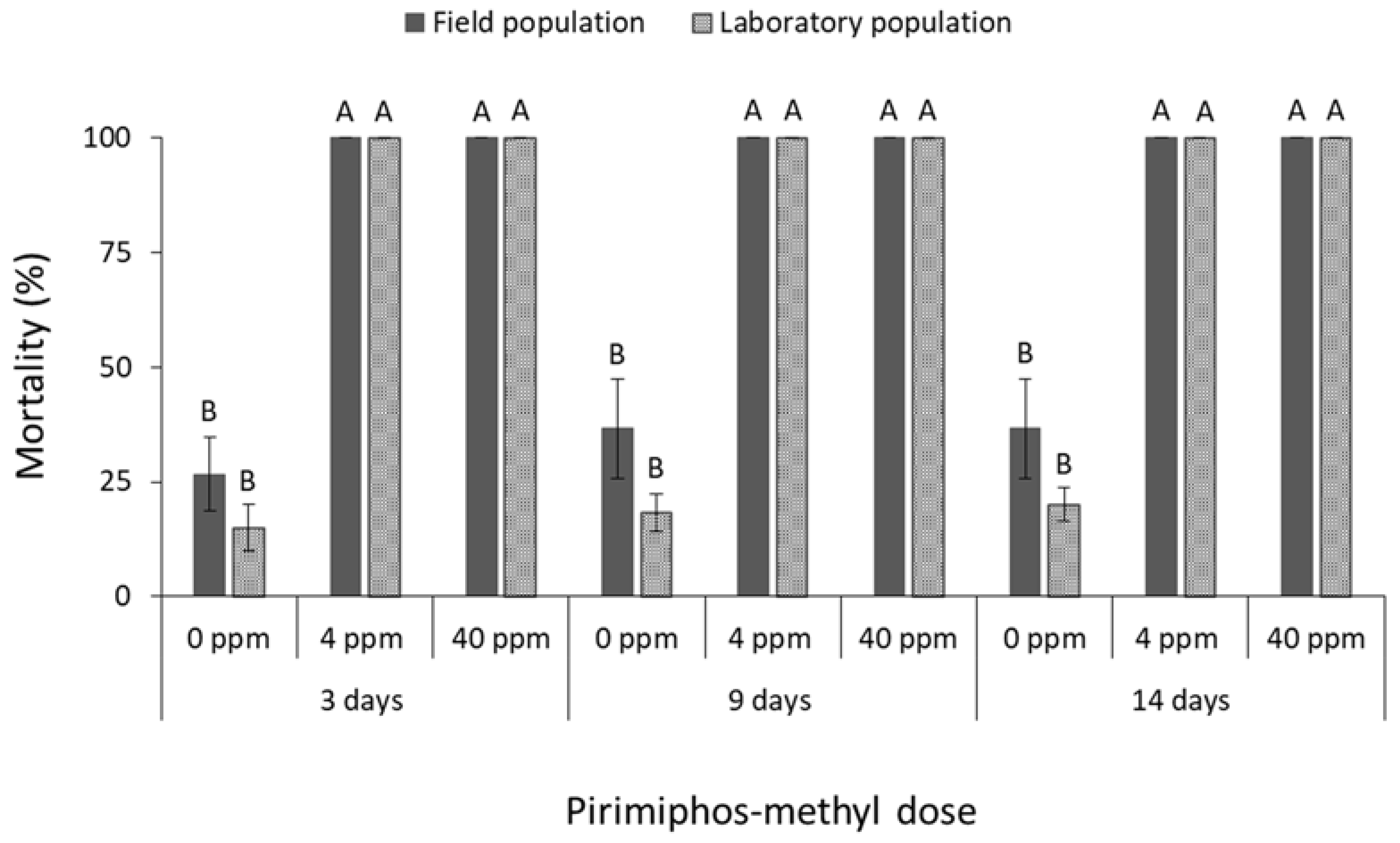
3.2. Phosphine
4. Discussion
Author Contributions
Funding

Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evered, D.; Collins, G.M. Origins and Development of Adaptation. In Proceedings of the Symposium on Origins and Development of Adaptation, Ciba Foundation, London, UK, 12–14 April 1983; Collins, E., Ed.; Pitman Publishing Ltd.: London, UK, 1984; pp. 152–166. [Google Scholar]
- Corrêa, A.S.; Vinson, C.C.; Braga, L.S.; Guedes, R.N.C.; de Oliveira, L.O. Ancient origin and recent range expansion of the maize weevil Sitophilus zeamais, and its genealogical relationship to the rice weevil S. Oryzae. Bul. Entomol. Res. 2017, 107, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melander, A.L. Can insects become resistant to sprays? J. Econ. Entomol. 1914, 7, 167–173. [Google Scholar] [CrossRef]
- Campbell, J.F.; Arthur, F.H.; Mullen, M.A. Insect management in food processing facilities. Adv. Food Nutr. Res. 2004, 48, 267–272. [Google Scholar]
- Hemingway, J. Resistance: A problem without an easy solution. Pest. Biochem. Physiol. 2018, 151, 73–75. [Google Scholar] [CrossRef]
- Helps, J.C.; Paveley, N.D.; White, S.; van den Bosch, F. Determinants of optimal insecticide resistant management strategies. J. Theor. Biol. 2020, 503, 110383. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Lima, J.O.G.; Santos, J.P.; Cruz, C.D. Resistance to DDT and pyrethroids in Brazilian populations of Sitophilus zeamais Motsch. (Coleoptera: Curculionidae). J. Stored Prod. Res. 1995, 31, 145–150. [Google Scholar] [CrossRef]
- Huang, F.; Subramanyam, B. Management of five stored-product insects in wheat with pirimiphos-methyl and pirimiphos-methyl plus synergized pyrethrins. Pest. Manag. Sci. 2005, 61, 356–362. [Google Scholar] [CrossRef]
- Kljajić, P.; Perić, I. Effectiveness of wheat-applied contact insecticides against Sitophilus granarius (L.) originating from different populations. J. Stored Prod. Res. 2007, 43, 523–529. [Google Scholar] [CrossRef]
- Collins, P.J.; Nayak, M.K.; Kopittke, R. Residual efficacy of four organophosphate insecticides on concrete and galvanized steel against three liposcelid psocid species (Psocoptera: Liposcelidae) infesting stored products. J. Econ. Entomol. 2000, 93, 1357–1363. [Google Scholar] [CrossRef]
- Lagisz, M.; Wolff, K.; Port, G. Time matters: Delayed toxicity of pirimiphos-methyl on Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) and its effects on efficacy estimation of residual treatments. J. Stored Prod. Res. 2010, 46, 161–165. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Dutton, A.C.; Athanassiou, C.G. Comparison of two pirimiphos-methyl formulations against major stored-product insect species. J. Stored Prod. Res. 2013, 55, 106–115. [Google Scholar] [CrossRef]
- Limoee, M.; Davari, B.; Moosa-Kazemi, S.H. Toxicity of pyrethroid and organophosphorous insecticides against two field collected strains of the german cockroach Blattella germanica (Blattaria: Blattellidae). J. Arthr. Dis. 2012, 6, 112–118. [Google Scholar]
- Dusfour, I.; Zorrilla, P.; Guidez, A.; Issaly, J.; Girod, R.; Guillaumot, L.; Robello, C.; Strode, C. Deltamethrin resistance mechanisms in Aedes aegypti populations from three French overseas territories worldwide. PLoS Negl. Trop. Dis. 2015, 9, e0004226. [Google Scholar] [CrossRef] [Green Version]
- Gunning, C.E.; Okamoto, K.W.; Astete, H.; Vasquez, G.M.; Erhardt, E.; del Aguila, C.; Pinedo, R.; Cardenas, R.; Pacheco, C.; Chalco, E.; et al. Efficacy of Aedes aegypti control by indoor Ultra Low Volume (ULV) insecticide spraying in Iquitos, Peru. PLoS Negl. Trop. Dis. 2018, 12, 0006378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current research on the safety of pyrethroids used as insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef] [Green Version]
- Athanassiou, C.G.; Papagregoriou, A.S.; Buchelos, C.T. Insecticidal and residual effect of three pyrethroids against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) on stored wheat. J. Stored Prod. Res. 2004, 40, 289–297. [Google Scholar] [CrossRef]
- Gourgouta, M.; Rumbos, C.I.; Athanassiou, C.G. Residual toxicity of a commercial cypermethrin formulation on grains against four major storage beetles. J. Stored Prod. Res. 2019, 83, 103–109. [Google Scholar] [CrossRef]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef] [Green Version]
- Hagstrum, D.W.; Subramanyam, B. Fundamentals of Stored-Product Entomology; AACC International: St. Paul, MN, USA, 2006; pp. 1–323. [Google Scholar]
- Athanassiou, C.G.; Rumbos, C.I.; Sakka, M.; Sotiroudas, V. Insecticidal efficacy of phosphine fumigation at low pressure against major stored-product insect species in a commercial dried fig processing facility. Crop. Prot. 2016, 90, 177–185. [Google Scholar] [CrossRef]
- Agrafioti, P.; Athanassiou, C.; Sotiroudas, V. Lessons learned for phosphine distribution and efficacy by using wireless phosphine sensors. In Proceedings of the 12th international Working Conference on Stored Product Protection, Berlin, Germany, 7–11 October 2018. [Google Scholar]
- Phillips, T.W.; Thoms, E.M.; Demark, J.; Walse, D.S. Fumigation. In Stored Product Protection; Hahstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KS, USA, 2012; Chapter 14. [Google Scholar]
- Nayak, M.K.; Collins, P.J.; Holloway, J.K.; Emery, R.N.; Pavic, H.; Bartlet, J. Strong resistance to phosphine in the rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae): Its characterization, a rapid assay for diagnosis and its distribution in Australia. Pest. Manag. Sci. 2013, 69, 48–53. [Google Scholar] [CrossRef]
- Karaağaç, S.U.; Konuş, M. Determination of organophosphate resistance status and mechanism in Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) from Turkey. Turk. J. Biochem. 2015, 40, 417–422. [Google Scholar]
- Wallbank, B.E. Resistance to organophosphorus grain protectants in Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae) from off-farm grain storages in New South Wales. Aust. J. Entomol. 1996, 35, 193–195. [Google Scholar] [CrossRef]
- Attia, M.A.; Wahba, T.F.; Shaarawy, N.; Moustafa, F.I.; Guedes, R.N.C.; Dewer, Y. Stored grain pest prevalence and insecticide resistance in Egyptian populations of the red flour beetle Tribolium castaneum (Herbst) and the rice weevil Sitophilus oryzae (L.). J. Stored Prod. Res. 2020, 87, 101611. [Google Scholar] [CrossRef]
- Kljajić, P.; Perić, I. Susceptibility to contact insecticides of granary weevil Sitophilus granarius (L.) (Coleoptera: Curculionidae) originating from different locations in the former Yugoslavia. J. Stored Prod. Res. 2006, 42, 149–161. [Google Scholar] [CrossRef]
- Perez-Mendoza, J. Survey of insecticide resistance in Mexican populations of maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Stored Prod. Res. 1999, 35, 107–115. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Dover, B.A.; Kambhampati, S. Resistance to chlorpyrifos-methyl, pirimiphos-methyl, and malathion in Brazilian and U.S. populations of Rhyzopertha dominica (Coleoptera: Bostrichidae). J. Econ. Entomol. 1996, 89, 27–32. [Google Scholar] [CrossRef]
- Heather, N.W. Sex-linked resistance to pyrethroids in Sitophilus oryzae (L.) (Coleoptera: Curculionidae). J. Stored Prod. Res. 1986, 22, 15–20. [Google Scholar] [CrossRef]
- Ribeiro, B.M.; Guedes, R.N.C.; Oliveira, E.E.; Santos, J.P. Insecticide resistance and synergism in Brazilian populations of Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2003, 39, 21–31. [Google Scholar] [CrossRef]
- Daglish, G.J.; Nayak, M.K. Prevalence of resistance to deltamethrin in Rhyzopertha dominica (F.) in eastern Australia. J. Stored Prod. Res. 2018, 78, 45–49. [Google Scholar] [CrossRef]
- Agrafioti, P.; Athanassiou, C.G.; Nayak, M.K. Detection of phosphine resistance in major stored-product insects in Greece and evaluation of a field resistance test kit. J. Stored Prod. Res. 2019, 82, 40–47. [Google Scholar] [CrossRef]
- Afful, E.; Elliott, B.; Nayak, M.K.; Phillips, T.W. Phosphine resistance in north american field populations of the lesser grain borer, Rhyzopertha dominica (Coleoptera: Bostrichidae). J. Econ. Entomol. 2018, 111, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, P.; Zhang, H. Phosphine resistance in Rhyzopertha dominica (Fabricius) (Coleoptera: Bostrichidae) from different geographical populations in China. Afr. J. Biotechn. 2011, 10, 16367–16373. [Google Scholar] [CrossRef]
- Rajendran, S.; Narasimhan, K.S. Posphine resistance in the cigarette beetle Lasioderma serricorne (Coleoptera: Anobiidae) and overcoming control failures during fumigation of stored tobacco. Pest. Manag. Sci. 1994, 40, 207–210. [Google Scholar]
- Pimentel, M.A.G.; Guedes, R.N.C. Spread of phosphine among Brazilian populations of three species of stored products insects. Neotrop. Entomol. 2010, 39, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Collins, P.J.; Daglish, G.J.; Pavic, H.; Kopitkee, R.A. Response of mixed-age cultures of phosphine-resistant and susceptible strains of lesser grain borer, Rhyzopertha dominica, to phosphine at a range of concentration and exposure periods. J. Stored Prod. Res. 2005, 41, 373–385. [Google Scholar] [CrossRef]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W. Managing resistance to chemical treatments in stored products pests. Stewart Postharvest Rev. 2015, 1, 3. [Google Scholar]
- Collins, P.J.; Schlipalius, D.I. Insecticide Resistance. In Recent Advances in Stored Product Protection; Athanassiou, C.G., Arthur, F.H., Eds.; Springer: Verlag, Germany, 2018; pp. 169–182. [Google Scholar] [CrossRef]
- Gorham, J.R. Insect and Mite Pests in Food: An Illustrated Key; U.S. Department of Agriculture: Washington DC, USA, 1987. [Google Scholar]
- Steuerwald, R.; Dierks-Lange, H.; Schmitt, S. Rapid bioassay for determining the phosphine tolerance. In Proceedings of the 9th International Working Conference on Stored Product Protection, Campinas, Abrapos, Brasil, 15–18 October 1994; Lorini, B., Beckel, D., Sundfeld, S., Biagi, C., Faroni, B., Santori, E., Guedes, F.S., Eds.; CAB Int.: Wallingford, UK, 2006; pp. 306–311. Available online: http://refhub.elsevier.com/S0022–474X(18)30395–3/sref32 (accessed on 17 March 2022).
- Athanassiou, C.G.; Kavallieratos, N.G.; Brabec, D.L.; Agrafioti, P.; Sakka, M.; Campbell, J.F. Using immobilization as a quick diagnostic indicator for resistance to phosphine. J. Stored Prod. Res. 2019, 82, 17–26. [Google Scholar] [CrossRef]
- SAS Institute Inc. Using JMP® Software, Version 7.0; SAS Institute Inc.: Cary, Japan, 2011; 1989–2019. [Google Scholar]
- IBM Corp. IBM SPSS Statistics Version 24.0 for Windows; IBM Corp.: Armonk, NY, USA, 2016. [Google Scholar]
- Buchelos, C.T.; Athanassiou, C.G. Dominance and frequency of coleoptera found on stored cereals and cereal products in Central Greece. Entomol. Hell. 1993, 11, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Athanassiou, C.G.; Buchelos, C.T. Detection of stored-wheat beetle species and estimation of population density using unbaited probe traps and grain trier samples. Entomol. Exp. Appl. 2001, 98, 67–78. [Google Scholar] [CrossRef]
- Yao, J.; Chen, C.; Wu, H.; Chang, J.; Silver, K.; Campbell, J.F.; Arthur, F.H.; Zhu, K.Y. Differential susceptibilities of two closely-related stored product pests, the red flour beetle (Tribolium castaneum) and the confused flour beetle (Tribolium confusum), to five selected insecticides. J. Stored Prod. Res. 2019, 84, 101524. [Google Scholar] [CrossRef]
- Daglish, G.J.; Nayak, M.K. Potential of the neonicotinoid imidacloprid and the oxadiazine indoxacarb for controlling five coleopteran pests of stored grain. Insect Sci. 2012, 19, 96–101. [Google Scholar] [CrossRef]
- Daglish, G.J. Efficacy of six grain protectants applied alone or in combination against three species of Coleoptera. J. Stored Prod. Res. 1998, 34, 263–268. [Google Scholar] [CrossRef]
- Fang, L.; Subramanyam, B.; Arthur, F.H. Effectiveness of spinosad on four classes of wheat against five stored-product insects. J. Econ. Entomol. 2002, 95, 640–650. [Google Scholar] [CrossRef]
- Alleoni, B.; Ferreira, W. Control of Sitophilus zeamais Mots., 1958 and Sitophilus oryzae (L.; 1763) weevils (Coleoptera, Curculionidae) in stored corn grain (Zea mays L.) with insecticide pirimiphos methyl (Actellic 500 CE). In Proceedings of the 9th International Working Conference on Stored-Product Protection, Passo Fundo, Abrapos, Brasil, 15–18 October 2006; Lorini, B., Beckel, D., Sundfeld, S., Biagi, C., Faroni, B., Santori, E., Guedes, F.S., Eds.; ABRAPOS: Passo Fundo, Brazil, 2006. [Google Scholar]
- Rumbos, C.I.; Dutton, A.C.; Athanassiou, C.G. Insecticidal efficacy of two pirimiphos-methyl formulations for the control of three stored-product beetle species: Effect of commodity. Crop Prot. 2016, 80, 94–100. [Google Scholar] [CrossRef]
- Ortega, D.S.; Bacca, T.; Silva, A.P.N.; Canal, N.A.; Haddi, K. Control failure and insecticides resistance in populations of Rhyzopertha dominica (Coleoptera: Bostrichidae) from Colombia. J. Stored Prod. Res. 2021, 92, 101802. [Google Scholar] [CrossRef]
- Agrafioti, P.; Athanassiou, C.G. Insecticidal effect of contact insecticides against stored product beetle populations with different susceptibility to phosphine. J. Stored Prod. Res. 2018, 79, 9–15. [Google Scholar] [CrossRef]
- Lorini, I.; Galley, D. The Cross-Resistance Spectrum in Deltamethrin Resistance Strains of Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae). Neotr. Entomol. 2001, 30, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Nayak, M.K.; Collins, P.J.; Throne, J.E.; Wang, J. Biology and Management of Psocids Infesting Stored Products. Annu. Rev. Entomol. 2014, 59, 279–297. [Google Scholar] [CrossRef] [Green Version]





| Species | R. dominica | S. zeamais | C. ferrugineus | |||||
|---|---|---|---|---|---|---|---|---|
| Effect (Source) | df | F | p | F | p | F | p | |
| Intercept | 1 | 5494.8 | <0.01 | 3080.2 | <0.01 | 1763.2 | <0.01 | |
| Between variabiles | Population | 1 | 14.2 | <0.01 | 0.5 | 0.47 | <0.1 | 0.92 |
| Insecticide | 2 | 85.8 | <0.01 | 153.6 | <0.01 | 30.2 | <0.01 | |
| Population × Insecticide | 2 | 28.6 | <0.01 | 8.9 | <0.01 | 1.0 | 0.39 | |
| Dose | 2 | 1302.8 | <0.01 | 317.6 | <0.01 | 236.6 | <0.01 | |
| Population × Dose | 2 | 3.8 | 0.02 | 0.9 | 0.40 | 4.3 | 0.02 | |
| Insecticide × Dose | 4 | 64.1 | <0.01 | 45.4 | <0.01 | 4.6 | <0.01 | |
| Population × Insecticide × Dose | 4 | 8.6 | <0.01 | 6.3 | <0.01 | 5.8 | <0.01 | |
| Within variables | Time | 2 | 232.8 | <0.01 | 214.3 | <0.01 | 137.5 | <0.01 |
| Time × Population | 2 | 1.8 | 0.17 | 9.0 | <0.01 | 0.3 | 0.77 | |
| Time × Insecticide | 4 | 6.0 | <0.01 | 5.0 | <0.01 | 47.2 | <0.01 | |
| Time × Population × Insecticide | 4 | 44.0 | <0.01 | 30.1 | <0.01 | 1.7 | 0.15 | |
| Time × Dose | 4 | 36.9 | <0.01 | 16.3 | <0.01 | 18.7 | <0.01 | |
| Time × Population × Dose | 4 | 7.3 | <0.01 | 9.3 | <0.01 | 0.7 | 0.62 | |
| Time × Insecticide × Dose | 8 | 12.0 | <0.01 | 20.0 | <0.01 | 21.1 | <0.01 | |
| Time × Population × Insecticide × Dose | 8 | 19.2 | <0.01 | 10.2 | <0.01 | 1.5 | 0.17 | |
| Species | KDt50 | KDt95 | KDt99 | Slope ± SE | X2 | p |
|---|---|---|---|---|---|---|
| R. dominica (field) | 4.5 (−10.1–9.2) | 12.2 (6.2–15.7) | 15.4 (11.4–20.0) | 5.2 ± 0.4 | 100.9 | <0.01 |
| S. zeamais (field) | 70.1 (43.8–91.5) | 151.1 (122.2–221.2) | 184.7 (146.7–282.8) | 8.5 ± 0.0 | 178.5 | <0.01 |
| C. ferrugineus (field) | 91.6 (78.2–115.0) | 166.9 (134.8–260.3) | 198.1 (156.0–322.8) | 6.5 ± 0.0 | 119.8 | <0.01 |
| R. dominica (lab) | 8.9 (4.2–10.4) | 13.4 (11.9–16.9) | 15.2 (13.4–21.4) | 3.2 ± 0.1 | 3.1 | 1.0 |
| S. zeamais (lab) | a | a | a | a | a | a |
| C. ferrugineus (lab) | 8.8 (6.9–9.6) | 12.6 (11.7–14.7) | 14.2 (12.9–17.7) | 3.9 ± 0.1 | 25.8 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baliota, G.V.; Lampiri, E.; Batzogianni, E.N.; Athanassiou, C.G. Insecticidal Effect of Four Insecticides for the Control of Different Populations of Three Stored-Product Beetle Species. Insects 2022, 13, 325. https://doi.org/10.3390/insects13040325
Baliota GV, Lampiri E, Batzogianni EN, Athanassiou CG. Insecticidal Effect of Four Insecticides for the Control of Different Populations of Three Stored-Product Beetle Species. Insects. 2022; 13(4):325. https://doi.org/10.3390/insects13040325
Chicago/Turabian StyleBaliota, Georgia V., Evagelia Lampiri, Evanthia N. Batzogianni, and Christos G. Athanassiou. 2022. "Insecticidal Effect of Four Insecticides for the Control of Different Populations of Three Stored-Product Beetle Species" Insects 13, no. 4: 325. https://doi.org/10.3390/insects13040325
APA StyleBaliota, G. V., Lampiri, E., Batzogianni, E. N., & Athanassiou, C. G. (2022). Insecticidal Effect of Four Insecticides for the Control of Different Populations of Three Stored-Product Beetle Species. Insects, 13(4), 325. https://doi.org/10.3390/insects13040325







