The Effect of an Irradiation-Induced Recombination Suppressing Inversion on the Genetic Stability and Biological Quality of a White Eye-Based Aedes aegypti Genetic Sexing Strain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Genetic Stability
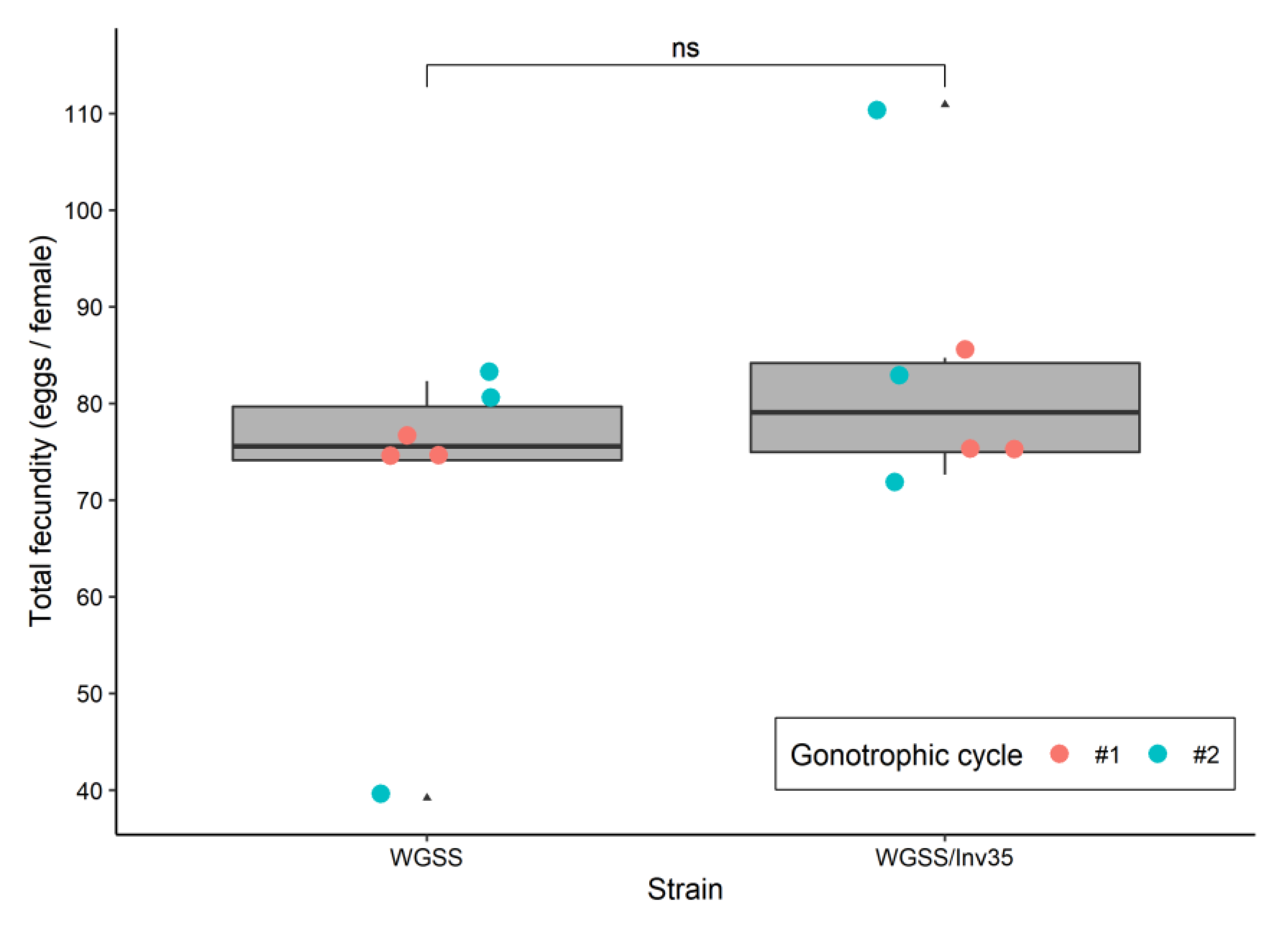
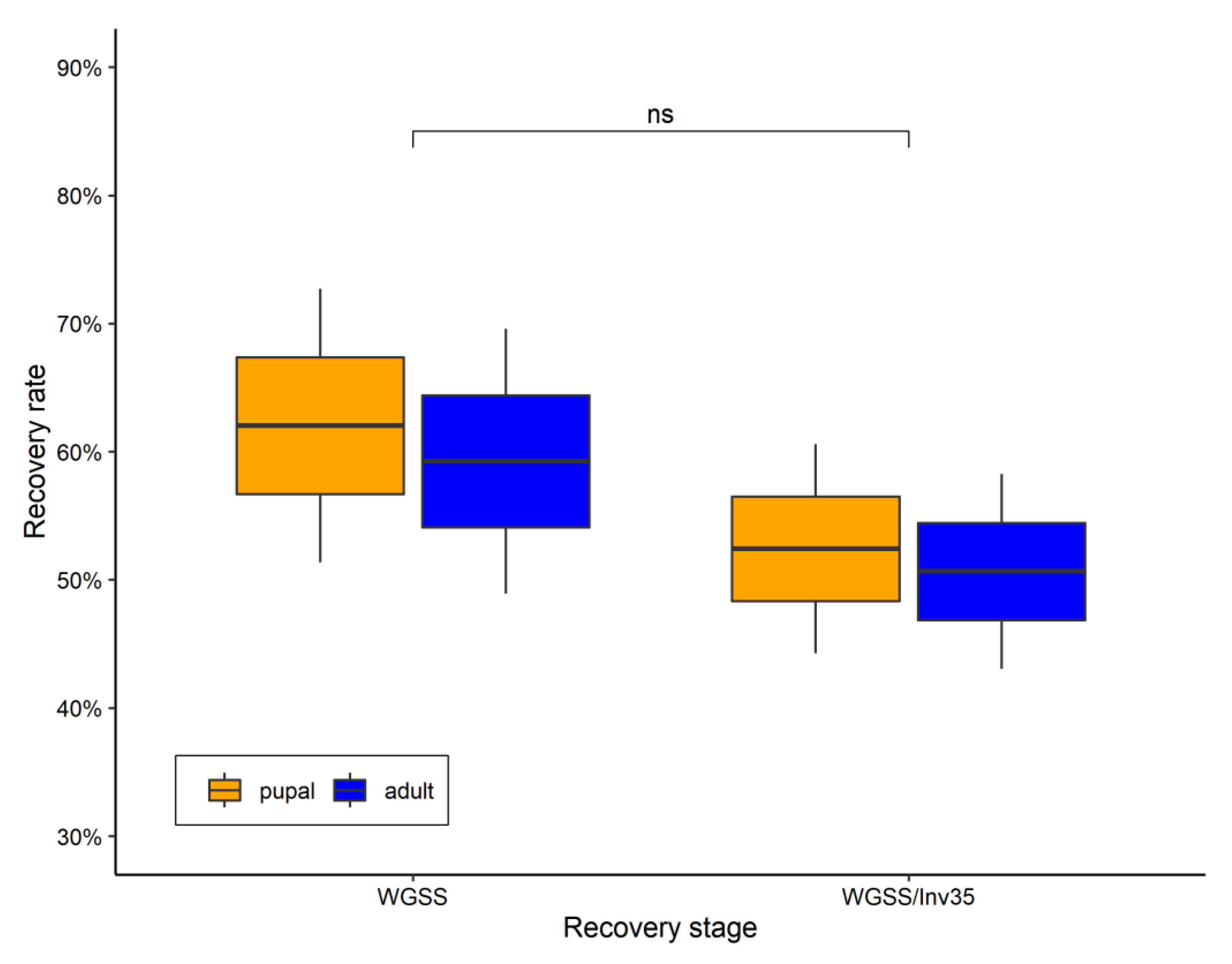
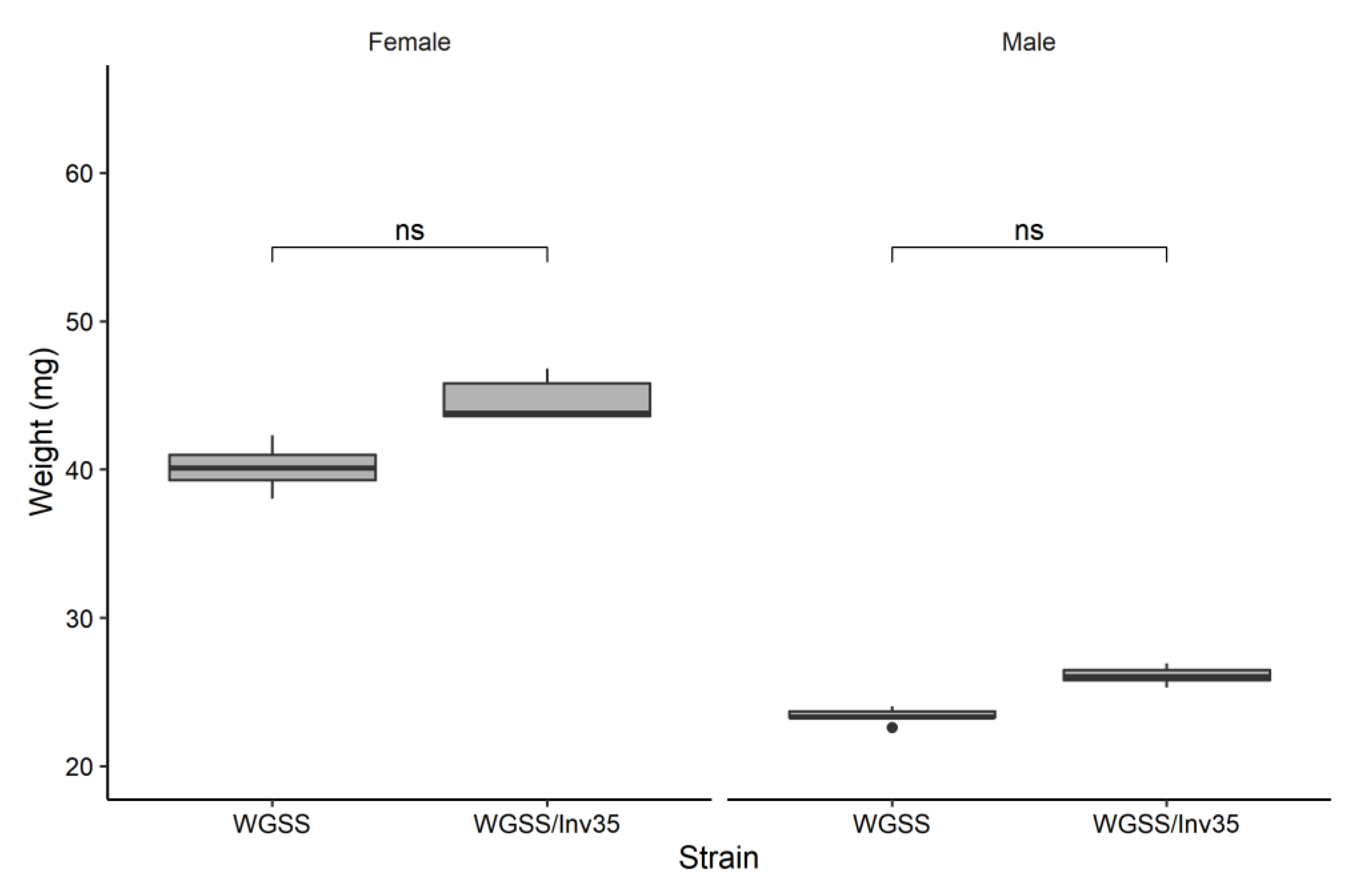
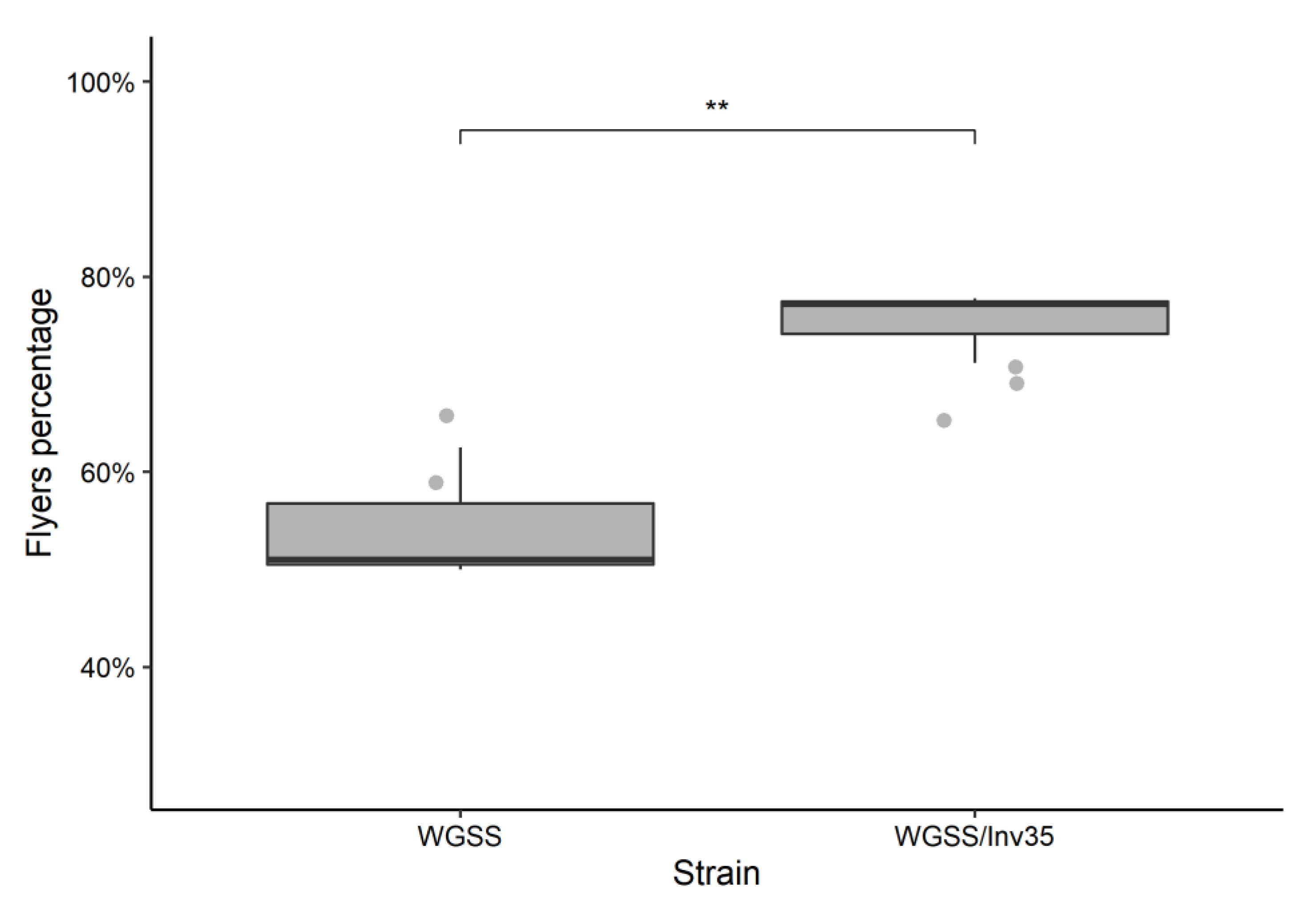
3.2. Quality Control Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-NG, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative Strategies for Mosquito-Borne Arbovirus Control. PLoS Negl. Trop. Dis. 2019, 13, e0006822. [Google Scholar] [CrossRef]
- Franklinos, L.H.V.; Jones, K.E.; Redding, D.W.; Abubakar, I. The Effect of Global Change on Mosquito-Borne Disease. Lancet Infect. Dis. 2019, 19, e302–e312. [Google Scholar] [CrossRef]
- Swei, A.; Couper, L.I.; Coffey, L.L.; Kapan, D.; Bennett, S. Patterns, Drivers, and Challenges of Vector-Borne Disease Emergence. Vector-Borne Zoonotic Dis. 2020, 20, 159–170. [Google Scholar] [CrossRef]
- Colón-González, F.J.; Sewe, M.O.; Tompkins, A.M.; Sjödin, H.; Casallas, A.; Rocklöv, J.; Caminade, C.; Lowe, R. Projecting the Risk of Mosquito-Borne Diseases in a Warmer and More Populated World: A Multi-Model, Multi-Scenario Intercomparison Modelling Study. Lancet Planet. Health 2021, 5, e404–e414. [Google Scholar] [CrossRef]
- Kim, K.-S. Current Challenges in the Development of Vaccines and Drugs Against Emerging Vector-Borne Diseases. Curr. Med. Chem. 2019, 26, 2974–2986. [Google Scholar] [CrossRef]
- Baldacchino, F.; Caputo, B.; Chandre, F.; Drago, A.; della Torre, A.; Montarsi, F.; Rizzoli, A. Control Methods against Invasive Aedes Mosquitoes in Europe: A Review. Pest Manag. Sci. 2015, 71, 1471–1485. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes Aegypti and Ae. Albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes Aegypti and Aedes Albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Powell, J.R. Mosquito-Borne Human Viral Diseases: Why Aedes Aegypti? Am. J. Trop Med. Hyg. 2018, 98, 1563–1565. [Google Scholar] [CrossRef]
- Vontas, J.; Kioulos, E.; Pavlidi, N.; Morou, E.; della Torre, A.; Ranson, H. Insecticide Resistance in the Major Dengue Vectors Aedes Albopictus and Aedes Aegypti. Pestic. Biochem. Physiol. 2012, 104, 126–131. [Google Scholar] [CrossRef]
- Lees, R.S.; Gilles, J.R.; Hendrichs, J.; Vreysen, M.J.; Bourtzis, K. Back to the Future: The Sterile Insect Technique against Mosquito Disease Vectors. Curr. Opin. Insect Sci. 2015, 10, 156–162. [Google Scholar] [CrossRef]
- Hoang, K.P.; Teo, T.M.; Ho, T.X.; Le, V.S. Mechanisms of Sex Determination and Transmission Ratio Distortion in Aedes Aegypti. Parasit. Vectors 2016, 9, 49. [Google Scholar] [CrossRef]
- Bouyer, J.; Culbert, N.J.; Dicko, A.H.; Pacheco, M.G.; Virginio, J.; Pedrosa, M.C.; Garziera, L.; Pinto, A.T.M.; Klaptocz, A.; Germann, J.; et al. Field Performance of Sterile Male Mosquitoes Released from an Uncrewed Aerial Vehicle. Sci. Robot. 2020, 5. [Google Scholar] [CrossRef]
- Pagendam, D.; Snoad, N.; Yang, W.-H.; Segoli, M.; Ritchie, S.; Trewin, B.; Beebe, N. Improving Estimates of Fried’s Index from Mating Competitiveness Experiments. JABES 2018, 23, 446–462. [Google Scholar] [CrossRef]
- Dyck, V.A.; Reyes, J.; Vreysen, M.J.B.; Fernandez, E.; Teruya, T.; Barnes, B.N.; Gomez-Riera, P.; Lindguist, D.; Loosjes, M. Management of Area-Wide Integrated Pest Management Programmes That Integrate the Sterile Insect Technique. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: New York, NY, USA, 2021; pp. 781–814. ISBN 978-1-00-303557-2. [Google Scholar]
- Hendrichs, J.; Pereira, R.; Vreysen, M.J.B. (Eds.) Area-Wide Integrated Pest Management: Development and Field Application; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-316923-9. [Google Scholar]
- Hendrichs, J.; Franz, G.; Rendon, P. Increased Effectiveness and Applicability of the Sterile Insect Technique through Male-only Releases for Control of Mediterranean Fruit Flies during Fruiting Seasons. J. Appl. Entomol. 1995, 119, 371–377. [Google Scholar] [CrossRef]
- Rendón, P.; McInnis, D.; Lance, D.; Stewart, J. Medfly (Diptera: Tephritidae) Genetic Sexing: Large-Scale Field Comparison of Males-Only and Bisexual Sterile Fly Releases in Guatemala. J. Econ. Entomol. 2004, 97, 1547–1553. [Google Scholar] [CrossRef]
- Lutrat, C.; Giesbrecht, D.; Marois, E.; Whyard, S.; Baldet, T.; Bouyer, J. Sex Sorting for Pest Control: It’s Raining Men! Trends Parasitol. 2019, 35, 649–662. [Google Scholar] [CrossRef]
- World Health Organization. The International Atomic Energy Agency Guidance Framework for Testing the Sterile Insect Technique as a Vector Control Tool against Aedes-Borne Diseases; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Culbert, N.J.; Balestrino, F.; Dor, A.; Herranz, G.S.; Yamada, H.; Wallner, T.; Bouyer, J. A Rapid Quality Control Test to Foster the Development of Genetic Control in Mosquitoes. Sci. Rep. 2018, 8, 16179. [Google Scholar] [CrossRef]
- Crawford, J.E.; Clarke, D.W.; Criswell, V.; Desnoyer, M.; Cornel, D.; Deegan, B.; Gong, K.; Hopkins, K.C.; Howell, P.; Hyde, J.S.; et al. Efficient Production of Male Wolbachia -Infected Aedes Aegypti Mosquitoes Enables Large-Scale Suppression of Wild Populations. Nat. Biotechnol. 2020, 38, 482–492. [Google Scholar] [CrossRef]
- Koskinioti, P.; Augustinos, A.A.; Carvalho, D.O.; Misbah-ul-Haq, M.; Pillwax, G.; de la Fuente, L.D.; Salvador-Herranz, G.; Herrero, R.A.; Bourtzis, K. Genetic Sexing Strains for the Population Suppression of the Mosquito Vector Aedes Aegypti. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20190808. [Google Scholar] [CrossRef] [PubMed]
- Franz, G.; Bourtzis, K.; Caceres, C. Practical and Operational Genetic Sexing Systems Based on Classical Genetic Approaches in Fruit Flies, an Example for Other Species Amenable to Large-Scale Rearing for the Sterile Insect Technique. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2021; pp. 575–604. ISBN 978-1-00-303557-2. [Google Scholar]
- Fisher, K.; Caceres, C. A Filter Rearing System for Mass Reared Genetic Sexing Strains of Mediterranean Fruit Fly (Diptera: Tephritidae). In Area-wide Control of Fruit Flies and other Insect Pests; Joint Proceedings of the International Conference on Area-Wide Control of Insect Pests, 28 May–2 June 1998 and the Fifth International Symposium on Fruit Flies of Economic Importance, Penang, Malaysia, 1–5 June 1998; Penerbit Universiti Sains Malaysia: Gelugor, Malaysia, 2000; pp. 543–550, ISBN 983-861-195-6. [Google Scholar]
- John, A.; Vinayan, K.; Varghese, J. Achiasmy: Male Fruit Flies Are Not Ready to Mix. Front. Cell Dev. Biol. 2016, 4, 75. [Google Scholar] [CrossRef]
- Satomura, K.; Osada, N.; Endo, T. Achiasmy and Sex Chromosome Evolution. Ecol. Genet. Genom. 2019, 13, 100046. [Google Scholar] [CrossRef]
- Rössler, Y. Genetic Recombination in Males of the Mediterranean Fruit Fly,1 and Its Relation to Automated Sexing Methods. Ann. Entomol. Soc. Am. 1982, 75, 28–31. [Google Scholar] [CrossRef]
- Cladera, J.L. Absence of Recombination in the Male of Ceratitis Capitata. Experientia 1981, 37, 342–343. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Misbah-ul-Haq, M.; Carvalho, D.O.; de la Fuente, L.D.; Koskinioti, P.; Bourtzis, K. Irradiation Induced Inversions Suppress Recombination between the M Locus and Morphological Markers in Aedes Aegypti. BMC Genet. 2020, 21, 142. [Google Scholar] [CrossRef]
- Foster, G.G. Chromosomal Inversions and Genetic Control Revisited: The Use of Inversions in Sexing Systems for Higher Diptera. Theoret. Appl. Genet. 1991, 81, 619–623. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Nikolouli, K.; Duran de la Fuente, L.; Misbah-ul-Haq, M.; Carvalho, D.O.; Bourtzis, K. Introgression of the Aedes Aegypti Red-Eye Genetic Sexing Strains Into Different Genomic Backgrounds for Sterile Insect Technique Applications. Front. Bioeng. Biotechnol. 2022, 10, 821428. [Google Scholar] [CrossRef]
- Misbah-ul-Haq, M.; Carvalho, D.O.; Duran De La Fuente, L.; Augustinos, A.A.; Bourtzis, K. Genetic Stability and Fitness of Aedes Aegypti Red-Eye Genetic Sexing Strains With Pakistani Genomic Background for Sterile Insect Technique Applications. Front. Bioeng. Biotechnol. 2022, 10, 871703. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The International Atomic Energy Agency Guidelines for Routine Colony Maintenance of Aedes Mosquito Species V1. 2017. Available online: https://www.iaea.org/sites/default/files/21/06/nafa-ipc-manual-guidelines-for-routine-colony-maintenance-of-aedes-mosquito-species-v1.0 (accessed on 30 July 2022).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project (accessed on 30 July 2022).
- RStudio Team. RStudio: Integrated Development Environment for R 2016. PBC: Boston, MA, USA, 2016; Available online: http://www.rstudio (accessed on 30 July 2022).
- Therneau, T. A Package for Survival Analysis in R. R Package Version 3.2-3. 2020. 2020. Available online: https://CRAN.R-project.org/package=survival (accessed on 30 July 2022).
- Parker, A.G.; Vreysen, M.J.B.; Bouyer, J.; Calkins, C.O. Sterile Insect Quality Control/Assurance. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Robinson, A.S., Hendrichs, J., Eds.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-303557-2. [Google Scholar]
- McBroome, J.; Liang, D.; Corbett-Detig, R. Fine-Scale Position Effects Shape the Distribution of Inversion Breakpoints in Drosophila Melanogaster. Genome Biol. Evol. 2020, 12, 1378–1391. [Google Scholar] [CrossRef]
- Mérot, C.; Llaurens, V.; Normandeau, E.; Bernatchez, L.; Wellenreuther, M. Balancing Selection via Life-History Trade-Offs Maintains an Inversion Polymorphism in a Seaweed Fly. Nat. Commun. 2020, 11, 670. [Google Scholar] [CrossRef]
- Jay, P.; Chouteau, M.; Whibley, A.; Bastide, H.; Parrinello, H.; Llaurens, V.; Joron, M. Mutation Load at a Mimicry Supergene Sheds New Light on the Evolution of Inversion Polymorphisms. Nat. Genet. 2021, 53, 288–293. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misbah-ul-Haq, M.; Augustinos, A.A.; Carvalho, D.O.; Duran de la Fuente, L.; Bourtzis, K. The Effect of an Irradiation-Induced Recombination Suppressing Inversion on the Genetic Stability and Biological Quality of a White Eye-Based Aedes aegypti Genetic Sexing Strain. Insects 2022, 13, 946. https://doi.org/10.3390/insects13100946
Misbah-ul-Haq M, Augustinos AA, Carvalho DO, Duran de la Fuente L, Bourtzis K. The Effect of an Irradiation-Induced Recombination Suppressing Inversion on the Genetic Stability and Biological Quality of a White Eye-Based Aedes aegypti Genetic Sexing Strain. Insects. 2022; 13(10):946. https://doi.org/10.3390/insects13100946
Chicago/Turabian StyleMisbah-ul-Haq, Muhammad, Antonios A. Augustinos, Danilo O. Carvalho, Lucia Duran de la Fuente, and Kostas Bourtzis. 2022. "The Effect of an Irradiation-Induced Recombination Suppressing Inversion on the Genetic Stability and Biological Quality of a White Eye-Based Aedes aegypti Genetic Sexing Strain" Insects 13, no. 10: 946. https://doi.org/10.3390/insects13100946
APA StyleMisbah-ul-Haq, M., Augustinos, A. A., Carvalho, D. O., Duran de la Fuente, L., & Bourtzis, K. (2022). The Effect of an Irradiation-Induced Recombination Suppressing Inversion on the Genetic Stability and Biological Quality of a White Eye-Based Aedes aegypti Genetic Sexing Strain. Insects, 13(10), 946. https://doi.org/10.3390/insects13100946





