Epidemiological, Entomological, and Climatological Investigation of the 2019 Dengue Fever Outbreak in Gewane District, Afar Region, North-East Ethiopia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
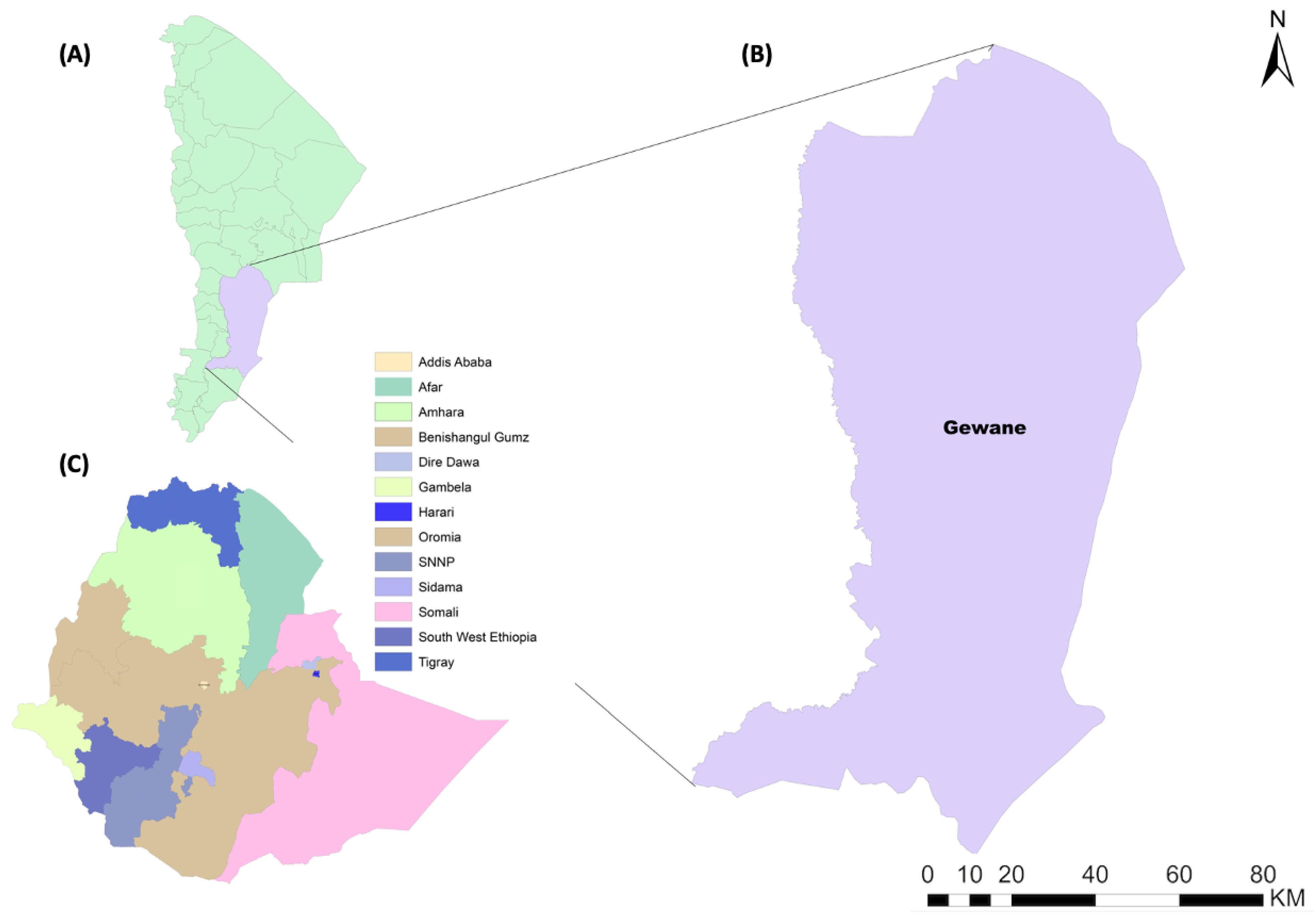
2.1. Study Area
2.2. Study Design, Period and Population
2.3. Entomological Investigation
2.4. Laboratory Investigation
2.5. Climatological Data
2.6. Data Analysis
3. Results
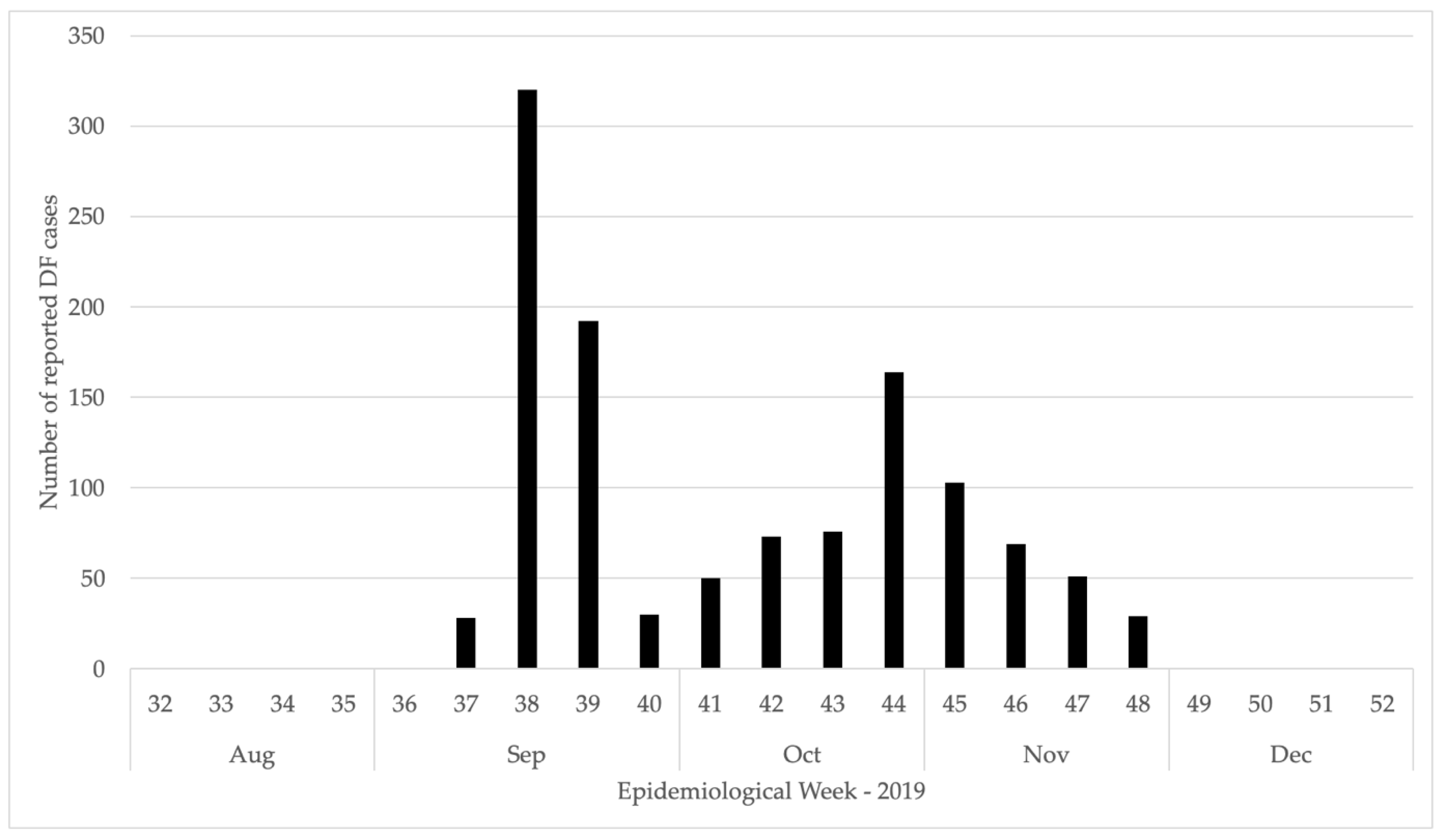
3.1. Epidemiological Investigation
3.2. Entomological Investigation
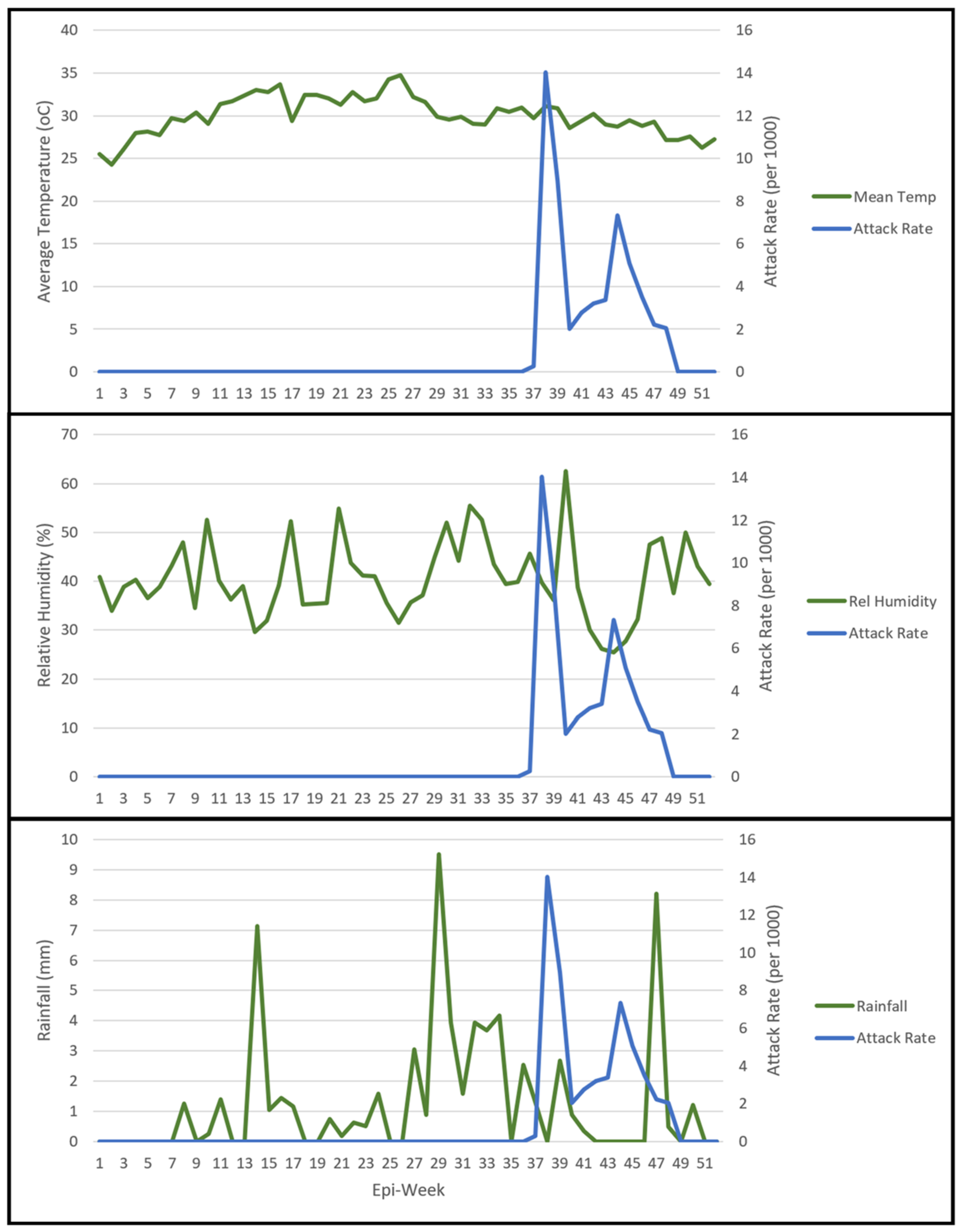
3.3. Climatological Data
3.4. Outbreak Response Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woyessa, A.B.; Mengesha, M.; Kassa, W.; Kifle, E.; Wondabeku, M.; Girmay, A.; Kebede, A.; Jima, D. The first acute febrile illness investigation associated with dengue fever in Ethiopia, 2013: A descriptive analysis. Ethiop. J. Health Dev. 2014, 28, 155–161. [Google Scholar]
- Degife, L.H.; Worku, Y.; Belay, D.; Bekele, A.; Hailemariam, Z. Factors associated with dengue fever outbreak in Dire Dawa administration city, October, 2015, Ethiopia-case control study. BMC Public Health 2019, 19, 650. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dengue and Severe Dengue: Global Burden of Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 15 July 2022).
- Campbell, L.P.; Luther, C.; Moo-Llanes, D.; Ramsey, J.M.; Danis-Lozano, R.; Peterson, A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140135. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Kulkarni, M.A.; Duguay, C.; Ost, K. Charting the evidence for climate change impacts on the global spread of malaria and dengue and adaptive responses: A scoping review of reviews. Glob. Health 2022, 18, 1. [Google Scholar] [CrossRef]
- CDC. About Dengue: What You Need to Know. Available online: https://www.cdc.gov/dengue/about/index.html (accessed on 15 July 2022).
- Rigau-Perez, J.G.; Vorndam, A.V.; Clark, G.G. The dengue and dengue hemorrhagic fever epidemic in Puerto Rico, 1994–1995. Am. J. Trop. Med. Hyg. 2001, 64, 67–74. [Google Scholar] [CrossRef]
- Guillena, J.B.; Opena, E.L.; Baguio, M. Prevalence of dengue fever (DF) and dengue hemorrhagic fever (DHF): A description and forecasting. In Proceedings of the 11th National Convention on Statistics (NCS’10), Manila, Philippines, 4–5 October 2010. [Google Scholar]
- Were, F. The dengue situation in Africa. Paediatr. Int. Child Health 2012, 32, 18–21. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef]
- Narayanan, M.; Aravind, M.; Thilothammal, N.; Prema, R.; Sargunam, C.R.; Ramamurty, N. Dengue fever epidemic in Chennai-a study of clinical profile and outcome. Indian Pediatrics 2002, 39, 1027–1033. [Google Scholar]
- Baba, M.; Talle, M. The effect of climate on dengue virus infections in Nigeria. N. Y. Sci. J. 2011, 4, 28–33. [Google Scholar]
- WHO. Dengue Control: The Mosquito. Available online: https://www.who.int/denguecontrol/mosquito/en/ (accessed on 8 May 2020).
- Faull, K.J.; Williams, C.R. Intraspecific variation in desiccation survival time of Aedes aegypti (L.) mosquito eggs of Australian origin. J Vector Ecol. 2015, 40, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Pancharoen, C.; Mekmullica, J.; Thisyakorn, U. Primary dengue infection: What are the clinical distinctions from secondary infection? Southeast Asian J. Trop. Med. Public Health 2001, 32, 476–480. [Google Scholar] [PubMed]
- Ngoagouni, C.; Kamgang, B.; Nakouné, E.; Paupy, C.; Kazanji, M. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: What consequences for emerging diseases? Parasites Vectors 2015, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Suri, S.; Bhasin, A.; Srivastava, L.; Bharadwaj, M. An epidemic of dengue haemorrhagic fever and dengue shock syndrome in Delhi: A clinical study. Ann. Trop. Paediatr. 1990, 10, 329–334. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Kabra, S.; Verma, I.; Arora, N.; Jain, Y.; Kalra, V. Dengue haemorrhagic fever in children in Delhi. Bull. World Health Organ. 1992, 70, 105. [Google Scholar] [PubMed]
- Choi, Y.; Tang, C.S.; McIver, L.; Hashizume, M.; Chan, V.; Abeyasinghe, R.R.; Iddings, S.; Huy, R. Effects of weather factors on dengue fever incidence and implications for interventions in Cambodia. BMC Public Health 2016, 16, 241. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Seriki, A.A.; Irekeola, A.A.; Ogah, J.I. The neglect and fast spread of some arboviruses: A note for healthcare providers in Nigeria. Diseases 2018, 6, 99. [Google Scholar] [CrossRef]
- Lalani, T.; Yun, H.; Tribble, D.; Ganesan, A.; Kunz, A.; Fairchok, M.; Schnaubelt, E.; Fraser, J.; Mitra, I.; Kronmann, K.C.; et al. A comparison of compliance rates with anti-vectorial protective measures during travel to regions with dengue or chikungunya activity, and regions endemic for Plasmodium falciparum malaria. J. Travel Med. 2016, 23, taw043. [Google Scholar] [CrossRef]
- Gutu, M.A.; Bekele, A.; Seid, Y.; Mohammed, Y.; Gemechu, F.; Woyessa, A.B.; Tayachew, A.; Dugasa, Y.; Gizachew, L.; Idosa, M.; et al. Another dengue fever outbreak in Eastern Ethiopia-An emerging public health threat. PLoS Negl. Trop. Dis. 2021, 15, e0008992. [Google Scholar] [CrossRef]
- Amarasinghe, A.; Kuritsky, J.N.; Letson, G.W.; Margolis, H.S. Dengue virus infection in Africa. Emerg. Infect. Dis. 2011, 17, 1349. [Google Scholar] [CrossRef]
- Ahmed, Y.M.; Salah, A.A. Epidemiology of dengue fever in Ethiopian Somali region: Retrospective health facility based study. Cent. Afr. J. Public Health 2016, 2, 51–56. [Google Scholar]
- Yusuf, A.M.; Ibrahim, A.M. Knowledge, attitude and practice towards dengue fever prevention and associated factors among public health sector health-care professionals: In Dire Dawa, eastern Ethiopia. Risk Manag Healthc Policy 2019, 12, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Ferede, G.; Tiruneh, M.; Abate, E.; Wondimeneh, Y.; Damtie, D.; Gadisa, E.; Howe, R.; Aseffa, A.; Tessema, B. A serologic study of dengue in northwest Ethiopia: Suggesting preventive and control measures. PLoS Negl. Trop. Dis. 2018, 12, 0006430. [Google Scholar] [CrossRef] [PubMed]
- Geleta, E.N. Serological evidence of dengue fever and its associated factors in health facilities in the Borena Zone, South Ethiopia. Res. Rep. Trop. Med. 2019, 10, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Eshetu, D.; Shimelis, T.; Nigussie, E.; Shumie, G.; Chali, W.; Yeshitela, B.; Assefa, A.; Gadisa, E. Seropositivity to dengue and associated risk factors among non-malarias acute febrile patients in Arba Minch districts, southern Ethiopia. BMC Infect. Dis. 2020, 20, 639. [Google Scholar] [CrossRef]
- Akelew, Y.; Pareyn, M.; Lemma, M.; Negash, M.; Bewket, G.; Derbew, A.; Belay, G.; Pollmann, J.; Adriaensen, W.; Peeters, M.; et al. Aetiologies of acute undifferentiated febrile illness at the emergency ward of the University of Gondar Hospital, Ethiopia. Trop. Med. Int. Health 2022, 27, 271–279. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Rueda, L.M. Pictorial keys to the sections, groups, and species of the Aedes (Finlaya) in the Afrotropical Region (Diptera: Culicidae). Zootaxa 2017, 4221, 131–141. [Google Scholar] [CrossRef]
- Rozendaal, J.A. Vector Control: Methods for Use by Individuals and Communities; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- CSA. Population Projection for Ethiopia 2007–2037. The 2012 Inter-Censual Population Survey; Central Statistics Agency: Adddis Ababa, Ethiopia, 2013.
- Székely, G.J.; Rizzo, M.L. The Energy of Data. Annu. Rev. Stat. Its Appl. 2017, 4, 447–479. [Google Scholar] [CrossRef]
- Rizzo, M.L.; Székely, G.J. Energy distance. WIRES Comput. Stat. 2016, 8, 27–38. [Google Scholar] [CrossRef]
- Székely, G.J.; Rizzo, M.L. Energy statistics: A class of statistics based on distances. J. Stat. Plan. Inference 2013, 143, 1249–1272. [Google Scholar] [CrossRef]
- Székely, G.J.; Rizzo, M.L.; Bakirov, N.K. Measuring and testing dependence by correlation of distances. Ann. Stat. 2007, 35, 2769–2794. [Google Scholar] [CrossRef]
- Getachew, D.; Tekie, H.; Gebre-Michael, T.; Balkew, M.; Mesfin, A. Breeding Sites of Aedes aegypti: Potential Dengue Vectors in Dire Dawa, East Ethiopia. Interdiscip. Perspect. Infect. Dis. 2015, 2015, 706276. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Fakhor, H.; Nimavat, N.; Wara, U.U.; Lal, P.M.; Costa, A.C.D.S.; Ahmad, S.; Essar, M.Y. Dengue and COVID-19: A risk of coepidemic in Ethiopia. J. Med. Virol. 2021, 93, 5680–5681. [Google Scholar] [CrossRef] [PubMed]
- Focks, D.A. Special Programme for Research and Training in Tropical Diseases (TDR). In A Review of Entomological Sampling Methods and Indicators for Dengue Vectors; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Wegbreit, J. The Possible Effects of Temperature and Precipitation on Dengue Morbidity in Trinidad and Tobago: A Retrospective Longitudinal Study; Population-Environment Dynamics: Issues and Policy; University of Michigan, School of Natural Resources and Environment: Ann Arbor, MI, USA, 1997. [Google Scholar]
- Saeed, M.; Alshehri, A.; Fahed, K.; Arabia, S. Dengue fever outburst and its relationship with climatic factors. World Appl. Sci. J. 2013, 22, 506–515. [Google Scholar]
- Thammapalo, S.; Chongsuwiwatwong, V.; McNeil, D.; Geater, A. The climatic factors influencing the occurrence of dengue hemorrhagic fever in Thailand. Southeast Asian J. Trop. Med. Public Health 2005, 36, 191–196. [Google Scholar] [PubMed]
- Benedum, C.M.; Seidahmed, O.M.E.; Eltahir, E.A.B.; Markuzon, N. Statistical modeling of the effect of rainfall flushing on dengue transmission in Singapore. PLoS Negl. Trop. Dis. 2018, 12, e0006935. [Google Scholar] [CrossRef]
- Seidahmed, O.M.; Eltahir, E.A. A Sequence of Flushing and Drying of Breeding Habitats of Aedes aegypti (L.) Prior to the Low Dengue Season in Singapore. PLoS Negl. Trop. Dis. 2016, 10, e0004842. [Google Scholar] [CrossRef]
- Kakarla, S.G.; Caminade, C.; Mutheneni, S.R.; Morse, A.P.; Upadhyayula, S.M.; Kadiri, M.R.; Kumaraswamy, S. Lag effect of climatic variables on dengue burden in India. Epidemiol. Infect. 2019, 147, e170. [Google Scholar] [CrossRef]
- Schmidt, W.-P.; Suzuki, M.; Thiem, V.D.; White, R.G.; Tsuzuki, A.; Yoshida, L.-M.; Yanai, H.; Haque, U.; Anh, D.D.; Ariyoshi, K. Population density, water supply, and the risk of dengue fever in Vietnam: Cohort study and spatial analysis. PLoS Med. 2011, 8, e1001082. [Google Scholar] [CrossRef]
- Quintero, J.; García-Betancourt, T.; Cortés, S.; García, D.; Alcalá, L.; González-Uribe, C.; Brochero, H.; Carrasquilla, G. Effectiveness and feasibility of long-lasting insecticide-treated curtains and water container covers for dengue vector control in Colombia: A cluster randomised trial. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 116–125. [Google Scholar] [CrossRef][Green Version]
- Loroño-Pino, M.A.; García-Rejón, J.E.; Machain-Williams, C.; Gomez-Carro, S.; Nuñez-Ayala, G.; Nájera-Vázquez Mdel, R.; Losoya, A.A.L.; Saavedra-Rodriguez, K.; Lozano-Fuentes, S.; Beaty, M.K.; et al. Towards a Casa Segura: A consumer product study of the effect of insecticide-treated curtains on Aedes aegypti and dengue virus infections in the home. Am. J. Trop. Med. Hyg. 2013, 89, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, A.; Orelus, N.; Maskill, R.; Alexander, N.; Streit, T.; McCall, P.J. Insecticide-treated bednets to control dengue vectors: Preliminary evidence from a controlled trial in Haiti. Trop. Med. Int. Health 2008, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, A.; Morrison, A.C.; Paz-Soldan, V.A.; Forshey, B.M.; Cordova-Lopez, J.J.; Astete, H.; Elder, J.P.; Sihuincha, M.; Gotlieb, E.E.; Halsey, E.S.; et al. The impact of insecticide treated curtains on dengue virus transmission: A cluster randomized trial in Iquitos, Peru. PLoS Negl. Trop. Dis. 2020, 14, e0008097. [Google Scholar] [CrossRef] [PubMed]


| Age Category | Female (%) a | Male (%) a | Total Cases (%) a | Population (2019) a | Attack Rate (per 1000) |
|---|---|---|---|---|---|
| <5 | 12 (2.4) | 21 (3.1) | 33 (2.8) | 2522 (11.2) | 13.1 |
| 5–14 | 60 (11.9) | 70 (10.3) | 130 (11.0) | 4920 (22.0) | 26.4 |
| 15–49 | 394 (78.0) | 542 (79.7) | 936 (79.0) | 14,239 (63.8) | 65.7 |
| 49+ | 39 (7.7) | 47 (6.9) | 86 (6.9) | 641 (2.9) | 134.2 |
| Total | 505 | 680 | 1185 | 22,322 | 53.1 |
| Container | Negative (%) a | Positive (%) a | Total Samples (%) a | Positivity Rate (%) |
|---|---|---|---|---|
| Plastic or Metal Buckets/Bowls | 2 (2.4) | 10 (12.5) | 12 (7.4) | 83.3 |
| Clay Jars | 2 (2.4) | 2 (2.5) | 4 (2.5) | 50.0 |
| Plastic Tanks | 42 (51.2) | 40 (50.0) | 82 (50.6) | 48.8 |
| Tires | 36 (43.9) | 28 (35.0) | 64 (39.5) | 43.8 |
| Total | 82 | 80 | 162 | 49.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekuriaw, W.; Kinde, S.; Kindu, B.; Mulualem, Y.; Hailu, G.; Gebresilassie, A.; Sisay, C.; Bekele, F.; Amare, H.; Wossen, M.; et al. Epidemiological, Entomological, and Climatological Investigation of the 2019 Dengue Fever Outbreak in Gewane District, Afar Region, North-East Ethiopia. Insects 2022, 13, 1066. https://doi.org/10.3390/insects13111066
Mekuriaw W, Kinde S, Kindu B, Mulualem Y, Hailu G, Gebresilassie A, Sisay C, Bekele F, Amare H, Wossen M, et al. Epidemiological, Entomological, and Climatological Investigation of the 2019 Dengue Fever Outbreak in Gewane District, Afar Region, North-East Ethiopia. Insects. 2022; 13(11):1066. https://doi.org/10.3390/insects13111066
Chicago/Turabian StyleMekuriaw, Wondemeneh, Solomon Kinde, Bezabih Kindu, Yibeyin Mulualem, Girma Hailu, Araya Gebresilassie, Chalachw Sisay, Fitsum Bekele, Hiwot Amare, Mesfin Wossen, and et al. 2022. "Epidemiological, Entomological, and Climatological Investigation of the 2019 Dengue Fever Outbreak in Gewane District, Afar Region, North-East Ethiopia" Insects 13, no. 11: 1066. https://doi.org/10.3390/insects13111066
APA StyleMekuriaw, W., Kinde, S., Kindu, B., Mulualem, Y., Hailu, G., Gebresilassie, A., Sisay, C., Bekele, F., Amare, H., Wossen, M., Woyessa, A., Cross, C. L., & Messenger, L. A. (2022). Epidemiological, Entomological, and Climatological Investigation of the 2019 Dengue Fever Outbreak in Gewane District, Afar Region, North-East Ethiopia. Insects, 13(11), 1066. https://doi.org/10.3390/insects13111066










