Ultrastructure of Ejaculatory Ducts of Cerapanorpa nanwutaina and Furcatopanorpa longihypovalva (Mecoptera: Panorpidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Specimens
2.2. Gross Anatomy
2.3. Transmission Electron Microscopy (TEM)
2.4. Light Microscopy (LM)
3. Results
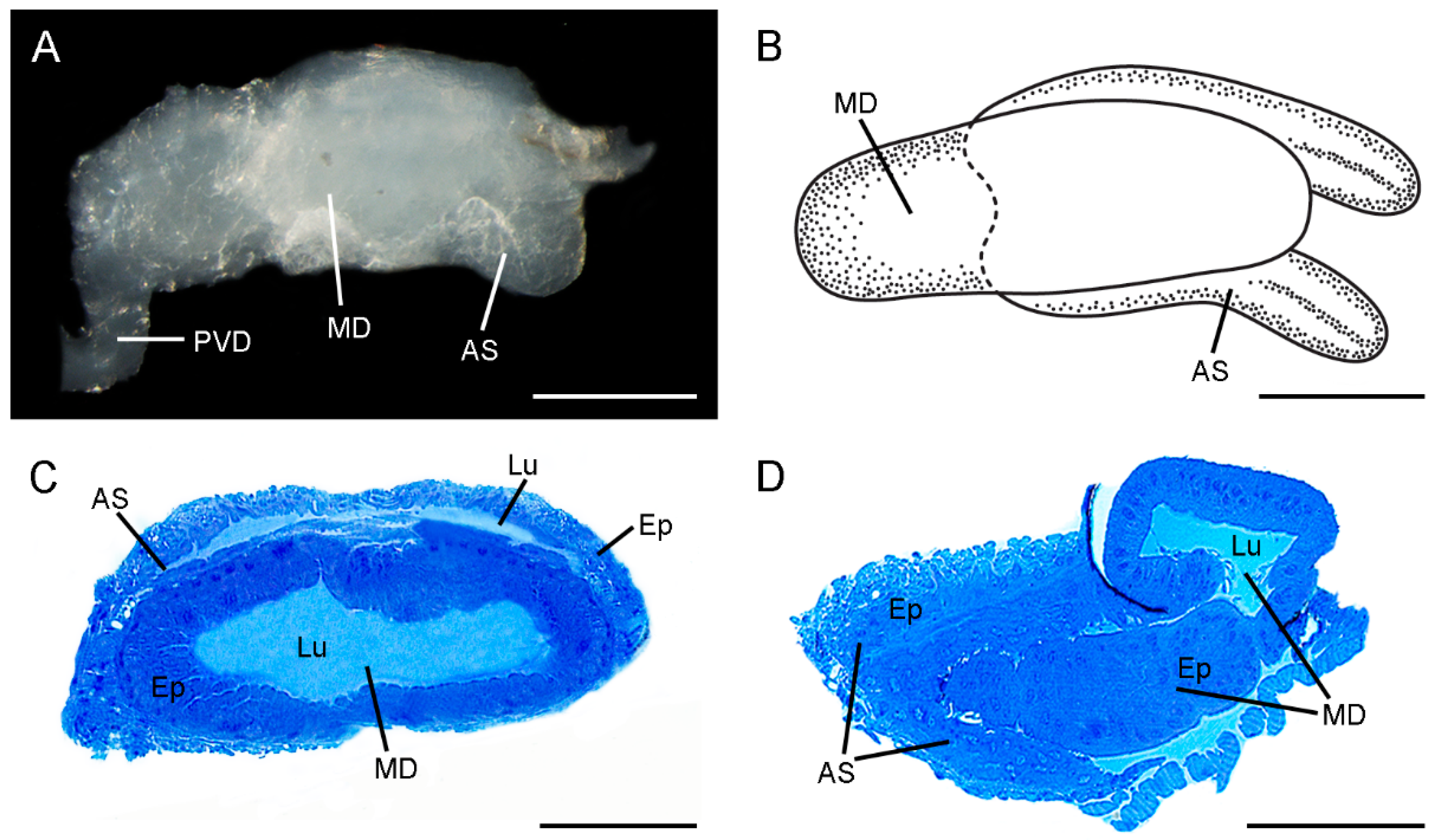
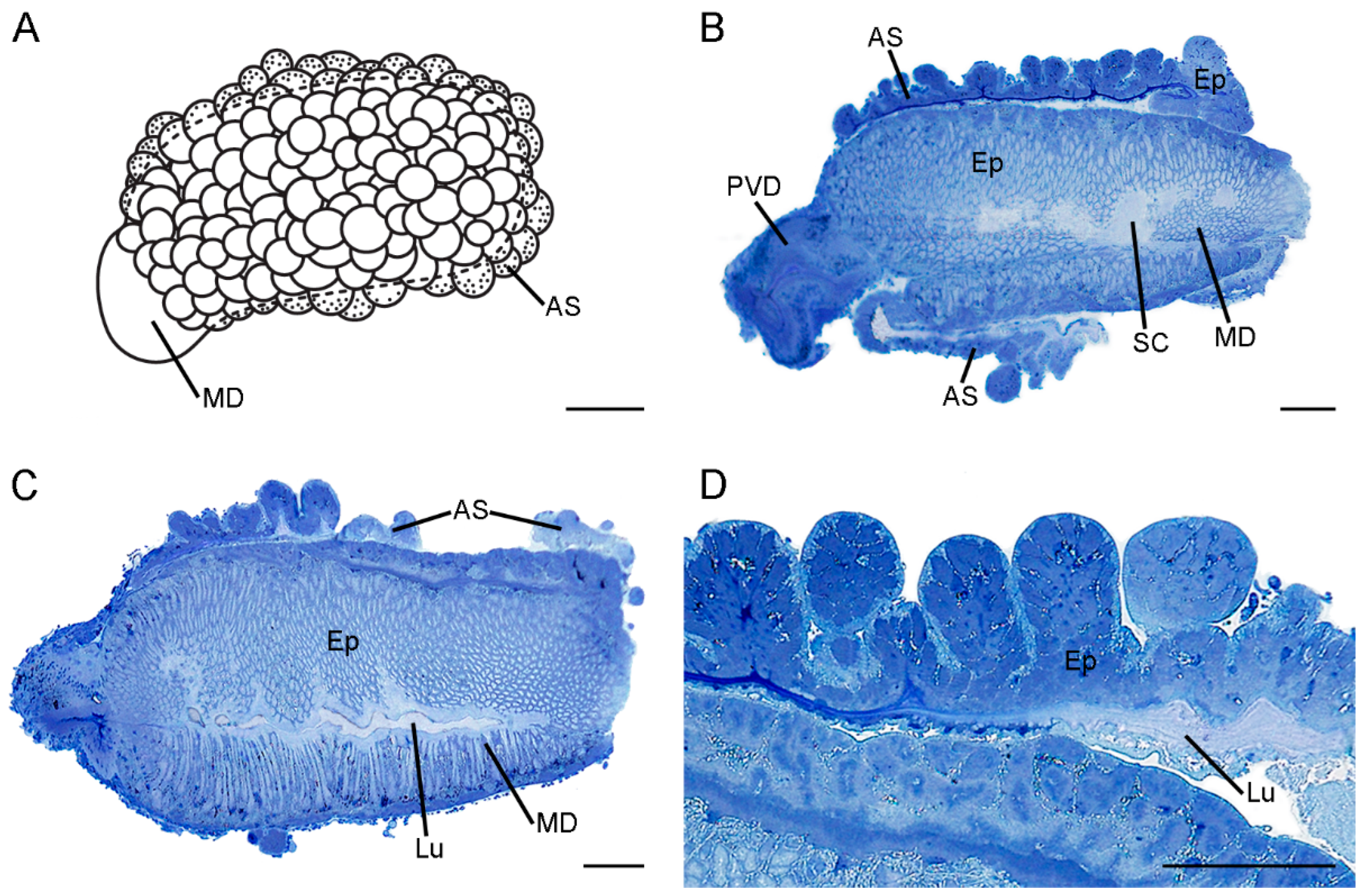
3.1. Gross Morphology of the Ejaculatory duct
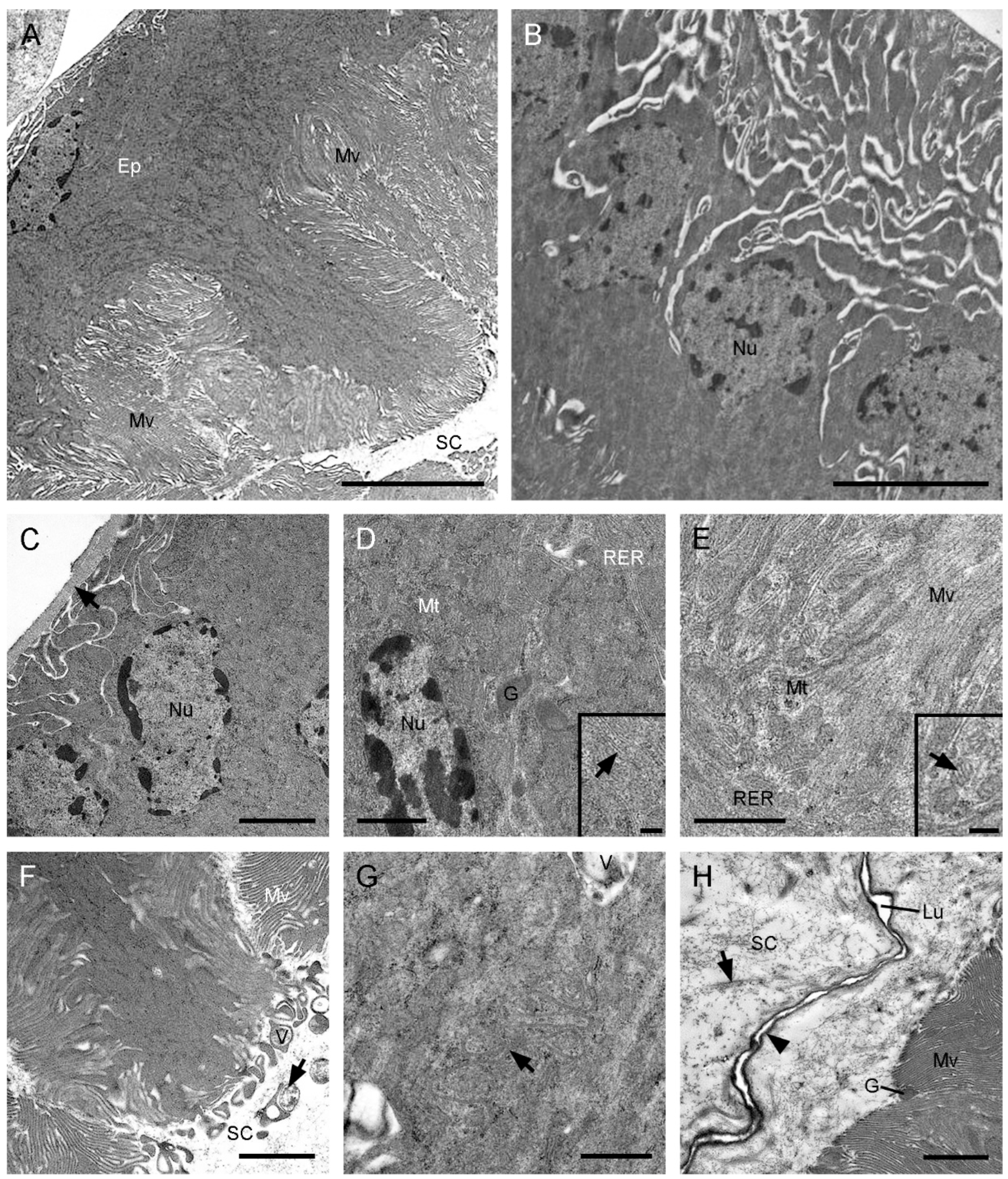
3.2. Histology and Ultrastructure of the Ejaculatory Duct in C. nanwutaina
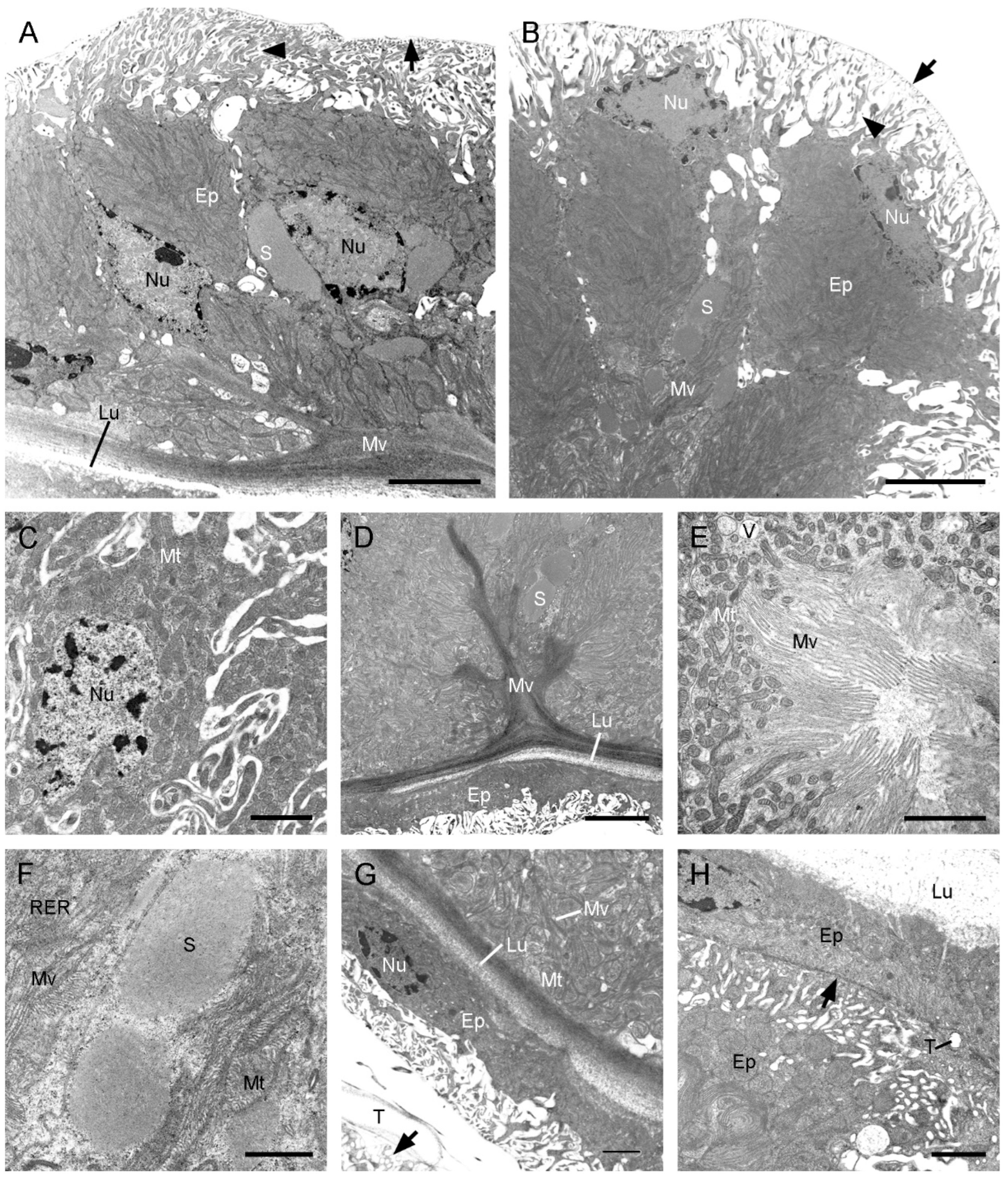
3.3. Histology and Ultrastructure of the Ejaculatory duct in F. longihypovalva
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapman, R.F. The Insects: Structure and Function, 5th ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Snodgrass, R.E. Principles of Insect Morphology; McGraw Hill: New York, NY, USA, 1935. [Google Scholar]
- Gillott, C. Arthropoda–insecta. In Reproductive Biology of Invertebrates; Adiyody, K.G., Adiyody, R.G., Eds.; Wiley: New York, NY, USA, 1988; pp. 319–471. [Google Scholar]
- Gullan, P.J.; Cranston, P.S. The Insects: An Outline of Entomology, 5th ed.; Blackwell Publishing: Oxford, UK, 2014. [Google Scholar]
- Wensler, R.J.D.; Rempel, J.G. The morphology of the male and female reproductive systems of the midge, Chironomus plumosus L. Can. J. Zool. 1962, 40, 199–229. [Google Scholar] [CrossRef]
- Gillott, C. Insect male mating systems. In Insect Reproduction; Leather, S.R., Hardie, J., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 33–56. [Google Scholar]
- Ômura, S. Studies on the reproductive system of the male of Bombyx Mori II. Post-testicular organs and post-yesticular behaviour of the spermatozoa. J. Fac. Agr. Hokkaido Imp. Univ. 1938, 40, 129–170. [Google Scholar]
- Shepherd, J.C. Activation of saturniid moth sperm by a secretion of the male reproductive tract. J. Insect Physiol. 1974, 20, 2107–2122. [Google Scholar] [CrossRef]
- Herman, W.S.; Peng, P. Juvenile hormone stimulation of sperm activator production in male monarch butterflies. J. Insect Physiol. 1976, 22, 579–581. [Google Scholar] [CrossRef]
- Lung, O.; Kuo, L.; Wolfner, M.F. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J. Insect Physiol. 2001, 47, 617–622. [Google Scholar] [CrossRef]
- Leopold, R.A. Cytological and cytochemical studies on the ejaculatory duct and accessory secretion in Musca domestica. J. Insect Physiol. 1970, 16, 1859–1872. [Google Scholar] [CrossRef]
- Morrison, P.E.; Venkatesh, K.; Thompson, B. The role of male accessory-gland substance on female reproduction with some observations of spermatogenesis in the stable fly. J. Insect Physiol. 1982, 28, 607–614. [Google Scholar] [CrossRef]
- Soldán, T. The structure and development of the male internal reproductive organs in six European species of Ephemeroptera. Acta Entomol. Bohemoslov. 1979, 76, 22–33. [Google Scholar]
- Brito, P.; Salles, F.F.; Dolder, H. Characteristics of the male reproductive system and spermatozoa of Leptophlebiidae (Ephemeroptera). Neotrop. Entomol. 2011, 40, 103–107. [Google Scholar] [CrossRef]
- Wheeler, D.E.; Krutzsch, P.H. Internal reproductive system in adult males of the genus Camponotus (Hymenoptera: Formicidae: Formicinae). J. Morphol. 1992, 211, 307–317. [Google Scholar] [CrossRef]
- Ramamurthi, B.N. Studies on the male genital tube in the Dermaptera. Proc. R. Entomol. Soc. Lond. 1958, 33, 186–190. [Google Scholar] [CrossRef]
- Ramamurthi, B.N. The male efferent system in Euborellia annulipes (Lucas) with special reference to the evolution of the gonopore in the Dermaptera. Proc. R. Entomol. Soc. Lond. 1959, 34, 90–96. [Google Scholar] [CrossRef]
- Gregory, G.E. The formation and fate of the spermatophore in the African migratory locust, Locusta migratoria migratorioides Reiche and Fairmaire. Trans. R. Entomol. Soc. Lond. 2009, 117, 33–66. [Google Scholar] [CrossRef]
- Bonhag, P.F.; Wick, J.R. The functional anatomy of the male and female reproductive systems of the milkweed bug, Oncopeltus fasciatus (Dallas) (Heteroptera: Lygaeidae). J. Morphol. 1953, 93, 177–283. [Google Scholar] [CrossRef]
- Lai-Fook, J. Structure of the accessory glands and duplex of the internal male reproductive system of Calpodes ethlius (Hesperiidae, Lepidoptera). Can. J. Zool. 1982, 60, 1202–1215. [Google Scholar] [CrossRef]
- Shen, J.; Hua, B.-Z. Fine structures of the ejaculatory sac and sperm pump of the scorpionfly Panorpa liui Hua (Mecoptera: Panorpidae). Micron 2013, 51, 41–47. [Google Scholar] [CrossRef]
- Wang, J.-S.; Hua, B.-Z. Morphological phylogeny of Panorpidae (Mecoptera: Panorpoidea). Syst. Entomol. 2021, 46, 526–557. [Google Scholar] [CrossRef]
- Thornhill, R. Panorpa (Mecoptera: Panorpidae) scorpionflies: Systems for understanding resource-defense polygyny and alternative male reproductive efforts. Annu. Rev. Ecol. Syst. 1981, 12, 355–386. [Google Scholar] [CrossRef]
- Byers, G.W.; Thornhill, R. Biology of the Mecoptera. Annu. Rev. Entomol. 1983, 28, 203–228. [Google Scholar] [CrossRef]
- Engqvist, L.; Sauer, K.P. Influence of nutrition on courtship and mating in the scorpionfly Panorpa cognata (Mecoptera, Insecta). Ethology 2003, 109, 911–928. [Google Scholar] [CrossRef]
- Thornhill, R.; Sauer, K.P. The notal organ of the scorpionfly (Panorpa vulgaris): An adaptation to coerce mating duration. Behav. Ecol. 1991, 2, 156–164. [Google Scholar] [CrossRef]
- Tong, X.; Zhong, W.; Hua, B.Z. Copulatory mechanism and functional morphology of genitalia and anal horn of the scorpionfly Cerapanorpa dubia (Mecoptera: Panorpidae). J. Morphol. 2018, 279, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Hua, B.-Z. Nuptial feeding and genital coupling of Neopanorpa scorpionflies (Insecta: Mecoptera: Panorpidae) with notal organs of various lengths. Contrib. Zool. 2019, 88, 498–512. [Google Scholar] [CrossRef]
- Chou, I.; Ran, R.B.; Wang, S.M. Study on the classification of Chinese Mecoptera (I, II). Entomotaxonomia 1981, 3, 1–22. [Google Scholar]
- Hua, B.-Z.; Cai, L.-J. A new species of the genus Panorpa (Mecoptera: Panorpidae) from China with notes on its biology. J. Nat. Hist. 2009, 43, 545–552. [Google Scholar] [CrossRef]
- Lyu, Q.-H.; Hua, B.-Z. Ultrastructure of the vasa deferentia of Terrobittacus implicatus and Cerapanorpa nanwutaina (Insecta: Mecoptera). Protoplasma 2019, 256, 883–891. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Lyu, Q.-H.; Hua, B.-Z. Male reproductive system and sperm ultrastructure of Furcatopanorpa longihypovalva (Hua and Cai, 2009) (Mecoptera: Panorpidae) and its phylogenetic implication. Zool. Anz. 2016, 264, 41–46. [Google Scholar] [CrossRef]
- Steiner, P. Beitrag zur Fortpflanzungsbiologie und Morphologie des Genitalapparates von Boreus hiemalis L. Z. Morph. Okol. Tiere 1937, 32, 276–288. [Google Scholar] [CrossRef]
- Potter, E. The internal anatomy of the order Mecoptera. Trans. R. Entomol. Soc. Lond. 1938, 87, 467–501. [Google Scholar] [CrossRef]
- Setty, L.R. Biology and morphology of some north American Bittacidae (Order Mecoptera). Am. Midl. Nat. 1940, 23, 257–353. [Google Scholar] [CrossRef]
- Grell, K.G. Der Genitalapparat von Panorpa communis L. Zool. Jb. Anat. 1942, 67, 513–588. [Google Scholar]
- Cooper, K.W. A Southern California Boreus, B. notoperates n. sp. I. Comparative morphology and systematics (Mecoptera: Boreidae). Psyche 1972, 79, 269–283. [Google Scholar] [CrossRef]
- Chen, P.S. The functional morphology and biochemistry of insect male accessory glands and their secretions. Annu. Rev. Entomol. 1984, 29, 233–255. [Google Scholar] [CrossRef]
- Noirot, C.; Quennedey, A. Fine structure of insect epidermal glands. Annu. Rev. Entomol. 1974, 19, 61–80. [Google Scholar] [CrossRef]
- Quennedey, A. Insect Epidermal Gland Cells: Ultrastructure and Morphgenesis; Wiley-Liss: New York, NY, USA, 1998. [Google Scholar]
- Šobotník, J.; Kutalová, K.; Vytisková, B.; Roisin, Y.; Bourguignon, T. Age-dependent changes in ultrastructure of the defensive glands of Neocapritermes taracua workers (Isoptera, Termitidae). Arthropod Struct. Dev. 2014, 43, 205–210. [Google Scholar] [CrossRef]
- Filimonova, S.A. Morpho-functional variety of the coxal glands in cheyletoid mites (Prostigmata). I. Syringophilidae. Arthropod Struct. Dev. 2016, 45, 356–367. [Google Scholar] [CrossRef]
- Crossley, A.C.; Waterhouse, D.F. The ultrastructure of the osmeterium and the nature of its secretion in Papilio larvae (Lepidoptera). Tissue Cell 1969, 1, 525–554. [Google Scholar] [CrossRef]
- Kölsch, G. The ultrastructure of glands and the production and function of the secretion in the adhesive capture apparatus of Stenus species (Coleoptera: Staphylinidae). Can. J. Zool. 2000, 78, 465–475. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Borkent, A.; Wood, D.M. The male genital tract and aedeagal components of the Diptera with a discussion of their phylogenetic significance. Zool. J. Linn. Soc. 2007, 150, 711–742. [Google Scholar] [CrossRef]
- Hünefeld, F.; Beutel, R.G. The sperm pumps of Strepsiptera and Antliophora (Hexapoda). J. Zool. Syst. Evol. Res. 2005, 43, 297–306. [Google Scholar] [CrossRef]
- Kokwaro, E.D.; Murithi, J.K. Ultrastructural characteristics of the ejaculatory duct of the male tsetse, Glossina morsitans morsitans Westwood. Insect Sci. Appl. 2011, 9, 475–482. [Google Scholar] [CrossRef]
- Brito, P.; Salles, F.F.; Dolder, H. Morphology of male reproductive systems in Ephemeroptera: Intrinsic musculature. Neotrop. Entomol. 2012, 41, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Susic-Jung, L.; Hornbruch-Freitag, C.; Kuckwa, J.; Rexer, K.-H.; Lammel, U.; Renkawitz-Pohl, R. Multinucleated smooth muscles and mononucleated as well as multinucleated striated muscles develop during establishment of the male reproductive organs of Drosophila melanogaster. Dev. Biol. 2012, 370, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Dallacqua, R.P.; da Cruz-Landim, C. Ultrastructure of the ducts of the reproductive tract of males of Melipona bicolor bicolor Lepeletier (Hymenoptera, Apinae, Meliponini). Anat. Histol. Embryol. 2003, 32, 276–281. [Google Scholar] [CrossRef]
- Moors, L.; Koeniger, G.; Billen, J. Ontogeny and morphology of the bulbus, part of the male reproductive organ in Apis mellifera carnica (Hymenoptera, Apidae). Apidologie 2012, 43, 201–211. [Google Scholar] [CrossRef]
- Tong, X.; Hua, B.-Z. The sperm pump and genital coupling of Panorpodes kuandianensis (Mecoptera: Panorpodidae). Arthropod Struct. Dev. 2019, 50, 15–23. [Google Scholar] [CrossRef]
- Zhong, W.; Qi, Z.-Y.; Hua, B.-Z. Atypical mating in a scorpionfly without a notal organ. Contrib. Zool. 2015, 84, 305–315. [Google Scholar] [CrossRef]
- Sauer, K.P.; Lubjuhn, T.; Sindern, J.; Kullmann, H.; Kurtz, J.; Epplen, C.; Epplen, J.T. Mating system and sexual selection in the scorpionfly Panorpa vulgaris (Mecoptera: Panorpidae). Naturwissenschaften 1998, 85, 219–228. [Google Scholar] [CrossRef]
- Kullmann, H.; Sauer, K.P. Mating tactic dependent sperm transfer rates in Panorpa similis (Mecoptera; Panorpidae): A case of female control? Ecol. Entomol. 2009, 34, 153–157. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Q.-H.; Chen, Q.-X.; Sun, Y.-L.; Dong, W.-J. Ultrastructure of Ejaculatory Ducts of Cerapanorpa nanwutaina and Furcatopanorpa longihypovalva (Mecoptera: Panorpidae). Insects 2022, 13, 1074. https://doi.org/10.3390/insects13111074
Lyu Q-H, Chen Q-X, Sun Y-L, Dong W-J. Ultrastructure of Ejaculatory Ducts of Cerapanorpa nanwutaina and Furcatopanorpa longihypovalva (Mecoptera: Panorpidae). Insects. 2022; 13(11):1074. https://doi.org/10.3390/insects13111074
Chicago/Turabian StyleLyu, Qi-Hui, Qing-Xiao Chen, Ya-Lan Sun, and Wen-Jie Dong. 2022. "Ultrastructure of Ejaculatory Ducts of Cerapanorpa nanwutaina and Furcatopanorpa longihypovalva (Mecoptera: Panorpidae)" Insects 13, no. 11: 1074. https://doi.org/10.3390/insects13111074
APA StyleLyu, Q.-H., Chen, Q.-X., Sun, Y.-L., & Dong, W.-J. (2022). Ultrastructure of Ejaculatory Ducts of Cerapanorpa nanwutaina and Furcatopanorpa longihypovalva (Mecoptera: Panorpidae). Insects, 13(11), 1074. https://doi.org/10.3390/insects13111074






