Assessment of Chestnut Gall Toughness: Implications for a Biocontrol Agent
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Sites
2.2. Collection and Dissection of the Galls
2.3. Gall Toughness Analysis
2.4. Insects
2.5. Oviposition Trials
2.6. Statistical Analysis
3. Results
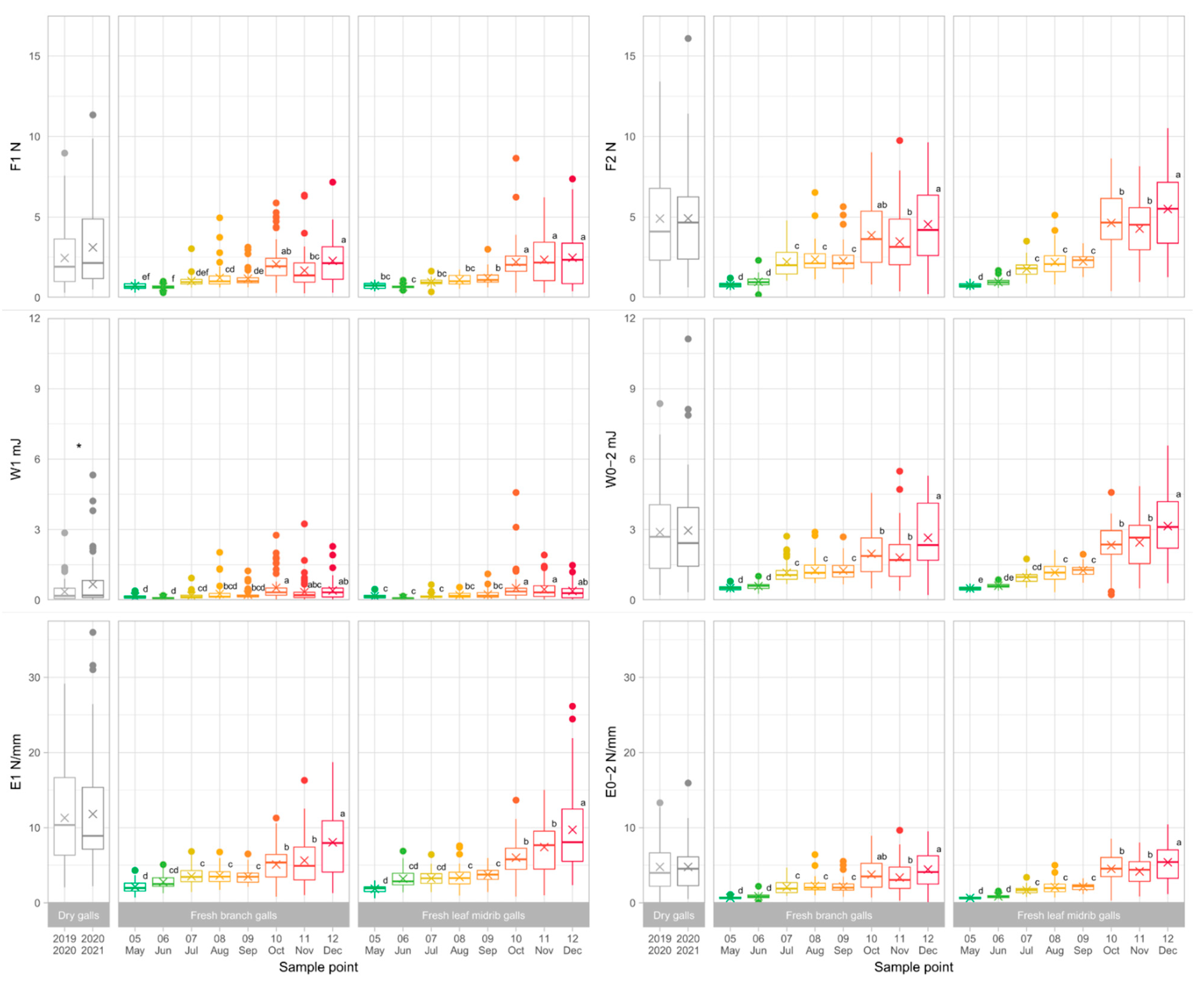
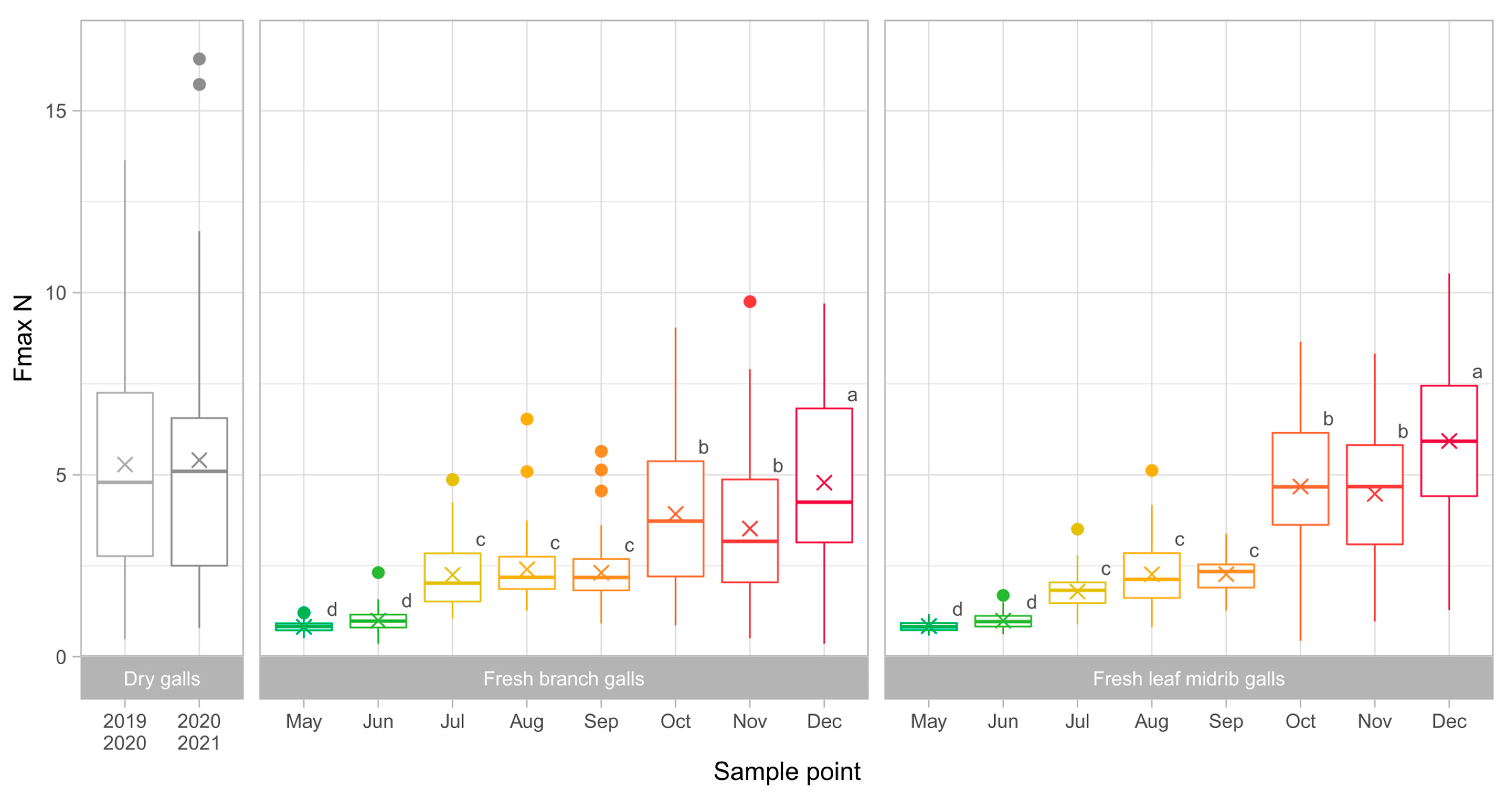
3.1. Collection and Dissection of the Galls
3.2. Gall Toughness Analysis
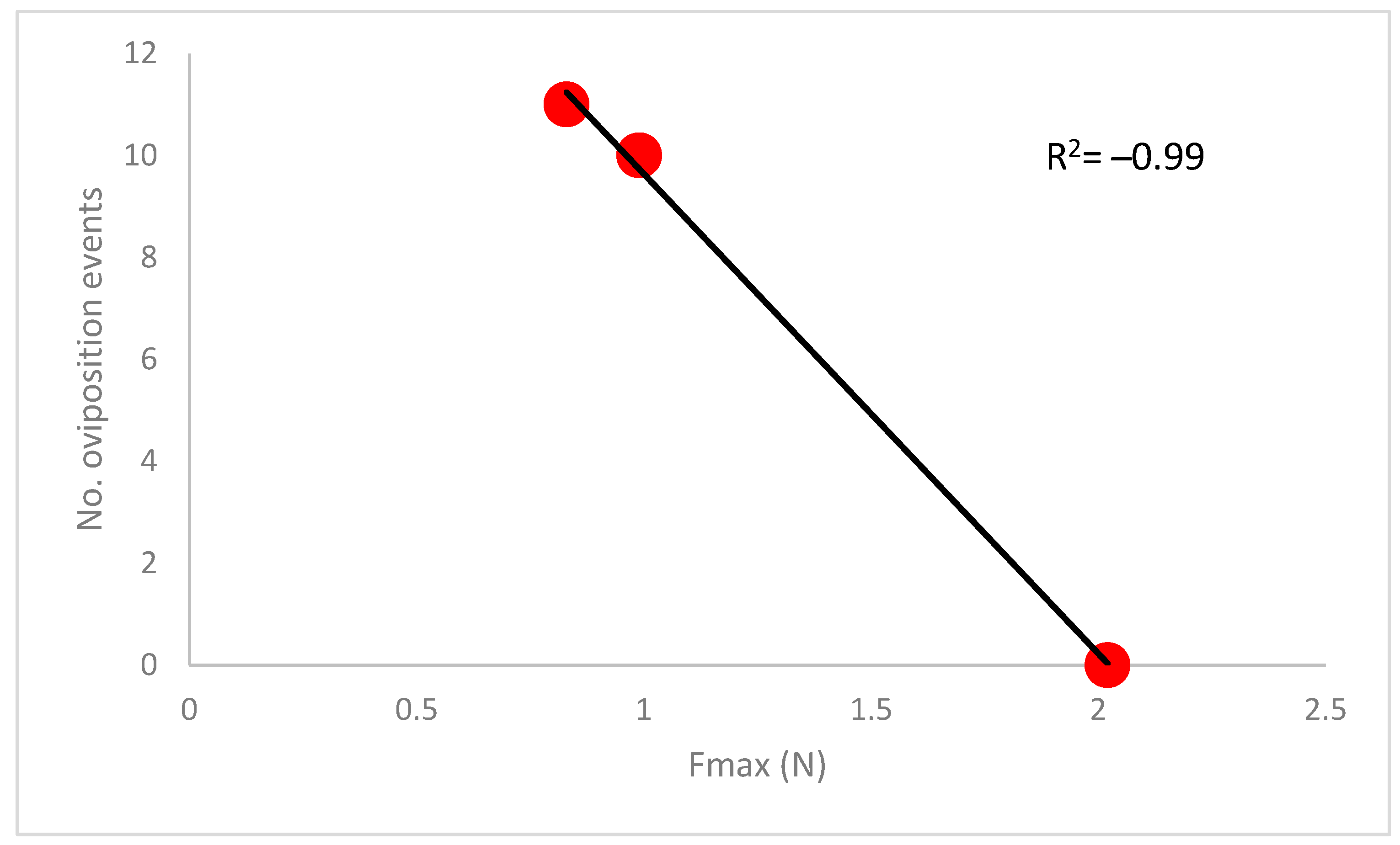
3.3. Oviposition Trials
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mani, M.S. Ecology of Plant Galls; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Stone, G.N.; Schönrogge, K. The adaptive significance of insect gall morphology. Trends Ecol. Evol. 2003, 18, 512–522. [Google Scholar] [CrossRef]
- Ronquist, F.; Liljeblad, J. Evolution of the gall wasp–host plant association. Evolution 2001, 55, 2503–2522. [Google Scholar] [PubMed]
- Abe, Y.; Melika, G.; Stone, G.H. The diversity and phylogeography of cynipid gall wasps (Hymenoptera: Cynipidae) of the oriental and eastern palearctic regions, and their associated communities. Orient. Insects 2007, 41, 169–212. [Google Scholar] [CrossRef]
- Hartley, S.E. The chemical composition of plant galls: Are levels of nutrients and secondary compounds controlled by the gall-former? Oecologia 1998, 113, 492–501. [Google Scholar] [CrossRef]
- Fay, P.A.; Hartnett, D.C.; Knapp, A.K. Plant tolerance of gall-insect attack and gall-insect performance. Ecology 1996, 77, 521–534. [Google Scholar] [CrossRef]
- Stone, G.N.; Schönrogge, K.; Atkinson, R.J.; Bellido, D.; Pujade-Villar, J. The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annu. Rev. Entomol. 2002, 47, 633–668. [Google Scholar] [CrossRef]
- Zargaran, M.R.; Safaralizadeh, M.H.; Pourmirza, A.A.; Valizadegan, O. Effect of cardinal directions on gall morphology and parasitization of the gall wasp, Cynips quercusfolii. J. Insect Sci. 2011, 11, 169. [Google Scholar] [CrossRef]
- Moriya, S.; Shiga, M.; Adachi, I. Classical biological control of the chestnut gall wasp in Japan. In Proceedings of the 1st International Symposium on Biological Control of Arthropods, Honolulu, HI, USA, 14–18 January 2002; Van Driesche, R.G., Ed.; USDA Forest Service: Washington, DC, USA, 2003; pp. 407–415. [Google Scholar]
- Brussino, G.; Bosio, G.; Baudino, M.; Giordano, R.; Ramello, F.; Melika, G. Pericoloso insetto esotico per il castagno europeo. Inf. Agrar. 2002, 58, 59–61. [Google Scholar]
- Payne, J.A. Oriental chestnut gall wasp: New nut pest in North America. In Proceedings of the American Chestnut Symposium, Morgantown, WV, USA, 4–5 January 1978; Macdonald, W.L., Cech, F.C., Luchok, J., Smith, C., Eds.; West Virginia University Press: Morgantown, WV, USA, 1978; pp. 86–88. [Google Scholar]
- Battisti, A.; Benvegnù, I.; Colombari, F.; Haack, R.A. Invasion by the chestnut gall wasp in Italy causes significant yield loss in Castanea sativa nut production. Agric. For. Entomol. 2013, 16, 75–79. [Google Scholar] [CrossRef]
- Avtzis, D.N.; Melika, G.; Matošević, D.; Coyle, D.R. The Asian chestnut gall wasp Dryocosmus kuriphilus: A global invader and a successful case of classical biological control. J. Pest Sci. 2019, 92, 107–115. [Google Scholar] [CrossRef]
- Kato, K.; Hijii, N. Optimal clutch size of the chestnut gall-wasp, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae). Popul. Ecol. 1993, 35, 1–14. [Google Scholar] [CrossRef]
- Gilbert, F.; Astbury, C.; Bedingfield, J.; Ennis, B.; Lawson, S.; Sitch, T. The ecology of the pea galls of Cynips divisa. In Plant Galls; Michele, A.J., Williams, A.J., Eds.; Clarendon Press: Oxford, UK, 1994; Volume 49, pp. 331–349. [Google Scholar]
- Bernardo, U.; Iodice, L.; Sasso, R.; Tutore, V.A.; Cascone, P.; Guerrieri, E. Biology and monitoring of Dryocosmus kuriphilus on Castanea sativa in southern Italy. Agric. For. Entomol. 2013, 15, 65–76. [Google Scholar] [CrossRef]
- Gehring, E.; Kast, C.; Kilchenmann, V.; Bieri, K.; Gehrig, R.; Pezzatti, G.B.; Conedera, M. Impact of the Asian Chestnut Gall Wasp, Dryocosmus kuriphilus (Hymenoptera, Cynipidae), on the Chestnut Component of Honey in the Southern Swiss Alps. J. Econ. Entomol. 2018, 111, 43–52. [Google Scholar] [CrossRef]
- Quacchia, A.; Ferracini, C.; Nicholls, J.A.; Piazza, E.; Saladini, M.A.; Tota, F.; Melika, G.; Alma, A. Chalcid parasitoid community associated with the invading pest Dryocosmus kuriphilus in North-Western Italy. Insect Conserv. Divers. 2013, 6, 114–123. [Google Scholar] [CrossRef]
- Panzavolta, T.; Bernardo, U.; Bracalini, M.; Cascone, P.; Croci, F.; Gebiola, M.; Iodice, L.; Tiberi, R.; Guerrieri, E. Native parasitoids associated with Dryocosmus kuriphilus in Tuscany, Italy. Bull. Insectology 2013, 66, 195–201. [Google Scholar]
- Ferracini, C.; Bertolino, S.; Bernardo, U.; Bonsignore, C.P.; Faccoli, M.; Ferrari, E.; Lupi, D.; Maini, S.; Mazzon, L.; Nugnes, F.; et al. Do Torymus sinensis (Hymenoptera: Torymidae) and agroforestry system affect native parasitoids associated with the Asian chestnut gall wasp? Biol. Control 2018, 121, 36–43. [Google Scholar] [CrossRef]
- Ferracini, C.; Ferrari, C.; Pontini, M.; Saladini, M.A.; Alma, A. Effectiveness of Torymus sinensis: A successful long-term control of the Asian chestnut gall wasp in Italy. J. Pest Sci. 2019, 92, 353–359. [Google Scholar] [CrossRef]
- Ferracini, C.; Gonella, E.; Ferrari, E.; Saladini, M.A.; Picciau, L.; Tota, F.; Pontini, M.; Alma, A. Novel insight in the life cycle of Torymus sinensis, biocontrol agent of the chestnut gall wasp. Biocontrol 2015, 60, 169–177. [Google Scholar] [CrossRef]
- Quacchia, A.; Moriya, S.; Askew, R.; Schönrogge, K. Torymus sinensis: Biology, host range and hybridization. Acta Hortic. 2014, 1043, 105–111. [Google Scholar] [CrossRef]
- Briggs, C.J.; Latto, J. The window of vulnerability and its effect on relative parasitoid abundance. Ecol. Entomol. 1996, 21, 128–140. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Espírito-Santo, M.M.; Faria, M.L. Cynipid gall growth dynamics and enemy attack: Effects of gall size, toughness and thickness. An. Soc. Entomol. Bras. 1999, 28, 211–218. [Google Scholar] [CrossRef]
- Price, P.W.; Clancy, K.M. Interactions Among Three Trophic Levels: Gall size and Parasitoid Attack. Ecology 1986, 67, 1593–1600. [Google Scholar] [CrossRef]
- Weis, A.E.; Abrahamson, W.G.; McCrea, K.D. Host gall size and oviposition success by the parasitoid Eurytoma gigantea. Ecol. Entomol. 1985, 10, 341–348. [Google Scholar] [CrossRef]
- Nilsson, M.; Corley, J.C.; Anderbrant, O. Factors affecting success of galls of Aditrochus coihuensis (Hymenoptera: Pteromalidae). Rev. Soc. Entomol. Argent. 2011, 70, 337–346. [Google Scholar]
- Dixon, K.A.; Lerma, R.R.; Craig, T.P.; Hughes, K.A. Gall morphology and community composition in Asphondylia flocosa (Cecidomyiidae) galls on Atriplex polycarpa (Chenopodiaceae). Environ. Entomol. 1998, 27, 592–599. [Google Scholar] [CrossRef]
- Ferracini, C.; Pogolotti, C.; Alma, A. A mismatch in the emergence of Torymus sinensis may affect the effectiveness of this biocontrol agent? Biol. Control 2022, 174, 105029. [Google Scholar] [CrossRef]
- Gil-Tapetado, D.; Cabrero-Sanudo, F.J.; Polidori, C.; Gomez, J.F.; Nieves-Aldrey, J.L. Climate as a possible driver of gall morphology in the chestnut pest Dryocosmus kuriphilus across Spanish invaded areas. Bull. Entomol. Res. 2021, 111, 160–173. [Google Scholar] [CrossRef]
- Valentini, N.; Moraglio, S.T.; Rolle, L.; Tavella, L.; Botta, R. Nut and kernel growth and shell hardening in eighteen hazelnut cultivars. Hortic. Sci. 2015, 42, 149–158. [Google Scholar] [CrossRef]
- Giacosa, S.; Belviso, S.; Bertolino, M.; Dal Bello, B.; Gerbi, V.; Ghirardello, D.; Giordano, M.; Zeppa, G.; Rolle, L. Hazelnut kernels (Corylus avellana L.) mechanical and acoustic properties determination: Comparison of test speed, compression or shear axis, roasting, and storage condition effect. J. Food Eng. 2016, 173, 59–68. [Google Scholar] [CrossRef]
- Ferracini, C.; Ferrari, E.; Pontini, M.; Hernández Nova, L.K.; Saladini, M.A.; Alma, A. Post-release evaluation of non-target effects of Torymus sinensis, the biological control agent of Dryocosmus kuriphilus in Italy. Biocontrol 2017, 62, 445–456. [Google Scholar] [CrossRef]
- Gil-Tapetado, D.; Castedo-Dorado, F.; Luis, J.; Nieves-Aldrey, M.; Lombardero, J. Gall size of Dryocosmus kuriphilus limits down-regulation by native parasitoids. Biol. Invasions 2021, 23, 1157–1174. [Google Scholar] [CrossRef]
- Craig, T.P.; Itami, J.K.; Price, P.W. The window of vulnerability of a shoot-galling sawfly to attack by a parasitoid. Ecology 1990, 71, 1471–1482. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A. Insect seasonality: Diapause maintenance, termination, and post diapause development. Annu. Rev. Entomol. 1976, 21, 81–107. [Google Scholar] [CrossRef]
- Ellers, J.; Van Alphen, J.J.M. A trade-off between diapause duration and fitness in female parasitoids. Ecol. Entomol. 2002, 27, 279–284. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferracini, C.; Pogolotti, C.; Giacosa, S.; Fontana, E.V.; Rolle, L.; Alma, A. Assessment of Chestnut Gall Toughness: Implications for a Biocontrol Agent. Insects 2022, 13, 1095. https://doi.org/10.3390/insects13121095
Ferracini C, Pogolotti C, Giacosa S, Fontana EV, Rolle L, Alma A. Assessment of Chestnut Gall Toughness: Implications for a Biocontrol Agent. Insects. 2022; 13(12):1095. https://doi.org/10.3390/insects13121095
Chicago/Turabian StyleFerracini, Chiara, Cristina Pogolotti, Simone Giacosa, Eleonora Vittoria Fontana, Luca Rolle, and Alberto Alma. 2022. "Assessment of Chestnut Gall Toughness: Implications for a Biocontrol Agent" Insects 13, no. 12: 1095. https://doi.org/10.3390/insects13121095
APA StyleFerracini, C., Pogolotti, C., Giacosa, S., Fontana, E. V., Rolle, L., & Alma, A. (2022). Assessment of Chestnut Gall Toughness: Implications for a Biocontrol Agent. Insects, 13(12), 1095. https://doi.org/10.3390/insects13121095









