Developmental Plasticity in Butterfly Eyespot Mutants: Variation in Thermal Reaction Norms across Genotypes and Pigmentation Traits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
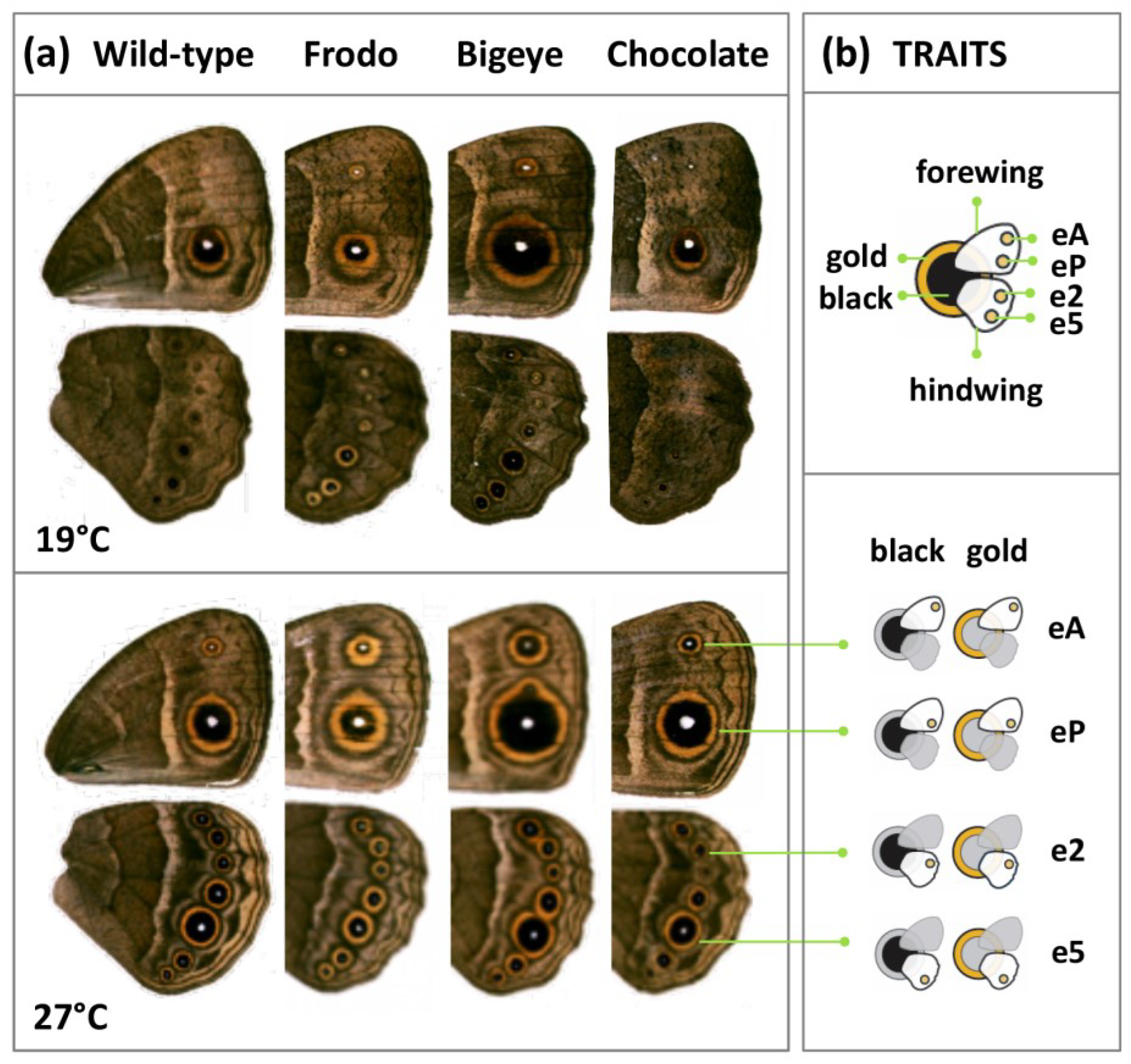
2.1. Animals
2.2. Pigmentation Traits
2.3. Statistical Analyses
3. Results
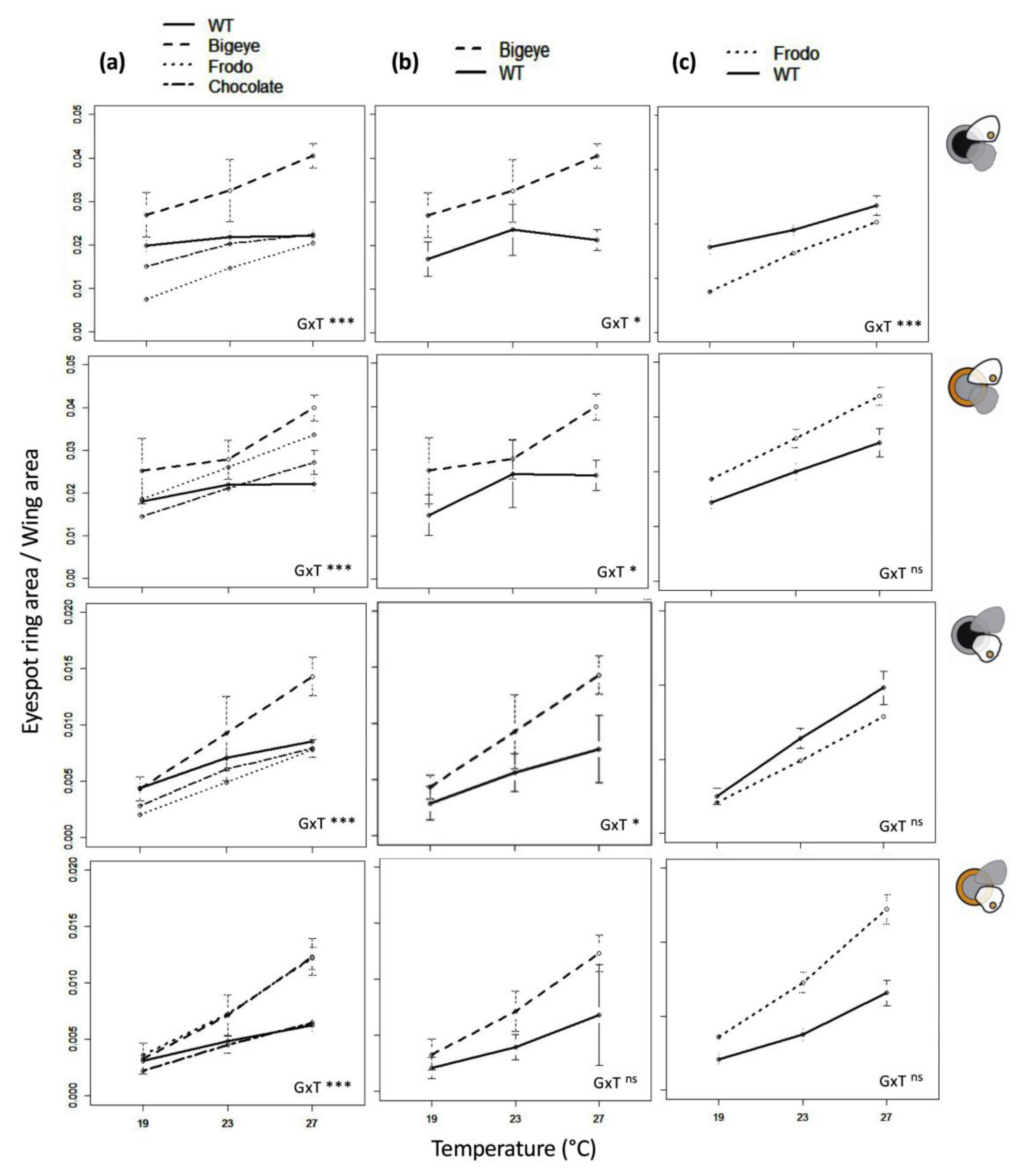
3.1. Genotype, Temperature, and Genotype-by-Temperature Effects on Eyespot Color Rings
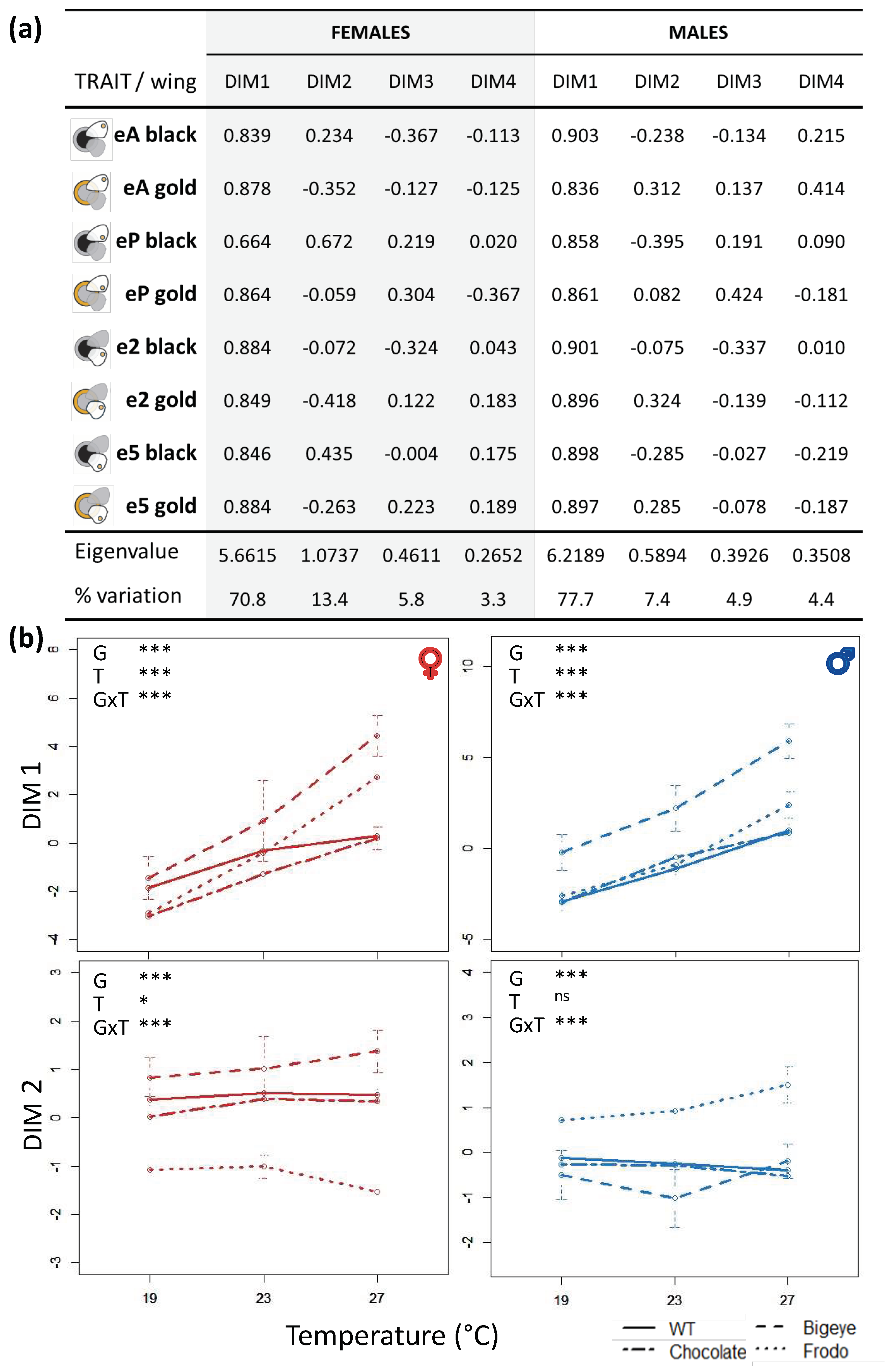
3.2. Principal Component Analysis
3.3. Comparing Individuals Differing at Specific Pigmentation Loci
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: New York, NY, USA, 2020; ISBN 978-0-19-988073-7. [Google Scholar]
- Beldade, P.; Mateus, A.R.A.; Keller, R.A. Evolution and molecular mechanisms of adaptive developmental plasticity. Mol. Ecol. 2011, 20, 1347–1363. [Google Scholar] [CrossRef]
- Gilbert, S.F. Ecological developmental biology: Environmental signals for normal animal development. Evol. Dev. 2012, 14, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Sword, G.A.; Lo, N. Polyphenism in Insects. Curr. Biol. 2011, 21, R738–R749. [Google Scholar] [CrossRef] [PubMed]
- Beldade, P.; Brakefield, P.M. The genetics and evo–devo of butterfly wing patterns. Nat. Rev. Genet. 2002, 3, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Brakefield, P.M.; Zwaan, B.J. Seasonal Polyphenisms and Environmentally Induced Plasticity in the Lepidoptera: The Coor-dinated Evolution of Many Traits on Multiple Levels. In Mechanisms of Life History Evolution: The Genetics and Physiology of Life History Traits and Trade-Offs; Flatt, T., Heyland, A., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 243–252. [Google Scholar]
- van der Burg, K.R.; Reed, R.D. Seasonal plasticity: How do butterfly wing pattern traits evolve environmental responsiveness? Curr. Opin. Genet. Dev. 2021, 69, 82–87. [Google Scholar] [CrossRef]
- Nijhout, H.F. Development and evolution of adaptive polyphenisms. Evol. Dev. 2003, 5, 9–18. [Google Scholar] [CrossRef]
- Daniels, E.V.; Murad, R.; Mortazavi, A.; Reed, R.D. Extensive transcriptional response associated with seasonal plasticity of butterfly wing patterns. Mol. Ecol. 2014, 23, 6123–6134. [Google Scholar] [CrossRef]
- Kijimoto, T.; Moczek, A.P. Hedgehog signaling enables nutrition-responsive inhibition of an alternative morph in a polyphenic beetle. Proc. Natl. Acad. Sci. USA 2016, 113, 5982–5987. [Google Scholar] [CrossRef]
- Schrader, L.; Simola, D.F.; Heinze, J.; Oettler, J. Sphingolipids, Transcription Factors, and Conserved Toolkit Genes: Developmental Plasticity in the Ant Cardiocondyla obscurior. Mol. Biol. Evol. 2015, 32, 1474–1486. [Google Scholar] [CrossRef]
- Moczek, A.P.; Sultan, S.; Foster, S.; Ledón-Rettig, C.; Dworkin, I.; Nijhout, H.F.; Abouheif, E.; Pfennig, D.W. The role of developmental plasticity in evolutionary innovation. Proc. R. Soc. B Boil. Sci. 2011, 278, 2705–2713. [Google Scholar] [CrossRef]
- Rodrigues, Y.K.; Beldade, P. Thermal Plasticity in Insects’ Response to Climate Change and to Multifactorial Environments. Front. Ecol. Evol. 2020, 8, 271. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nijhout, H.F. Evolution of a Polyphenism by Genetic Accommodation. Science 2006, 311, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F. To plasticity and back again. eLife 2015, 4, e06995. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Jolander, L.S.-H.; Wenk, M.R.; Oliver, J.C.; Nijhout, H.F.; Monteiro, A. Origin of the mechanism of phenotypic plasticity in satyrid butterfly eyespots. eLife 2020, 9, e49544. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, E.; Duneau, D.; Beldade, P. Genetic basis of thermal plasticity variation in Drosophila melanogaster body size. PLoS Genet. 2018, 14, e1007686. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, E.; Beldade, P. Genomics of Developmental Plasticity in Animals. Front. Genet. 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Stoehr, A.M.; Wojan, E.M. Multiple cues influence multiple traits in the phenotypically plastic melanization of the cabbage white butterfly. Oecologia 2016, 182, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, C.; Pigliucci, M. Phenotypic Evolution: A Reaction Norm Perspective; Sinauer: Sunderland, MA, USA, 1998; ISBN 978-0-87893-799-8. [Google Scholar]
- Murren, C.J.; Maclean, H.J.; Diamond, S.E.; Steiner, U.K.; Heskel, M.A.; Handelsman, C.A.; Ghalambor, C.K.; Auld, J.R.; Callahan, H.S.; Pfennig, D.W.; et al. Evolutionary Change in Continuous Reaction Norms. Am. Nat. 2014, 183, 453–467. [Google Scholar] [CrossRef]
- Brakefield, P.M.; Gates, J.; Keys, D.; Kesbeke, F.; Wijngaarden, P.J.; Montelro, A.; French, V.; Carroll, S.B. Development, plasticity and evolution of butterfly eyespot patterns. Nature 1996, 384, 236–242. [Google Scholar] [CrossRef]
- Aubret, F.; Shine, R. Genetic Assimilation and the Postcolonization Erosion of Phenotypic Plasticity in Island Tiger Snakes. Curr. Biol. 2009, 19, 1932–1936. [Google Scholar] [CrossRef]
- Bento, G.; Ogawa, A.; Sommer, R.J. Co-option of the hormone-signalling module dafachronic acid–DAF-12 in nematode evolution. Nature 2010, 466, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Hallsson, L.R.; Björklund, M. Selection in a fluctuating environment leads to decreased genetic variation and facilitates the evolution of phenotypic plasticity: Evolutionary Response in a Fluctuating Environment. J. Evol. Biol. 2012, 25, 1275–1290. [Google Scholar] [CrossRef] [PubMed]
- Oostra, V.; Brakefield, P.M.; Hiltemann, Y.; Zwaan, B.J.; Brattström, O. On the fate of seasonally plastic traits in a rainforest butterfly under relaxed selection. Ecol. Evol. 2014, 4, 2654–2667. [Google Scholar] [CrossRef]
- Courtier-Orgogozo, V.; Arnoult, L.; Prigent, S.R.; Wiltgen, S.; Martin, A. Gephebase, a database of genotype–phenotype relationships for natural and domesticated variation in Eukaryotes. Nucleic Acids Res. 2019, 48, D696–D703. [Google Scholar] [CrossRef] [PubMed]
- Via, S.; Gomulkiewicz, R.; De Jong, G.; Scheiner, S.M.; Schlichting, C.D.; Van Tienderen, P.H. Adaptive phenotypic plasticity: Consensus and controversy. Trends Ecol. Evol. 1995, 10, 212–217. [Google Scholar] [CrossRef]
- Brakefield, P.M.; Beldade, P.; Zwaan, B.J. The African Butterfly Bicyclus anynana: A Model for Evolutionary Genetics and Evolutionary Developmental Biology. Cold Spring Harb. Protoc. 2009, 2009, pdb.emo122. [Google Scholar] [CrossRef]
- Beldade, P.; Peralta, C.M. Developmental and evolutionary mechanisms shaping butterfly eyespots. Curr. Opin. Insect Sci. 2017, 19, 22–29. [Google Scholar] [CrossRef]
- Beldade, P.; Monteiro, A. Eco-evo-devo advances with butterfly eyespots. Curr. Opin. Genet. Dev. 2021, 69, 6–13. [Google Scholar] [CrossRef]
- Monteiro, A. Physiology and Evolution of Wing Pattern Plasticity in Bicyclus Butterflies: A Critical Review of the Literature. In Diversity and Evolution of Butterfly Wing Patterns; Sekimura, T., Nijhout, H.F., Eds.; Springer: Singapore, 2017; pp. 91–105. ISBN 978-9-81-104955-2. [Google Scholar]
- Brakefield, P.; Frankino, W. Polyphenisms in Lepidoptera: Multidisciplinary Approaches to Studies of Evolution and Devel-opment. In Phenotypic Plasticity of Insects; Whitman, D., Ananthakrishnan, T., Eds.; Science Publishers: Amsterdam, The Netherlands, 2009; ISBN 978-1-57-808423-4. [Google Scholar]
- Brakefield, P.M.; Larsen, T.B. The evolutionary significance of dry and wet season forms in some tropical butterflies. Biol. J. Linn. Soc. 1984, 22, 1–12. [Google Scholar] [CrossRef]
- Olofsson, M.; Vallin, A.; Jakobsson, S.; Wiklund, C. Marginal Eyespots on Butterfly Wings Deflect Bird Attacks Under Low Light Intensities with UV Wavelengths. PLoS ONE 2010, 5, e10798. [Google Scholar] [CrossRef]
- Prudic, K.L.; Stoehr, A.M.; Wasik, B.R.; Monteiro, A. Eyespots deflect predator attack increasing fitness and promoting the evolution of phenotypic plasticity. Proc. R. Soc. B Boil. Sci. 2015, 282, 20141531. [Google Scholar] [CrossRef] [PubMed]
- van Bergen, E.; Beldade, P. Seasonal plasticity in anti-predatory strategies: Matching of color and color preference for effective crypsis. Evol. Lett. 2019, 3, 313–320. [Google Scholar] [CrossRef]
- Monteiro, A.F.; Brakefield, P.M.; French, V. The Evolutionary Genetics and Developmental Basis of Wing Pattern Variation in the Butterfly Bicyclus anynana. Evolution 1994, 48, 1147. [Google Scholar] [CrossRef] [PubMed]
- Beldade, P.; Koops, K.; Brakefield, P.M. Modularity, individuality, and evo-devo in butterfly wings. Proc. Natl. Acad. Sci. USA 2002, 99, 14262–14267. [Google Scholar] [CrossRef]
- Beldade, P.; Koops, K.; Brakefield, P.M. Developmental constraints versus flexibility in morphological evolution. Nature 2002, 416, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Saenko, S.; French, V.; Brakefield, P.M.; Beldade, P. Conserved developmental processes and the formation of evolutionary novelties: Examples from butterfly wings. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Beldade, P.; Saenko, S.V.; Pul, N.; Long, A.D. A Gene-Based Linkage Map for Bicyclus anynana Butterflies Allows for a Comprehensive Analysis of Synteny with the Lepidopteran Reference Genome. PLoS Genet. 2009, 5, e1000366. [Google Scholar] [CrossRef] [PubMed]
- A Mateus, A.R.; Marques-Pita, M.; Oostra, V.; Lafuente, E.; Brakefield, P.M.; Zwaan, B.J.; Beldade, P. Adaptive developmental plasticity: Compartmentalized responses to environmental cues and to corresponding internal signals provide phenotypic flexibility. BMC Biol. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Monteiro, A.; Tong, X.; Bear, A.; Liew, S.F.; Bhardwaj, S.; Wasik, B.R.; Dinwiddie, A.; Bastianelli, C.; Cheong, W.F.; Wenk, M.R.; et al. Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs. PLoS Genet. 2015, 11, e1005529. [Google Scholar] [CrossRef]
- Wijngaarden, P.J.; Brakefield, P.M. The genetic basis of eyespot size in the butterfly Bicyclus anynana: An analysis of line crosses. Heredity 2000, 85, 471–479. [Google Scholar] [CrossRef][Green Version]
- Saenko, S.V.; A Jerónimo, M.; Beldade, P. Genetic basis of stage-specific melanism: A putative role for a cysteine sulfinic acid decarboxylase in insect pigmentation. Heredity 2012, 108, 594–601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saenko, S.V.; Brakefield, P.M.; Beldade, P. Single locus affects embryonic segment polarity and multiple aspects of an adult evolutionary novelty. BMC Biol. 2010, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Beldade, P.; Brakefield, P.M. Concerted evolution and developmental integration in modular butterfly wing patterns. Evol. Dev. 2003, 5, 169–179. [Google Scholar] [CrossRef]
- Rodrigues, Y.K.; Bergen, E.; Alves, F.; Duneau, D.; Beldade, P. Additive and non-additive effects of day and night temperatures on thermally plastic traits in a model for adaptive seasonal plasticity. Evolution 2021, 75, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- Brakefield, P.M.; Kesbeke, F.; Koch, P.B. The Regulation of Phenotypic Plasticity of Eyespots in the Butterfly Bicyclus anynana. Am. Nat. 1998, 152, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Wijngaarden, P.J.; Brakefield, P.M. Lack of response to artificial selection on the slope of reaction norms for seasonal polyphenism in the butterfly Bicyclus anynana. Heredity 2001, 87, 410–420. [Google Scholar] [CrossRef]
- R Development Core Team. R—A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2005. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer Series in Statistics; Springer: New York, NY, USA, 2002; ISBN 978-0-38-795442-4. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Josse, J.; Husson, F. missMDA: A Package for Handling Missing Values in Multivariate Data Analysis. J. Stat. Softw. 2016, 70, 1–31. [Google Scholar] [CrossRef]
- De Moed, G.H.; De Jong, G.; Scharloo, W. The phenotypic plasticity of wing size in Drosophila melanogaster: The cellular basis of its genetic variation. Heredity 1997, 79, 260–267. [Google Scholar] [CrossRef][Green Version]
- Mensch, J.; Lavagnino, N.; Carreira, V.P.; Massaldi, A.; Hasson, E.; Fanara, J.J. Identifying candidate genes affecting developmental time in Drosophila melanogaster: Pervasive pleiotropy and gene-by-environment interaction. BMC Dev. Biol. 2008, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver, J.G.; Ragland, G.J.; Shlichta, J.G. Quantitative Genetics of Continuous Reaction Norms: Thermal Sensitivity of Caterpillar Growth Rates. Evolution 2004, 58, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Knies, J.L.; Izem, R.; Supler, K.L.; Kingsolver, J.G.; Burch, C.L. The Genetic Basis of Thermal Reaction Norm Evolution in Lab and Natural Phage Populations. PLoS Biol. 2006, 4, e201. [Google Scholar] [CrossRef]
- Gutteling, E.W.; Riksen, J.A.G.; Bakker, J.; E Kammenga, J. Mapping phenotypic plasticity and genotype–environment interactions affecting life-history traits in Caenorhabditis elegans. Heredity 2006, 98, 28–37. [Google Scholar] [CrossRef] [PubMed]
- A Hutchings, J.; Swain, D.P.; Rowe, S.; Eddington, J.D.; Puvanendran, V.; A Brown, J. Genetic variation in life-history reaction norms in a marine fish. Proc. R. Soc. B Boil. Sci. 2007, 274, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Sureshkumar, S.; Lempe, J.; Weigel, D. Potent Induction of Arabidopsis thaliana Flowering by Elevated Growth Temperature. PLoS Genet. 2006, 2, e106. [Google Scholar] [CrossRef]
- Culumber, Z.W.; Schumer, M.; Monks, S.; Tobler, M. Environmental heterogeneity generates opposite gene-by-environment interactions for two fitness-related traits within a population: Brief Communication. Evolution 2015, 69, 541–550. [Google Scholar] [CrossRef]
- Czarnoleski, M.; Kramarz, P.; Małek, D.; Drobniak, S.M. Genetic components in a thermal developmental plasticity of the beetle Tribolium castaneum. J. Therm. Biol. 2017, 68, 55–62. [Google Scholar] [CrossRef]
- Gibert, J.-M.; Mouchel-Vielh, E.; De Castro, S.; Peronnet, F. Phenotypic Plasticity through Transcriptional Regulation of the Evolutionary Hotspot Gene tan in Drosophila melanogaster. PLoS Genet. 2016, 12, e1006218. [Google Scholar] [CrossRef]
- De Castro, S.; Peronnet, F.; Gilles, J.-F.; Mouchel-Vielh, E.; Gibert, J.-M. bric à brac (bab), a central player in the gene regulatory network that mediates thermal plasticity of pigmentation in Drosophila melanogaster. PLoS Genet. 2018, 14, e1007573. [Google Scholar] [CrossRef]
- Endler, L.; Gibert, J.-M.; Nolte, V.; Schlötterer, C. Pleiotropic effects of regulatory variation in tan result in correlation of two pigmentation traits in Drosophila melanogaster. Mol. Ecol. 2018, 27, 3207–3218. [Google Scholar] [CrossRef] [PubMed]
- Gibert, J.-M.; Mouchel-Vielh, E.; Peronnet, F. Modulation of yellow expression contributes to thermal plasticity of female abdominal pigmentation in Drosophila melanogaster. Sci. Rep. 2017, 7, srep43370. [Google Scholar] [CrossRef]
- Monteiro, A.; Brakefield, P.M.; French, V. Butterfly Eyespots: The Genetics and Development of the Color Rings. Evolution 1997, 51, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- E Allen, C.; Beldade, P.; Zwaan, B.J.; Brakefield, P.M. Differences in the selection response of serially repeated color pattern characters: Standing variation, development, and evolution. BMC Evol. Biol. 2008, 8, 94. [Google Scholar] [CrossRef]
- Oostra, V.; de Jong, M.; Invergo, B.; Kesbeke, F.; Wende, F.; Brakefield, P.M.; Zwaan, B. Translating environmental gradients into discontinuous reaction norms via hormone signalling in a polyphenic butterfly. Proc. R. Soc. B Boil. Sci. 2010, 278, 789–797. [Google Scholar] [CrossRef]
- David, J.R.; Capy, P.; Gauthier, J.-P. Abdominal pigmentation and growth temperature in Drosophila melanogaster: Similarities and differences in the norms of reaction of successive segments. J. Evol. Biol. 1990, 3, 429–445. [Google Scholar] [CrossRef]
- Lafuente, E.; Alves, F.; King, J.G.; Peralta, C.M.; Beldade, P. Many ways to make darker flies: Intra- and interspecific variation in Drosophila body pigmentation components. Ecol. Evol. 2021, 11, 8136–8155. [Google Scholar] [CrossRef]
- Oostra, V.; Mateus, A.R.A.; van der Burg, K.R.L.; Piessens, T.; van Eijk, M.; Brakefield, P.M.; Beldade, P.; Zwaan, B.J. Ecdysteroid Hormones Link the Juvenile Environment to Alternative Adult Life Histories in a Seasonal Insect. Am. Nat. 2014, 184, E79–E92. [Google Scholar] [CrossRef]
- David, J.R.; Gibert, P.; Moreteau, B. Evolution of Reaction Norms. In Phenotypic Plasticity: Functional and Conceptual Approaches; DeWitt, T.J., Scheiner, S.M., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 50–63. [Google Scholar]
- Gibert, P.; Capy, P.; Imasheva, A.; Moreteau, B.; Morin, J.; Pétavy, G.; David, J. Comparative analysis of morphological traits among Drosophila melanogaster and D. simulans: Genetic variability, clines and phenotypic plasticity. Genetica 2004, 120, 165–179. [Google Scholar] [CrossRef]
- Tang, H.Y.; Smith-Caldas, M.S.B.; Driscoll, M.V.; Salhadar, S.; Shingleton, A. FOXO Regulates Organ-Specific Phenotypic Plasticity In Drosophila. PLoS Genet. 2011, 7, e1002373. [Google Scholar] [CrossRef]
- Mirth, C.K.; Shingleton, A.W. Integrating Body and Organ Size in Drosophila: Recent Advances and Outstanding Problems. Front. Endocrinol. 2012, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Kijimoto, T.; Snell-Rood, E.C.; Pespeni, M.H.; Rocha, G.; Kafadar, K.; Moczek, A.P. The nutritionally responsive transcriptome of the polyphenic beetle Onthophagus taurus and the importance of sexual dimorphism and body region. Proc. R. Soc. B Boil. Sci. 2014, 281, 20142084. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, H.; Hust, J.A.; Miura, T.; Niimi, T.; Emlen, D.J.; Lavine, L.C. The Fat/Hippo signaling pathway links within-disc morphogen patterning to whole-animal signals during phenotypically plastic growth in insects: Fat/Hippo Signaling In Nu-trition-Sensitive Growth. Dev. Dyn. 2015, 244, 1039–1045. [Google Scholar] [CrossRef]
- Koyama, T.; A Rodrigues, M.; Athanasiadis, A.; Shingleton, A.W.; Mirth, C.K. Nutritional Control of Body Size through FoxO-Ultraspiracle Mediated Ecdysone Biosynthesis. eLife 2014, 3, e03091. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateus, A.R.A.; Beldade, P. Developmental Plasticity in Butterfly Eyespot Mutants: Variation in Thermal Reaction Norms across Genotypes and Pigmentation Traits. Insects 2022, 13, 1000. https://doi.org/10.3390/insects13111000
Mateus ARA, Beldade P. Developmental Plasticity in Butterfly Eyespot Mutants: Variation in Thermal Reaction Norms across Genotypes and Pigmentation Traits. Insects. 2022; 13(11):1000. https://doi.org/10.3390/insects13111000
Chicago/Turabian StyleMateus, Ana Rita Amaro, and Patrícia Beldade. 2022. "Developmental Plasticity in Butterfly Eyespot Mutants: Variation in Thermal Reaction Norms across Genotypes and Pigmentation Traits" Insects 13, no. 11: 1000. https://doi.org/10.3390/insects13111000
APA StyleMateus, A. R. A., & Beldade, P. (2022). Developmental Plasticity in Butterfly Eyespot Mutants: Variation in Thermal Reaction Norms across Genotypes and Pigmentation Traits. Insects, 13(11), 1000. https://doi.org/10.3390/insects13111000






