Chemical Control of Mosquitoes and the Pesticide Treadmill: A Case for Photosensitive Insecticides as Larvicides
Abstract
Simple Summary
Abstract
1. Introduction
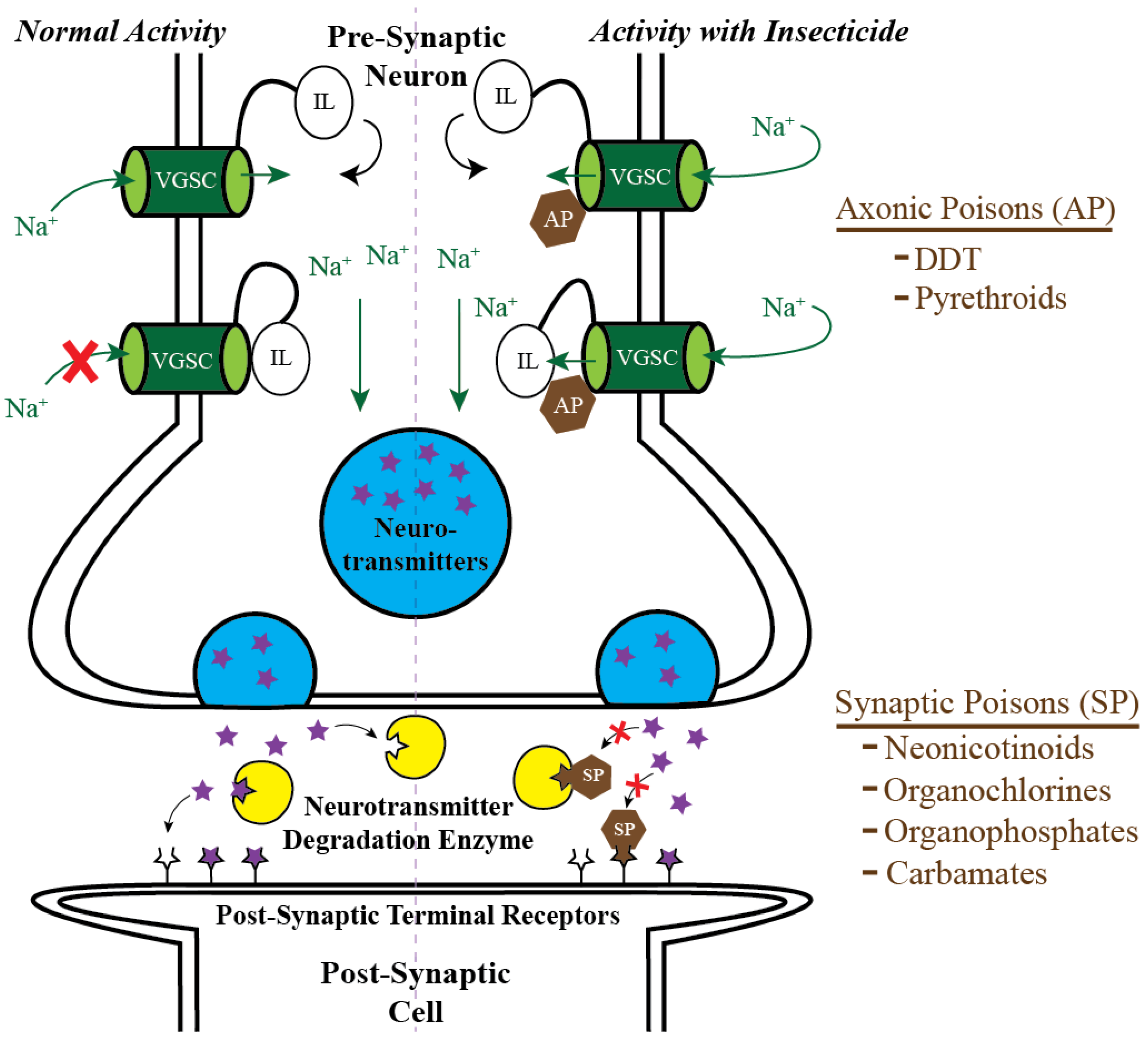
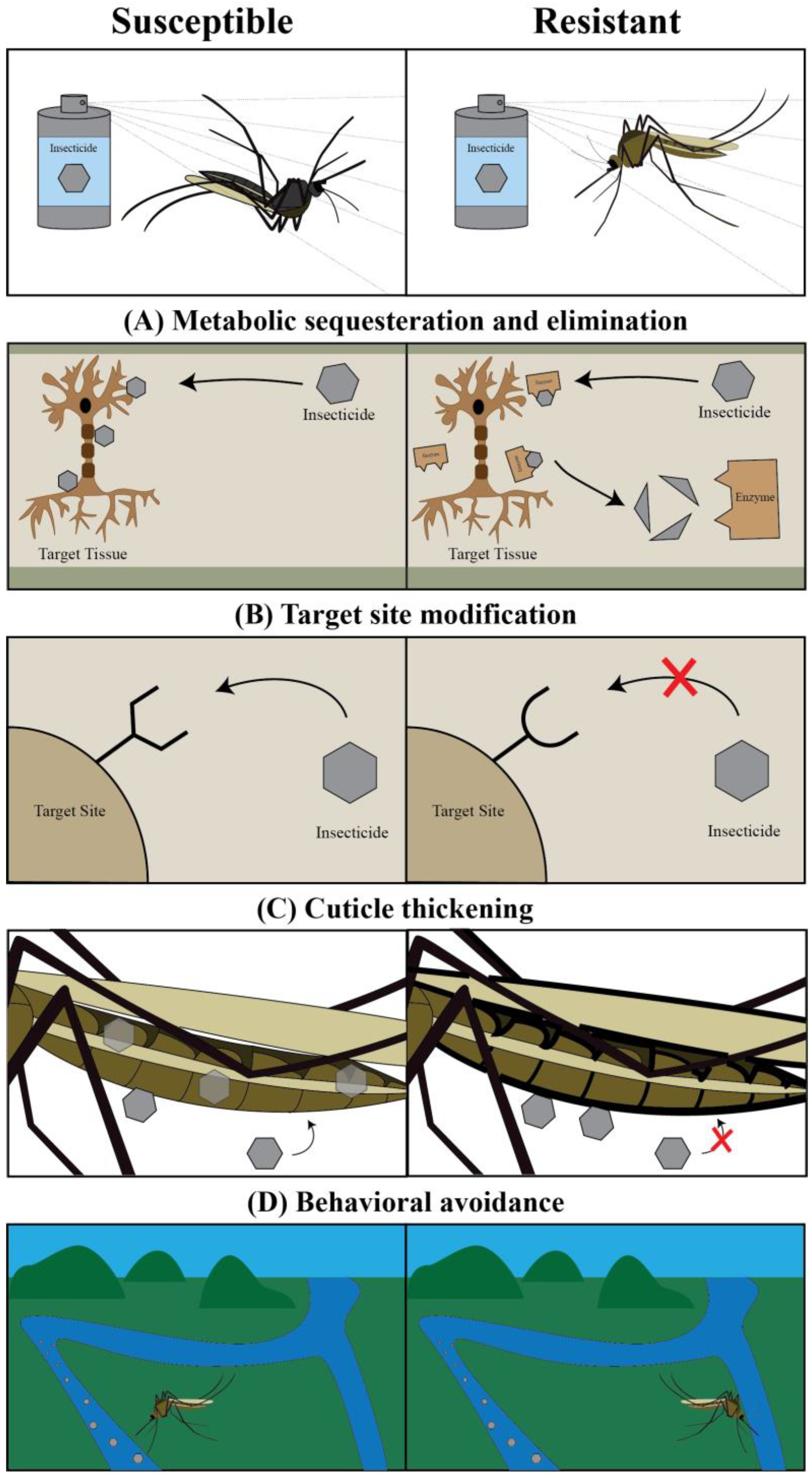
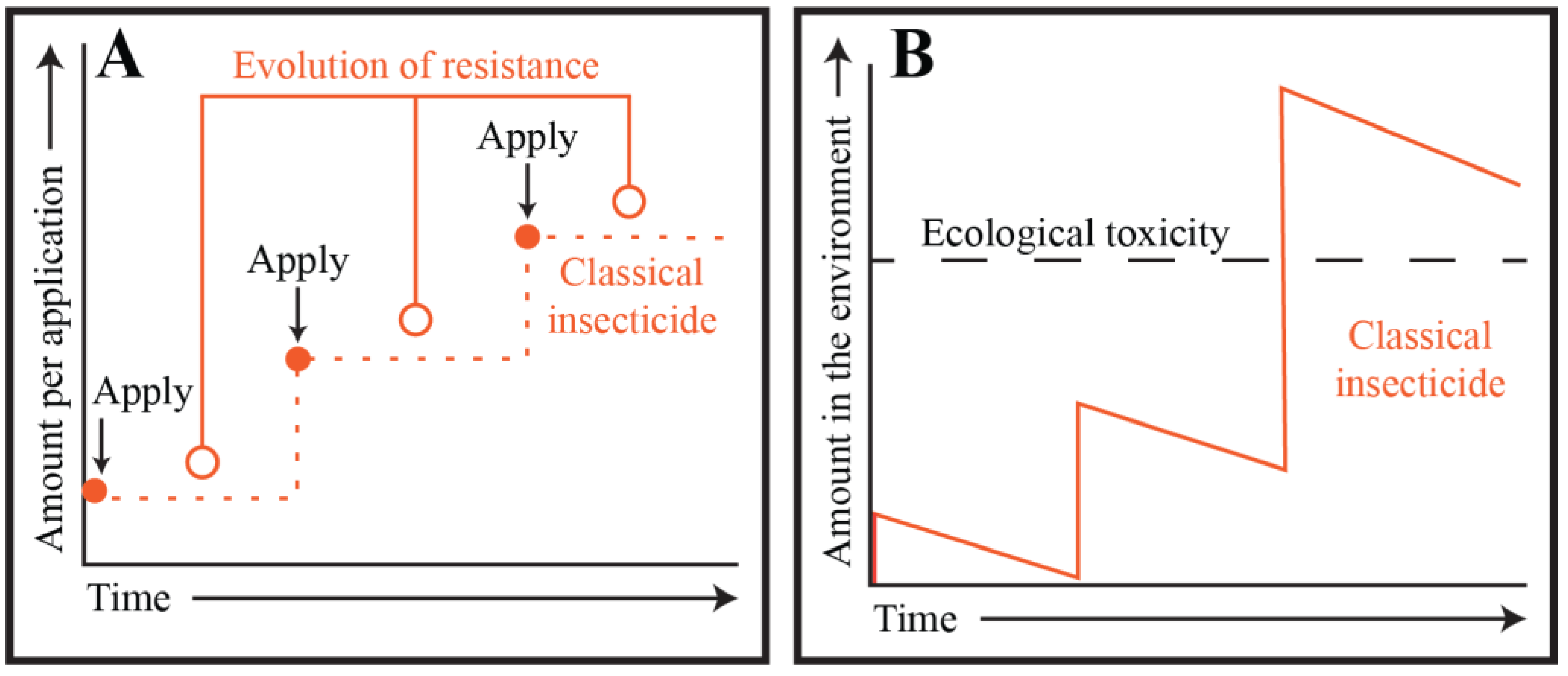
2. Targeting Mosquito Adults: Classical Chemical Insecticides and the Pesticide Treadmill
3. Targeting Mosquito Adults: The Ecological Cost of Classical Chemical Insecticides
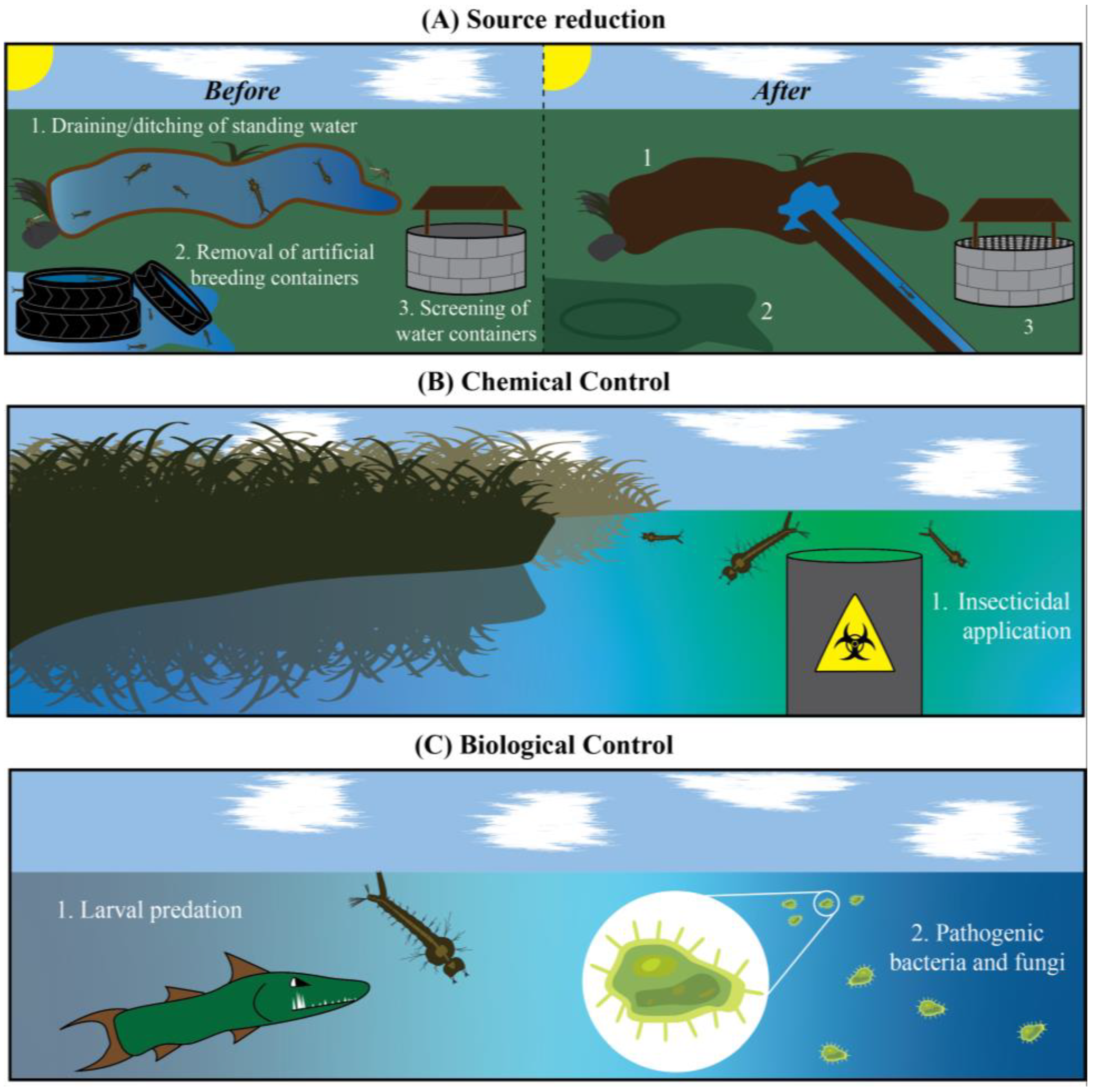
4. Targeting Mosquito Larvae: A Limited Arsenal of Insecticides
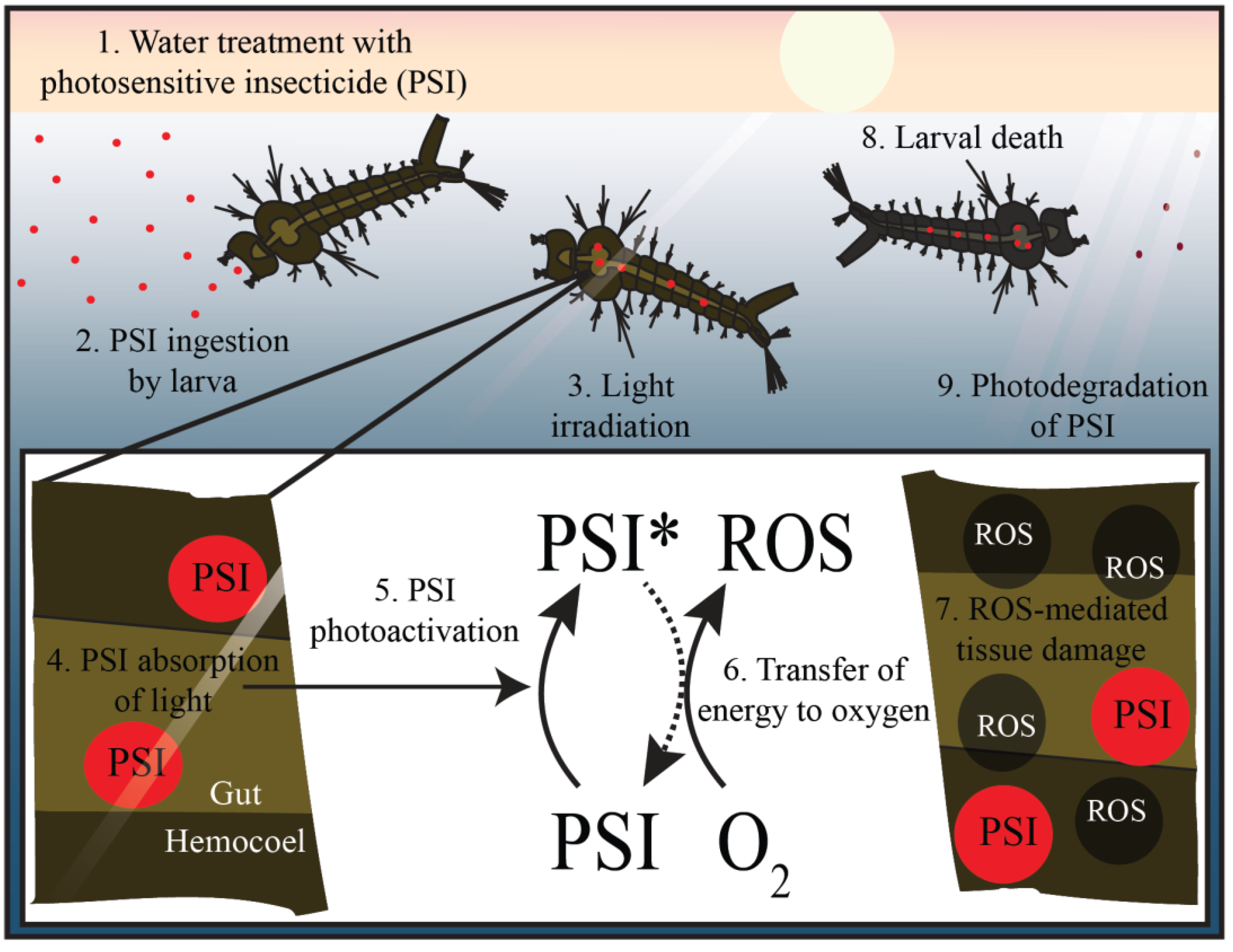
5. A Case for Photosensitive Insecticides as Mosquito Larvicides
6. Photosensitive Insecticides: Practical Outcomes and Outstanding Questions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Vector Control Response 2017–2030; WHO: Geneva, Switzerland, 2017; p. 53. [Google Scholar]
- da Silva, A.F.; Machado, L.C.; de Paula, M.B.; da Silva Pessoa Vieira, C.J.; de Morais Bronzoni, R.V.; de Melo Santos, M.A.V.; Wallau, G.L. Culicidae evolutionary history focusing on the Culicinae subfamily based on mitochondrial phylogenomics. Sci. Rep. 2020, 10, 18823. [Google Scholar] [CrossRef] [PubMed]
- Misof, B.; Liu, S.; Meusemann, K.; Peters, R.S.; Donath, A.; Mayer, C.; Frandsen, P.B.; Ware, J.; Flouri, T.; Beutel, R.G.; et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 2014, 346, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Dahl, C.; Madon, M.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; p. 577. [Google Scholar]
- Huang, Y.S.; Higgs, S.; Vanlandingham, D.L. Emergence and re-emergence of mosquito-borne arboviruses. Curr. Opin. Virol. 2019, 34, 104–109. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021; p. 322. [Google Scholar]
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Primers 2017, 3, 17050. [Google Scholar] [CrossRef] [PubMed]
- Talman, A.M.; Clain, J.; Duval, R.; Menard, R.; Ariey, F. Artemisinin bioactivity and resistance in malaria parasites. Trends Parasitol. 2019, 35, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Yellow fever vaccine. Expert Rev. Vaccines 2005, 4, 553–574. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; Lecuit, M. Zika, Chikungunya, and other emerging vector-borne viral diseases. Annu. Rev. Med. 2018, 69, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Walker, K.; Lynch, M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: Review of achievements and potential. Med. Vet. Entomol. 2007, 21, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Churcher, T.S.; Lissenden, N.; Griffin, J.T.; Worrall, E.; Ranson, H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife 2016, 5, e16090. [Google Scholar] [CrossRef]
- Bowman, L.R.; Donegan, S.; McCall, P.J. Is dengue vector control deficient in effectiveness or evidence?: Systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004551. [Google Scholar] [CrossRef]
- van den Berg, H.; da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, E.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef]
- WHO. Global Insecticide Use for Vector-Borne Disease Control, 4th ed.; WHO: Geneva, Switzerland, 2009; p. 91. [Google Scholar]
- WHO. Global Insecticide Use for Vector-Borne Disease Control: A 10-Year Assessment (2010–2019), 6th ed.; WHO: Geneva, Switzerland, 2021; p. 64. [Google Scholar]
- Brady, O.J.; Godfray, H.C.; Tatem, A.J.; Gething, P.W.; Cohen, J.M.; McKenzie, F.E.; Perkins, T.A.; Reiner, R.C., Jr.; Tusting, L.S.; Sinka, M.E.; et al. Vectorial capacity and vector control: Reconsidering sensitivity to parameters for malaria elimination. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 107–117. [Google Scholar] [CrossRef]
- EPA. Joint Statement on Mosquito Control in the United States. Available online: https://www.epa.gov/mosquitocontrol/joint-statement-mosquito-control-united-states (accessed on 10 November 2022).
- Peper, S.T.; Xue, R.D.; Presley, S.M. Status of vector control capabilities and capacities in Florida and Texas, and its potential public health consequences. J. Am. Mosq. Control Assoc. 2022, 38, 104–108. [Google Scholar] [CrossRef]
- Townson, H.; Nathan, M.B.; Zaim, M.; Guillet, P.; Manga, L.; Bos, R.; Kindhauser, M. Exploiting the potential of vector control for disease prevention. Bull. World Health Organ. 2005, 83, 942–947. [Google Scholar] [PubMed]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Giordano, G.; Guizzetti, M.; Vitalone, A. Neurotoxicity of pesticides: A brief review. Front. Biosci. 2008, 13, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.G.; Field, L.M.; Usherwood, P.N.; Williamson, M.S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef]
- Radcliffe, E.B.; Hutchison, W.D.; Cancelado, R.E. Integrated Pest Management: Concepts, Tactics, Strategies and Case Studies; Cambridge University Press: Cambridge, UK, 2009; p. 529. [Google Scholar]
- Stenersen, J. Chemical Pesticides Mode of Action and Toxicology; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Soderlund, D.M. State-dependent modification of voltage-gated sodium channels by pyrethroids. Pestic. Biochem. Physiol. 2010, 97, 78–86. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 2003, 48, 339–364. [Google Scholar] [CrossRef]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef] [PubMed]
- Namias, A.; Jobe, N.B.; Paaijmans, K.P.; Huijben, S. The need for practical insecticide-resistance guidelines to effectively inform mosquito-borne disease control programs. Elife 2021, 10, e65655. [Google Scholar] [CrossRef]
- Ranson, H.; Abdallah, H.; Badolo, A.; Guelbeogo, W.M.; Kerah-Hinzoumbe, C.; Yangalbe-Kalnone, E.; Sagnon, N.; Simard, F.; Coetzee, M. Insecticide resistance in Anopheles gambiae: Data from the first year of a multi-country study highlight the extent of the problem. Malar. J. 2009, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Burhani, J.; Lumjuan, N.; Black IV, W.C. Insecticide resistance in dengue vectors. TropIKA.net 2010, 1, 1–12. [Google Scholar]
- Whalon, M.E.; Mota-Sanchez, D.; Hollingworth, R.M. Analysis of global pesticide resistance in arthropods. In Global Pesticide Resistance in Arthropods; Whalon, M.E., Mota-Sanchez, D., Hollingworth, R.M., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 5–31. [Google Scholar]
- Carrasco, D.; Lefevre, T.; Moiroux, N.; Pennetier, C.; Chandre, F.; Cohuet, A. Behavioural adaptations of mosquito vectors to insecticide control. Curr. Opin. Insect Sci. 2019, 34, 48–54. [Google Scholar] [CrossRef]
- Despres, L.; David, J.P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Auteri, M.; La Russa, F.; Blanda, V.; Torina, A. Insecticide resistance associated with kdr mutations in Aedes albopictus: An update on worldwide evidences. Biomed. Res. Int. 2018, 2018, 3098575. [Google Scholar] [CrossRef]
- Saha, P.; Chatterjee, M.; Ballav, S.; Chowdhury, A.; Basu, N.; Maji, A.K. Prevalence of kdr mutations and insecticide susceptibility among natural population of Aedes aegypti in West Bengal. PLoS ONE 2019, 14, e0215541. [Google Scholar] [CrossRef]
- Guedes, R.N.; Cutler, G.C. Insecticide-induced hormesis and arthropod pest management. Pest. Manag. Sci. 2014, 70, 690–697. [Google Scholar] [CrossRef]
- Knight, A.L.; Norton, G.W. Economics of agricultural pesticide resistance in arthropods. Ann. Rev. Entomol. 1989, 34, 293–313. [Google Scholar] [CrossRef]
- Bisset, J.; Rodriguez, M.; Soca, A.; Pasteur, N.; Raymond, M. Cross-resistance to pyrethroid and organophosphorus insecticides in the southern house mosquito (Diptera:Culicidae) from Cuba. J. Med. Entomol. 1997, 34, 244–246. [Google Scholar] [CrossRef]
- Peshin, R.; Zhang, W. Integrated pest management and pesticide use. In Integrated Pest Management; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–46. [Google Scholar]
- Prasittisuk, C.; Busvine, J.R. DDT-resistant mosquito strains with cross-resistance to pyrethroids. Pestic. Sci. 1977, 8, 527–533. [Google Scholar] [CrossRef]
- Tabashnik, B.E. Managing resistance with multiple pesticide tactics: Theory, evidence, and recommendations. J. Econ. Entomol. 1989, 82, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, Q.; Zhu, F.; Zhang, L. Pyrethroid resistance in mosquitoes. Insect Sci. 2006, 13, 159–166. [Google Scholar] [CrossRef]
- Mitchell, S.N.; Stevenson, B.J.; Muller, P.; Wilding, C.S.; Egyir-Yawson, A.; Field, S.G.; Hemingway, J.; Paine, M.J.; Ranson, H.; Donnelly, M.J. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc. Natl. Acad. Sci. USA 2012, 109, 6147–6152. [Google Scholar] [CrossRef]
- Nauen, R.; Denholm, I. Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Arch. Insect Biochem. Physiol. 2005, 58, 200–215. [Google Scholar] [CrossRef]
- Eddleston, M.; Karalliedde, L.; Buckley, N.; Fernando, R.; Hutchinson, G.; Isbister, G.; Konradsen, F.; Murray, D.; Piola, J.C.; Senanayake, N.; et al. Pesticide poisoning in the developing world—A minimum pesticides list. Lancet 2002, 360, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Gonzalez, L.M.; Oria, J.; Sanchez, R.; Arroyo, B. Influence of contamination by organochlorine pesticides and polychlorinated biphenyls on the breeding of the spanish imperial eagle (Aquila adalberti). Environ. Toxicol. Chem. 2008, 27, 433–441. [Google Scholar] [CrossRef]
- Peakall, D.B. Pesticides and the reproduction of birds. Sci. Am. 1970, 222, 72–78. [Google Scholar] [CrossRef]
- Boone, M.D.; James, S.M. Interactions of an insecticide, herbicide, and natural stressors in amphibian community mesocosms. Ecol. Appl. 2003, 13, 829–841. [Google Scholar] [CrossRef]
- Bridges, C.M. Long-term effects of pesticide exposure at various life stages of the southern leopard frog (Rana sphenocephala). Arch. Environ. Contam. Toxicol. 2000, 39, 91–96. [Google Scholar] [CrossRef]
- Guillette, L.J., Jr.; Vonier, P.M.; McLachlan, J.A. Affinity of the alligator estrogen receptor for serum pesticide contaminants. Toxicology 2002, 181–182, 151–154. [Google Scholar] [CrossRef]
- Lind, P.M.; Milnes, M.R.; Lundberg, R.; Bermudez, D.; Orberg, J.A.; Guillette, L.J., Jr. Abnormal bone composition in female juvenile American alligators from a pesticide-polluted lake (Lake Apopka, Florida). Environ. Health Perspect. 2004, 112, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Milnes, M.R.; Bryan, T.A.; Medina, J.G.; Gunderson, M.P.; Guillette, L.J., Jr. Developmental alterations as a result of in ovo exposure to the pesticide metabolite p,p’-DDE in Alligator mississippiensis. Gen. Comp. Endocrinol. 2005, 144, 257–263. [Google Scholar] [CrossRef] [PubMed]
- De Silva, H.J.; Samarawickrema, N.A.; Wickremasinghe, A.R. Toxicity due to organophosphorus compounds: What about chronic exposure? Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Rattner, B.A.; Burton, G.A., Jr.; Cairns, J., Jr. Handbook of Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2002; p. 1312. [Google Scholar]
- Liang, H.C.; Razaviarani, V.; Buchanan, I. Pesticides and herbicides. Water Environ. Res. 2013, 85, 1601–1644. [Google Scholar] [CrossRef]
- Saaristo, M.; Brodin, T.; Balshine, S.; Bertram, M.G.; Brooks, B.W.; Ehlman, S.M.; McCallum, E.S.; Sih, A.; Sundin, J.; Wong, B.B.M.; et al. Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. Biol. Sci. 2018, 285, 20181297. [Google Scholar] [CrossRef]
- Clark, R.D. Predicting mammalian metabolism and toxicity of pesticides in silico. Pest. Manag. Sci. 2018, 74, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M. Properties and applications of pyrethroids. Environ. Health Perspect. 1976, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E. Pesticide interactions: Mechanisms, benefits, and risks. J. Agric. Food Chem. 2017, 65, 4553–4561. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.B.; Case, P.; Chui, S.; Chung, D.; Haeffele, C.; Haston, K.; Lee, M.; Mai, V.P.; Marjuoa, Y.; Parker, J.; et al. Pesticide mixtures, endocrine disruption, and amphibian declines: Are we underestimating the impact? Environ. Health Perspect. 2006, 114 (Suppl. S1), 40–50. [Google Scholar] [CrossRef]
- Howe, G.E.; Gillis, R.; Mowbray, R.C. Effect of chemical synergy and larval stage on the toxicity of atrazine and alachlor to amphibian larvae. Environ. Toxicol. Chem. 1998, 17, 519–525. [Google Scholar] [CrossRef]
- Munn, M.D.; Gilliom, R.J. Pesticide Toxicity Index for Freshwater Aquatic Organisms; US Geological Survey: Sacramento, CA, 2001; p. 61. [Google Scholar]
- Relyea, R.A. Growth and survival of five amphibian species exposed to combinations of pesticides. Environ. Toxicol. Chem. 2004, 23, 1737–1742. [Google Scholar] [CrossRef]
- Wendt-Rasch, L.; Van den Brink, P.J.; Crum, S.J.; Woin, P. The effects of a pesticide mixture on aquatic ecosystems differing in trophic status: Responses of the macrophyte Myriophyllum spicatum and the periphytic algal community. Ecotoxicol. Environ. Saf. 2004, 57, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef]
- Ahtiainen, J.; Aalto, M.; Pessala, P. Biodegradation of chemicals in a standardized test and in environmental conditions. Chemosphere 2003, 51, 529–537. [Google Scholar] [CrossRef]
- Copley, S.D. Evolution of efficient pathways for degradation of anthropogenic chemicals. Nat. Chem. Biol. 2009, 5, 559–566. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Martin, T.J.; Price, O.R.; Snape, J.R.; van Egmond, R.A.; Finnegan, C.J.; Schafer, H.; Davenport, R.J.; Bending, G.D. Refinement of biodegradation tests methodologies and the proposed utility of new microbial ecology techniques. Ecotoxicol. Environ. Saf. 2015, 111, 9–22. [Google Scholar] [CrossRef]
- Reemtsma, T.; Alder, L.; Banasiak, U. Emerging pesticide metabolites in groundwater and surface water as determined by the application of a multimethod for 150 pesticide metabolites. Water Res. 2013, 47, 5535–5545. [Google Scholar] [CrossRef]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. USA 2015, 112, 5750–5755. [Google Scholar] [CrossRef] [PubMed]
- Caragata, E.P.; Dutra, H.L.C.; Sucupira, P.H.F.; Ferreira, A.G.A.; Moreira, L.A. Wolbachia as translational science: Controlling mosquito-borne pathogens. Trends Parasitol. 2021, 37, 1050–1067. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Walker, T.; SL, O.N. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011, 12, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T. Control of malaria-transmitting mosquitoes using gene drives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20190803. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Nyawira, K.T.; Xia, A. New discoveries and applications of mosquito fungal pathogens. Curr. Opin. Insect Sci. 2020, 40, 111–116. [Google Scholar] [CrossRef]
- Yen, P.S.; Failloux, A.B. A review: Wolbachia-based population replacement for mosquito control shares common points with genetically modified control approaches. Pathogens 2020, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Kaya, H.K. Insect Pathology; Academic Press: Boston, MA, USA, 2012; p. 508. [Google Scholar]
- Pener, M.P.; Dhadialla, T.S. An overview of insect growth disruptors; applied aspects. Adv. Insect Phys. 2012, 43, 1–162. [Google Scholar] [CrossRef]
- Floore, T.G. Mosquito larval control practices: Past and present. J. Am. Mosq. Control Assoc. 2006, 22, 527–533. [Google Scholar] [CrossRef]
- Beketov, M.A.; Liess, M. Potential of 11 pesticides to initiate downstream drift of stream macroinvertebrates. Arch. Environ. Contam Toxicol 2008, 55, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.M.; Hiraldo, F. Organochlorine and heavy metal contamination in the eggs of the Spanish Imperial Eagle (Aquila (heliaca) adalberti) and accompanying changes in eggshell morphology and chemistry. Environ. Pollut. 1988, 51, 241–258. [Google Scholar] [CrossRef]
- Muturi, E.J.; Selling, G.W.; Doll, K.M.; Hay, W.T.; Ramirez, J.L. Leptospermum scoparium essential oil is a promising source of mosquito larvicide and its toxicity is enhanced by a biobased emulsifier. PLoS ONE 2020, 15, e0229076. [Google Scholar] [CrossRef]
- Sarma, R.; Adhikari, K.; Mahanta, S.; Khanikor, B. Combinations of plant essential oil based terpene compounds as larvicidal and adulticidal agent against Aedes aegypti (Diptera: Culicidae). Sci. Rep. 2019, 9, 9471. [Google Scholar] [CrossRef]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberon, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Bruhl, C.A.; Despres, L.; Fror, O.; Patil, C.D.; Poulin, B.; Tetreau, G.; Allgeier, S. Environmental and socioeconomic effects of mosquito control in Europe using the biocide Bacillus thuringiensis subsp. israelensis (Bti). Sci. Total Environ. 2020, 724, 137800. [Google Scholar] [CrossRef]
- Dambach, P.; Baernighausen, T.; Traore, I.; Ouedraogo, S.; Sie, A.; Sauerborn, R.; Becker, N.; Louis, V.R. Reduction of malaria vector mosquitoes in a large-scale intervention trial in rural Burkina Faso using Bti based larval source management. Malar. J. 2019, 18, 311. [Google Scholar] [CrossRef]
- Raymond, B.; Johnston, P.R.; Nielsen-LeRoux, C.; Lereclus, D.; Crickmore, N. Bacillus thuringiensis: An impotent pathogen? Trends Microbiol. 2010, 18, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hua, G.; Adang, M.J. Effects and mechanisms of Bacillus thuringiensis crystal toxins for mosquito larvae. Insect Sci. 2017, 24, 714–729. [Google Scholar] [CrossRef]
- Georghiou, G.P.; Wirth, M.C. Influence of exposure to single versus multiple toxins of Bacillus thuringiensis subsp. israelensis on development of resistance in the mosquito Culex quinquefasciatus (Diptera: Culicidae). Appl. Environ. Microbiol. 1997, 63, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Griffitts, J.S.; Aroian, R.V. Many roads to resistance: How invertebrates adapt to Bt toxins. Bioessays 2005, 27, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Tetreau, G.; Bayyareddy, K.; Jones, C.M.; Stalinski, R.; Riaz, M.A.; Paris, M.; David, J.P.; Adang, M.J.; Despres, L. Larval midgut modifications associated with Bti resistance in the yellow fever mosquito using proteomic and transcriptomic approaches. BMC Genom. 2012, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Tilquin, M.; Paris, M.; Reynaud, S.; Despres, L.; Ravanel, P.; Geremia, R.A.; Gury, J. Long lasting persistence of Bacillus thuringiensis Subsp. israelensis (Bti) in mosquito natural habitats. PLoS ONE 2008, 3, e3432. [Google Scholar] [CrossRef]
- Wirth, M.C.; Park, H.W.; Walton, W.E.; Federici, B.A. Cyt1A of Bacillus thuringiensis delays evolution of resistance to Cry11A in the mosquito Culex quinquefasciatus. Appl. Environ. Microbiol. 2005, 71, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Goldman, I.F.; Arnold, J.; Carlton, B.C. Selection for resistance to Bacillus thuringiensis subspecies israelensis in field and laboratory populations of the mosquito Aedes aegypti. J. Invertebr. Pathol. 1986, 47, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Paris, M.; Tetreau, G.; Laurent, F.; Lelu, M.; Despres, L.; David, J.P. Persistence of Bacillus thuringiensis israelensis (Bti) in the environment induces resistance to multiple Bti toxins in mosquitoes. Pest. Manag. Sci. 2011, 67, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Harrington, L.C.; Zhang, L.; Scott, J.G. Insecticide resistance in Culex pipiens from New York. J. Am. Mosq. Control Assoc. 2005, 21, 305–309. [Google Scholar] [CrossRef]
- Saleh, M.S.; El-Meniawi, F.A.; Kelada, N.L.; Zahran, H.M. Resistance development in mosquito larvae Culex pipiens to the bacterial agent Bacillus thuringiensis var. israelensis. J. Appl. Entomol. 2003, 127, 29–32. [Google Scholar] [CrossRef]
- Moltini-Conclois, I.; Stalinski, R.; Tetreau, G.; Despres, L.; Lambrechts, L. Larval exposure to the bacterial insecticide Bti enhances dengue virus susceptibility of adult Aedes aegypti mosquitoes. Insects 2018, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Jablonski, A. Efficiency of anti-stokes fluorescence in dyes. Nature 1933, 131, 839–840. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy and the development of metal-based photosensitisers. Met. Based Drugs 2008, 2008, 276109. [Google Scholar] [CrossRef]
- McLean, A.J.; McGarvey, D.J.; Truscott, T.G.; Lambert, C.R.; Land, E.J. Effect of oxygen-enhanced intersystem crossing on the observed efficiency of formation of singlet oxygen. J. Chem. Soc. Faraday Trans. 1990, 86, 3075–3080. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology; Blackwell Science Oxford: Oxford, UK, 1997. [Google Scholar]
- Barbieri, A. Fluorescent sensitising substances as larvicides. The photodynamic action of light. Riv. Malariol. 1928, 7, 456–463. [Google Scholar]
- Ben Amor, T.; Jori, G. Sunlight-activated insecticides: Historical background and mechanisms of phototoxic activity. Insect Biochem. Mol. Biol. 2000, 30, 915–925. [Google Scholar] [CrossRef]
- Borovsky, D.; Linley, J.R.; Kagan, J. Polycyclic aromatic compounds as phototoxic mosquito larvicides. J. Am. Mosq. Control Assoc. 1987, 3, 246–250. [Google Scholar]
- Heitz, J.R. Pesticidal applications of photoactivated molecules. In Light-Activated Pest Control; American Chemical Society: Washington, DC, USA, 1995; Volume 616, pp. 1–16. [Google Scholar]
- Lima, A.R.; Silva, C.M.; Caires, C.S.A.; Prado, E.D.; Rocha, L.R.P.; Cabrini, I.; Arruda, E.J.; Oliveira, S.L.; Caires, A.R.L. Evaluation of eosin-methylene blue as a photosensitizer for larval control of Aedes aegypti by a photodynamic process. Insects 2018, 9, 109. [Google Scholar] [CrossRef]
- Mezzacappo, N.F.; de Souza, L.M.; Inada, N.M.; Dias, L.D.; Garbuio, M.; Venturini, F.P.; Correa, T.Q.; Moura, L.; Blanco, K.C.; de Oliveira, K.T.; et al. Curcumin/d-mannitol as photolarvicide: Induced delay in larval development time, changes in sex ratio and reduced longevity of Aedes aegypti. Pest. Manag. Sci. 2021, 77, 2530–2538. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.H.; Weng, S.C.; Luan, L.; Vicente, M.; Jiang, X.J.; Ng, D.K.P.; Kolli, B.K.; Chang, K.P. Novel phthalocyanines activated by dim light for mosquito larva- and cell-inactivation with inference for their potential as broad-spectrum photodynamic insecticides. PLoS ONE 2019, 14, e0217355. [Google Scholar] [CrossRef]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.; Ogilby, P.R. Lifetime and diffusion of singlet oxygen in a cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef]
- Venturini, F.P.; de Souza, L.M.; Garbuio, M.; Inada, N.M.; de Souza, J.P.; Kurachi, C.; de Oliveira, K.T.; Bagnato, V.S. Environmental safety and mode of action of a novel curcumin-based photolarvicide. Environ. Sci. Pollut. Res. Int. 2020, 27, 29204–29217. [Google Scholar] [CrossRef]
- Maisch, T. Resistance in antimicrobial photodynamic inactivation of bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Onoue, S.; Tsuda, Y. Analytical studies on the prediction of photosensitive/phototoxic potential of pharmaceutical substances. Pharm. Res. 2006, 23, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cabo, B.; Rodriguez-Palmeiro, I.; Corchero, R.; Rodil, R.; Rodil, E.; Arce, A.; Soto, A. Photocatalytic degradation of methyl orange, methylene blue and rhodamine B with AgCl nanocatalyst synthesised from its bulk material in the ionic liquid [P6 6 6 14]Cl. Water Sci. Technol. 2017, 75, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Weber, J. Study of the influence of triplet quencher on the photobleaching of rhodamine-6G. Opt. Commun. 1973, 7, 420–422. [Google Scholar] [CrossRef]
- Burrows, H.D.; Canle, L.M.; Santaballa, J.A.; Steenken, S. Reaction pathways and mechanisms of photodegradation of pesticides. J. Photochem. Photobiol. B 2002, 67, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Fagier, M.A. Plant-mediated biosynthesis and photocatalysis activities of zinc oxide nanoparticles: A prospect towards dyes mineralization. J. Nanotechnol. 2021, 2021, 6629180. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Plants, 3rd ed.; Academic Press: Boston, MA, USA, 2011; p. 672. [Google Scholar]
- de Souza, L.M.; Inada, N.M.; Venturini, F.P.; Carmona-Vargas, C.C.; Pratavieira, S.; de Oliveira, K.T.; Kurachi, C.; Bagnato, V.S. Photolarvicidal effect of curcuminoids from Curcuma longa Linn. against Aedes aegypti larvae. J. Asia-Pac. Entomol. 2019, 22, 151–158. [Google Scholar] [CrossRef]
- Lima, A.R.; Silva, C.M.; da Silva, L.M.; Machulek, A., Jr.; de Souza, A.P.; de Oliveira, K.T.; Souza, L.M.; Inada, N.M.; Bagnato, V.S.; Oliveira, S.L.; et al. Environmentally safe photodynamic control of Aedes aegypti using sunlight-activated synthetic curcumin: Photodegradation, aquatic ecotoxicity, and field trial. Molecules 2022, 27, 5699. [Google Scholar] [CrossRef]
- Dondji, B.; Duchon, S.; Diabate, A.; Herve, J.P.; Corbel, V.; Hougard, J.M.; Santus, R.; Schrevel, J. Assessment of laboratory and field assays of sunlight-induced killing of mosquito larvae by photosensitizers. J. Med. Entomol. 2005, 42, 652–656. [Google Scholar] [CrossRef]
- Capinera, J.L.; Squitier, J.M. Insecticidal activity of photoactive dyes to American and migratory grasshoppers (Orthoptera: Acrididae). J. Econ. Entomol. 2000, 93, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Mangan, R.L.; Moreno, D.S. Photoactive dye insecticide formulations: Adjuvants increase toxicity to Mexican fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2001, 94, 150–156. [Google Scholar] [CrossRef]
- Preuss, A.; Pfitzner, M.; Roder, B. Mosquito larvae control by photodynamic inactivation of their intestinal flora—A proof of principal study on Chaoborus sp. Photochem. Photobiol. Sci. 2019, 18, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Nagbanshi, M.; Goldau, N.; Mendes Jorge, M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Muller, O. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef]
- Omar, S.; Zedan, A.; Nugent, K. Cardiac vasoplegia syndrome: Pathophysiology, risk factors and treatment. Am. J. Med. Sci. 2015, 349, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Puntillo, F.; Giglio, M.; Pasqualucci, A.; Brienza, N.; Paladini, A.; Varrassi, G. Vasopressor-sparing action of methylene blue in severe sepsis and shock: A narrative review. Adv. Ther. 2020, 37, 3692–3706. [Google Scholar] [CrossRef]
- Dhillon, S.K.; Porter, S.L.; Rizk, N.; Sheng, Y.; McKaig, T.; Burnett, K.; White, B.; Nesbitt, H.; Matin, R.N.; McHale, A.P.; et al. Rose bengal-amphiphilic peptide conjugate for enhanced photodynamic therapy of malignant melanoma. J. Med. Chem. 2020, 63, 1328–1336. [Google Scholar] [CrossRef]
- Durkee, H.; Arboleda, A.; Aguilar, M.C.; Martinez, J.D.; Alawa, K.A.; Relhan, N.; Maestre-Mesa, J.; Amescua, G.; Miller, D.; Parel, J.M. Rose bengal photodynamic antimicrobial therapy to inhibit Pseudomonas aeruginosa keratitis isolates. Lasers Med. Sci. 2020, 35, 861–866. [Google Scholar] [CrossRef]
- Naranjo, A.; Arboleda, A.; Martinez, J.D.; Durkee, H.; Aguilar, M.C.; Relhan, N.; Nikpoor, N.; Galor, A.; Dubovy, S.R.; Leblanc, R.; et al. Rose bengal photodynamic antimicrobial therapy for patients with progressive infectious keratitis: A pilot clinical study. Am. J. Ophthalmol. 2019, 208, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.M.; MacRobert, A.J.; Mosse, C.A.; Periera, B.; Bown, S.G.; Keshtgar, M.R.S. Photodynamic therapy: Inception to application in breast cancer. Breast 2017, 31, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Boen, M.; Brownell, J.; Patel, P.; Tsoukas, M.M. The role of photodynamic therapy in acne: An evidence-based review. Am. J. Clin. Dermatol. 2017, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarthi, U.; Giribabu, L. Photodynamic therapy: Past, present and future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, N.; Kruger, C.A.; Abrahamse, H. Targeted photodynamic therapy as potential treatment modality for the eradication of colon cancer and colon cancer stem cells. Tumour. Biol. 2017, 39, 1010428317734691. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Ozog, D.M.; Rkein, A.M.; Fabi, S.G.; Gold, M.H.; Goldman, M.P.; Lowe, N.J.; Martin, G.M.; Munavalli, G.S. Photodynamic Therapy: A Clinical Consensus Guide. Dermatol. Surg. 2016, 42, 804–827. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Robertson, P.K.; Officer, S.; Pollard, P.M.; Prabhu, R.; McCullagh, C.; Robertson, J.M. Photobactericidal effects of TiO2 thin films at low temperatures—A preliminary study. J. Photochem. Photobiol. A 2010, 216, 290–294. [Google Scholar] [CrossRef]
- Hochmannova, L.; Vytrasova, J. Photocatalytic and antimicrobial effects of interior paints. Prog. Org. Coat. 2010, 67, 1–5. [Google Scholar] [CrossRef]
- Noimark, S.; Salvadori, E.; Gomez-Bombarelli, R.; MacRobert, A.J.; Parkin, I.P.; Kay, C.W. Comparative study of singlet oxygen production by photosensitiser dyes encapsulated in silicone: Towards rational design of anti-microbial surfaces. Phys. Chem. Chem. Phys. 2016, 18, 28101–28109. [Google Scholar] [CrossRef]
- Workman, M.J.; Gomes, B.; Weng, J.L.; Ista, L.K.; Jesus, C.P.; David, M.R.; Ramalho-Ortigao, M.; Genta, F.A.; Matthews, S.K.; Durvasula, R.; et al. Yeast-encapsulated essential oils: A new perspective as an environmentally friendly larvicide. Parasit. Vectors 2020, 13, 19. [Google Scholar] [CrossRef]
- Respicio, N.C.; Heitz, J.R. Development of resistance to erythrosin B in the house fly (Diptera: Muscidae). J. Econ. Entomol. 1983, 76, 1005–1008. [Google Scholar] [CrossRef]
- Giuliani, F.; Martinelli, M.; Cocchi, A.; Arbia, D.; Fantetti, L.; Roncucci, G. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob. Agents Chemother. 2010, 54, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Ikai, H.; Odashima, Y.; Kanno, T.; Nakamura, K.; Shirato, M.; Sasaki, K.; Niwano, Y. In vitro evaluation of the risk of inducing bacterial resistance to disinfection treatment with photolysis of hydrogen peroxide. PLoS ONE 2013, 8, e81316. [Google Scholar] [CrossRef] [PubMed]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updat. 2017, 31, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lauro, F.M.; Pretto, P.; Covolo, L.; Jori, G.; Bertoloni, G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem. Photobiol. Sci. 2002, 1, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Pedigo, L.A.; Gibbs, A.J.; Scott, R.J.; Street, C.N. Absence of bacterial resistance following repeat exposure to photodynamic therapy. Proc. SPIE 2009, 7380, 73803H. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.M.; Faustino, M.A.; Neves, M.G.; Tome, J.P.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Gomes, N.C.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Bolognese, F.; Chiodaroli, L.; Tolker-Nielsen, T.; Barbieri, P. Pigments influence the tolerance of Pseudomonas aeruginosa PAO1 to photodynamically induced oxidative stress. Microbiology 2015, 161, 2298–2309. [Google Scholar] [CrossRef] [PubMed]
- Johansson, F.; Nilsson-Örtman, V. Predation and the relative importance of larval colour polymorphisms and colour polyphenism in a damselfly. Evol. Ecol. 2013, 27, 579–591. [Google Scholar] [CrossRef]
- Zettler, J.A.; Adler, P.H.; McCreadie, J.W. Factors influencing larval color in the Simulium vittatum complex (Diptera: Simuliidae). Invertebr. Biol. 1998, 117, 245–252. [Google Scholar] [CrossRef]
- Champion, C.J.; Xu, J. Redox state affects fecundity and insecticide susceptibility in Anopheles gambiae. Sci. Rep. 2018, 8, 13054. [Google Scholar] [CrossRef]
- Oliver, S.V.; Brooke, B.D. The role of oxidative stress in the longevity and insecticide resistance phenotype of the major malaria vectors Anopheles arabiensis and Anopheles funestus. PLoS ONE 2016, 11, e0151049. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Walse, S.S.; Throne, J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017, 21, 47–53. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meier, C.J.; Rouhier, M.F.; Hillyer, J.F. Chemical Control of Mosquitoes and the Pesticide Treadmill: A Case for Photosensitive Insecticides as Larvicides. Insects 2022, 13, 1093. https://doi.org/10.3390/insects13121093
Meier CJ, Rouhier MF, Hillyer JF. Chemical Control of Mosquitoes and the Pesticide Treadmill: A Case for Photosensitive Insecticides as Larvicides. Insects. 2022; 13(12):1093. https://doi.org/10.3390/insects13121093
Chicago/Turabian StyleMeier, Cole J., Matthew F. Rouhier, and Julián F. Hillyer. 2022. "Chemical Control of Mosquitoes and the Pesticide Treadmill: A Case for Photosensitive Insecticides as Larvicides" Insects 13, no. 12: 1093. https://doi.org/10.3390/insects13121093
APA StyleMeier, C. J., Rouhier, M. F., & Hillyer, J. F. (2022). Chemical Control of Mosquitoes and the Pesticide Treadmill: A Case for Photosensitive Insecticides as Larvicides. Insects, 13(12), 1093. https://doi.org/10.3390/insects13121093







