Conservation Biological Control of Codling Moth (Cydia pomonella): Effects of Two Aromatic Plants, Basil (Ocimum basilicum) and French Marigolds (Tagetes patula)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
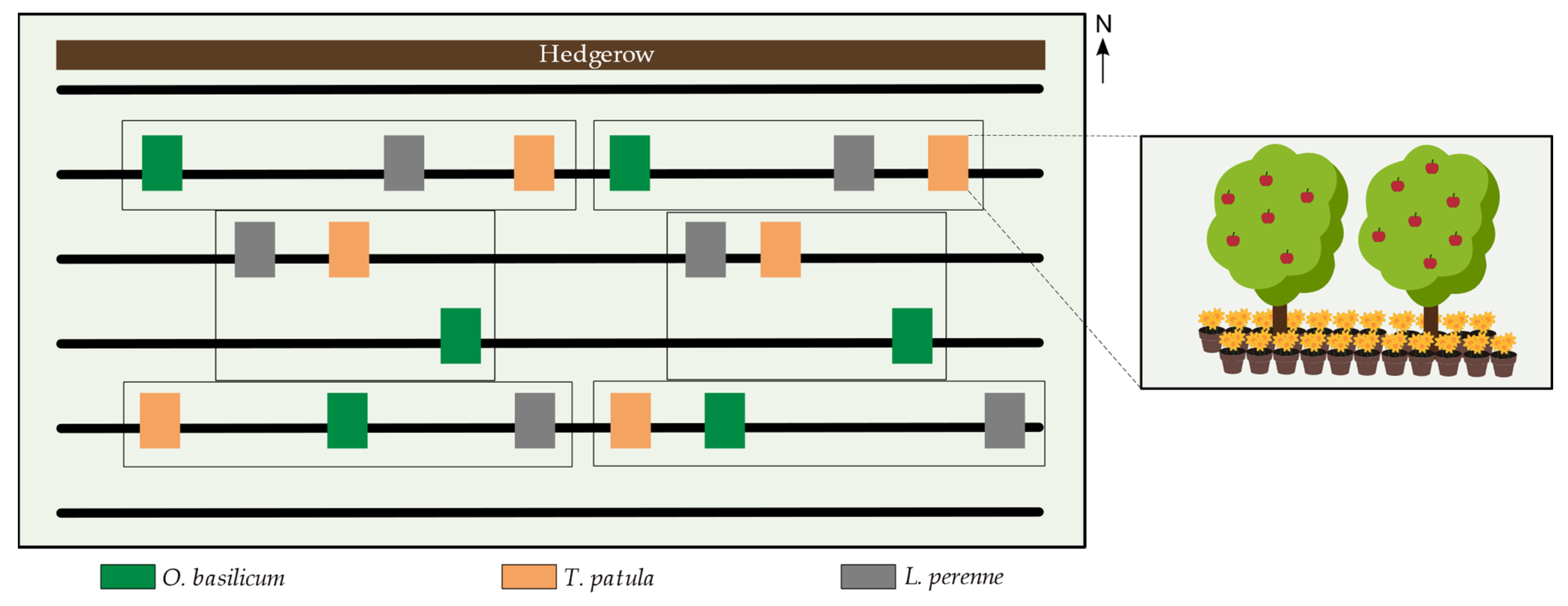
2.1. Study Site and Experimental Setup
2.2. Field Surveys
2.3. Adult Emergence Monitoring
2.4. Statistical Analysis
3. Results
3.1. Codling Moth Larvae and Damages
3.2. Codling Moth Parasitoids
3.3. Arthropod Predators
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| O. basilicum | T. patula | L. perenne | Total | ||||
|---|---|---|---|---|---|---|---|
| Total | Mean (±SE) | Total | Mean (±SE) | Total | Mean (±SE) | ||
| Araneae (all) | 236 | 19.67 (±2.46) | 216 | 18.0 (±2.18) | 251 | 20.91 (±1.94) | 703 |
| Miturgidae | 21 | 1.75 (±0.56) | 12 | 1.00 (±0.33) | 14 | 1.17 (±0.40) | 47 |
| Salticidae | 148 | 12.33 (±2.12) | 126 | 10.5 (±1.55) | 157 | 13.08 (±1.77) | 431 |
| Gnaphosidae | 27 | 2.25 (±0.33) | 21 | 1.75 (±0.39) | 39 | 3.25 (±0.64) | 87 |
| Thomisidae | 17 | 1.42 (±0.38) | 17 | 1.42 (±0.26) | 11 | 0.92 (±0.26) | 45 |
| Other Araneae | 23 | 1.92 (±0.29) | 40 | 3.33 (±0.75) | 30 | 2.5 (±0.45) | 93 |
| Coccinellidae | 32 | 2.67 (±0.78) | 19 | 1.58 (±0.76) | 18 | 1.5 (±0.53) | 69 |
| Carabidae | 14 | 1.17 (±0.40) | 11 | 0.92 (±0.31) | 12 | 1.00 (±0.27) | 37 |
| Forficulidae | 5090 | 349.58 (±16.09) | 4280 | 315.83 (±11.33) | 4860 | 342.08 (±16.00) | 14,230 |
| Shannon index | - | 1.36 (±0.08) | - | 1.21 (±0.12) | - | 1.23 (±0.10) | - |
References
- Dudley, N.; Alexander, S. Agriculture and biodiversity: A review. Biodiversity 2017, 18, 45–49. [Google Scholar] [CrossRef]
- Baude, M.; Kunin, W.E.; Boatman, N.D.; Conyers, S.; Davies, N.; Gillespie, M.A.K.; Morton, R.D.; Smart, S.M.; Memmott, J. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 2016, 530, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.D.; Scherber, C.; Borer, E.T.; Ebeling, A.; Gauzens, B.; Gilling, D.P.; Hines, J.; Isbell, F.; Ristok, C.; Tilman, D.; et al. Biodiversity enhances the multitrophic control of arthropod herbivory. Sci. Adv. 2020, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Meehan, T.D.; Werling, B.P.; Landis, D.A.; Gratton, C. Agricultural landscape simplification and insecticide use in the Midwestern United States. Proc. Natl. Acad. Sci. USA 2011, 108, 11500–11505. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, L.M. Engineering natural enemy shelters to enhance conservation biological control in field crops. Biol. Control 2019, 130, 155–163. [Google Scholar] [CrossRef]
- Gontijo, L.M.; Beers, E.H.; Snyder, W.E. Flowers promote aphid suppression in apple orchards. Biol. Control 2013, 66, 8–15. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: A review. Agron. Sustain. Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef]
- Campbell, A.; Wilby, A.; Sutton, P.; Wäckers, F. Getting More Power from Your Flowers: Multi-Functional Flower Strips Enhance Pollinators and Pest Control Agents in Apple Orchards. Insects 2017, 8, 101. [Google Scholar] [CrossRef]
- McKerchar, M.; Potts, S.G.; Fountain, M.T.; Garratt, M.P.D.; Westbury, D.B. The potential for wildflower interventions to enhance natural enemies and pollinators in commercial apple orchards is limited by other management practices. Agric. Ecosyst. Environ. 2020, 301, 107034. [Google Scholar] [CrossRef]
- Wäckers, F.L.; van Rijn, P.C.J. Pick and Mix: Selecting Flowering Plants to Meet the Requirements of Target Biological Control Insects. In Biodiversity and Insect Pests; Gurr, G.M., Wratten, S.D., Snyder, W.E., Read, D.M.Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 139–165. [Google Scholar] [CrossRef]
- Karpiński, T. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tian, L.; Han, X.; Yang, Y. Research advances in allelopathy of volatile organic compounds (VOCs) of plants. Horticulturae 2021, 7, 278. [Google Scholar] [CrossRef]
- Hogg, B.N.; Bugg, R.L.; Daane, K.M. Attractiveness of common insectary and harvestable floral resources to beneficial insects. Biol. Control 2011, 56, 76–84. [Google Scholar] [CrossRef]
- Hatt, S.; Xu, Q.; Francis, F.; Osawa, N. Aromatic plants of East Asia to enhance natural enemies towards biological control of insect pests. A review. Entomol. Gen. 2019, 38, 275–315. [Google Scholar] [CrossRef]
- Adeleye, V.O.; Seal, D.R.; Liburd, O.E.; McAuslane, H.; Alborn, H. Pepper weevil, Anthonomus eugenii (Coleoptera: Curculionidae) suppression on jalapeño peppers using non-host insect repellent plants. Crop Prot. 2021, 154, 105893. [Google Scholar] [CrossRef]
- Jankowska, B.; Poniedziałek, M.; Jędrszczyk, E. Effect of intercropping white cabbage with French Marigold (Tagetes patula nana L.) and Pot Marigold (Calendula officinalis L.) on the colonization of plants by pest insects. Folia Hortic. 2009, 21, 95–103. [Google Scholar] [CrossRef]
- Basedow, T.; Hua, L.; Aggarwal, N. The infestation of Vicia faba L. (Fabaceae) by Aphis fabae (Scop.) (Homoptera: Aphididae) under the influence of Lamiaceae (Ocimum basilicum L. and Satureja hortensis L.). J. Pest Sci. 2006, 79, 149–154. [Google Scholar] [CrossRef]
- Conboy, N.J.A.; McDaniel, T.; Ormerod, A.; George, D.; Gatehouse, A.M.R.; Wharton, E.; Donohoe, P.; Curtis, R.; Tosh, C.R. Companion planting with French marigolds protects tomato plants from glasshouse whiteflies through the emission of airborne limonene. PLoS ONE 2019, 14, e0213071. [Google Scholar] [CrossRef]
- Togni, P.H.B.; Venzon, M.; Muniz, C.A.; Martins, E.F.; Pallini, A.; Sujii, E.R. Mechanisms underlying the innate attraction of an aphidophagous coccinellid to coriander plants: Implications for conservation biological control. Biol. Control 2016, 92, 77–84. [Google Scholar] [CrossRef]
- Fang, Y.; Li, S.; Xu, Q.; Wang, J.; Mi, Y.; Jin, Z.; Desneux, N.; Wang, S. Optimizing the use of basil as a functional plant for the biological control of aphhids by Chrysopa pallens (Neuroptera: Chrysopidae) in Greenhouse. Insects 2022, 13, 552. [Google Scholar] [CrossRef]
- Barbir, J.; Azpiazu, C.; Badenes-Pérez, F.R.; Fernández-Quintanilla, C.; Dorado, J. Functionality of Selected Aromatic Lamiaceae in Attracting Pollinators in Central Spain. J. Econ. Entomol. 2016, 109, 529–536. [Google Scholar] [CrossRef]
- Song, B.; Jie, Z.; Wiggins, N.L.; Yuncong, Y.; Guangbo, T.; Xusheng, S. Intercropping with Aromatic Plants Decreases Herbivore Abundance, Species Richness, and Shifts Arthropod Community Trophic Structure. Environ. Entomol. 2012, 41, 872–879. [Google Scholar] [CrossRef]
- Blommers, L.H.M. Integrated pest management in European apple orchards. Annu. Rev. Entomol. 1994, 39, 213–241. [Google Scholar] [CrossRef]
- Shel’Deshova, G.G. Ecological factors determining distribution of the codling moth Lapspeyresia pomonella L. in the northern and southern hemispheres. Entomol. Rev. 1967, 46, 349–361. [Google Scholar]
- Audemard, H. Population dynamics of the codling moth. In World Crop Pests: Tortricid Pest, Their Biologie, Natural Enemies and Control; Van der Guest, L.P.S., Evenhuis, H.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Unruh, T.R.; Miliczky, E.R.; Horton, D.R.; Thomsen-Archer, K.; Rehfield-Ray, L.; Jones, V.P. Gut content analysis of arthropod predators of codling moth in Washington apple orchards. Biol. Control 2016, 102, 85–92. [Google Scholar] [CrossRef]
- Cross, J.V.; Solomon, M.G.; Babandreier, D.; Blommers, L.; Easterbrook, M.A.; Jay, C.N.; Jenser, G.; Jolly, R.L.; Kuhlmann, U.; Lilley, R.; et al. Biocontrol of Pests of Apples and Pears in Northern and Central Europe: 2. Parasitoids. Biocontrol Sci. Technol. 1999, 9, 277–314. [Google Scholar] [CrossRef]
- Lacey, L.A.; Unruh, T.R. Biological control of codling moth (Cydia pomonella, Lepidoptera: Tortricidae) and its role in integrated pest management, with emphasis on entomopathogens. Vedalia 2005, 12, 33–60. [Google Scholar]
- Mátray, S.; Herz, A. Flowering plants serve nutritional needs of Ascogaster quadridentata (Hymenoptera: Braconidae), a key parasitoid of codling moth. Biol. Control 2022, 171, 104950. [Google Scholar] [CrossRef]
- Belz, E.; Kölliker, M.; Balmer, O. Olfactory attractiveness of flowering plants to the parasitoid Microplitis mediator: Potential implications for biological control. BioControl 2013, 58, 163–173. [Google Scholar] [CrossRef]
- Rahat, S.; Gurr, G.M.; Wratten, S.D.; Mo, J.; Neeson, R. Effect of plant nectars on adult longevity of the stinkbug parasitoid, Trissolcus basalis. Int. J. Pest Manag. 2005, 51, 321–324. [Google Scholar] [CrossRef]
- Ricci, B.; Franck, P.; Bouchier, J.-C.; Casado, D.; Lavigne, C. Effects of hedgerow characteristics on intra-orchard distribution of larval codling moth. Agric. Ecosyst. Environ. 2011, 140, 395–400. [Google Scholar] [CrossRef]
- Maalouly, M.; Franck, P.; Lavigne, C. Temporal dynamics of parasitoid assemblages parasitizing the codling moth. Biol. Control 2015, 82, 31–39. [Google Scholar] [CrossRef]
- Franck, P.; Maalouly-Matar, M.; Olivares, J. Molecular Tools for the Detection and the Identification of Hymenoptera Parasitoids in Tortricid Fruit Pests. Int. J. Mol. Sci. 2017, 18, 2031. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, M.; Franck, P.; Olivares, J.; Ricard, J.-M.; Mandrin, J.-F.; Lavigne, C. Spider predation on rosy apple aphid in conventional, organic and insecticide-free orchards and its impact on aphid populations. Biol. Control 2017, 104, 57–65. [Google Scholar] [CrossRef]
- Happe, A.-K.; Roquer-Beni, L.; Bosch, J.; Alins, G.; Mody, K. Earwigs and woolly apple aphids in integrated and organic apple orchards_ responses of a generalist predator and a pest prey to local and landscape factors. Agric. Ecosyst. Environ. 2018, 268, 44–51. [Google Scholar] [CrossRef]
- Athanassov, A.; Charmillot, P.-J.; Jeanneret, P.; Renard, D. Les parasitoïdes des larves et des chrysalides de carpocapse Cydia pomonella L. Rev. Suisse De Vitic. Darboriculture Et Dhorticulture 1997, 29, 99–106. [Google Scholar]
- Martínez-Sastre, R.; Peña, R.; González-Ibáñez, A.; García, D.; Miñarro, M. Top-down and bottom-up regulation of codling moth populations in cider apple orchards. Crop Prot. 2021, 143, 105545. [Google Scholar] [CrossRef]
- Tang, G.B.; Song, B.Z.; Zhao, L.L.; Sang, X.S.; Wan, H.H.; Zhang, J.; Yao, Y.C. Repellent and attractive effects of herbs on insects in pear orchards intercropped with aromatic plants. Agrofor. Syst. 2013, 87, 273–285. [Google Scholar] [CrossRef]
- Souza, I.L.; Tomazella, V.B.; Santos, A.J.N.; Moraes, T.; Silveira, L.C.P. Parasitoids diversity in organic Sweet Pepper (Capsicum annuum) associated with Basil (Ocimum basilicum) and Marigold (Tagetes erecta). Braz. J. Biol. 2019, 79, 603–611. [Google Scholar] [CrossRef]
- Foti, M.C.; Rostás, M.; Peri, E.; Park, K.C.; Slimani, T.; Wratten, S.D.; Colazza, S. Chemical ecology meets conservation biological control: Identifying plant volatiles as predictors of floral resource suitability for an egg parasitoid of stink bugs. J. Pest Sci. 2017, 90, 299–310. [Google Scholar] [CrossRef]
- Batista, M.C.; Fonseca, M.C.M.; Teodoro, A.V.; Martins, E.F.; Pallini, A.; Venzon, M. Basil (Ocimum basilicum L.) attracts and benefits the green lacewing Ceraeochrysa cubana Hagen. Biol. Control 2017, 110, 98–106. [Google Scholar] [CrossRef]
- López, M.D.; Jordán, M.J.; Pascual-Villalobos, M.J. Toxic compounds in essential oils of coriander, caraway and basil active against stored rice pests. J. Stored Prod. Res. 2008, 44, 273–278. [Google Scholar] [CrossRef]
- Mccormick, C.A.; Gershenzon, J.; Unsicker, S.B. Little peaks with big effects: Establishing the role of minor plant volatiles in plant-insect interactions: Minor plant volatiles. Plant Cell Environ. 2014, 37, 1836–1844. [Google Scholar] [CrossRef]
- Song, B.; Tang, G.; Sang, X.; Zhang, J.; Yao, Y.; Wiggins, N. Intercropping with aromatic plants hindered the occurrence of Aphis citricola in an apple orchard system by shifting predator–prey abundances. Biocontrol Sci. Technol. 2013, 23, 381–395. [Google Scholar] [CrossRef]
- Wan, H.H.; Song, B.Z.; Tang, G.B.; Zhang, J.; Yao, Y.C. What are the effects of aromatic plants and meteorological factors on Pseudococcus comstocki and its predators in pear orchards? Agrofor. Syst. 2015, 89, 537–547. [Google Scholar] [CrossRef]
- Huang, D.; Sun, M.; Han, M.; Zhang, Z.; Miao, Y.; Zhang, J.; Yao, Y. Volatile organic compounds (VOCs) regulate the spatial distribution of Lepidoptera insects in an orchard ecosystem. Biol. Control 2020, 149, 104311. [Google Scholar] [CrossRef]
- Song, B.; Liang, Y.; Liu, S.; Zhang, L.; Tang, G.; Ma, T.; Yao, Y. Behavioral Responses of Aphis citricola (Hemiptera: Aphididae) and Its Natural Enemy Harmonia axyridis (Coleoptera: Coccinellidae) to Non-Host Plant Volatiles. Fla. Entomol. 2017, 100, 411–421. [Google Scholar] [CrossRef]
- Boetzl, F.A.; Krauss, J.; Heinze, J.; Hoffmann, H.; Juffa, J.; König, S.; Krimmer, E.; Prante, M.; Martin, E.A.; Holzschuh, A.; et al. A multitaxa assessment of the effectiveness of agri-environmental schemes for biodiversity management. Proc. Natl. Acad. Sci. USA 2021, 118, 10. [Google Scholar] [CrossRef]

| Ocimum basilicum | Tagetes patula | Lolium perenne | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 574 | n = 446 | n = 486 | |||||||||||
| Total | Asco. | Peri. | Pristo. | CM | Asco. | Peri. | Pristo. | CM | Asco. | Peri. | Pristo. | CM | |
| Small CM n = 470 | 292 | 88 | 32 | 3 | 10 | 56 | 20 | 1 | 3 | 56 | 14 | 4 | 5 |
| Large CM n = 1036 | 745 | 4 | 10 | 4 | 229 | 3 | 3 | 0 | 228 | 7 | 7 | 0 | 250 |
| Companion Plants | |||
|---|---|---|---|
| O. basilicum | T. patula | L. perenne | |
| CM larvae abundance | 2.49 (±0.20) b | 1.98 (±0.19) a | 2.13 (±0.18) ab |
| Proportion of small larvae (<30 mg) | 0.37 (±0.03) a | 0.29 (±0.03) ab | 0.26 (±0.03) b |
| Ratio parasitoid/codling moth (adults) | 0.36 (±0.03) a | 0.26 (±0.03) b | 0.26 (±0.03) b |
| A. quadridentata | 0.40 (±0.05) a | 0.26 (±0.05) a | 0.28 (±0.04) a |
| P. tristis | 0.18 (±0.03) a | 0.11 (±0.03) a | 0.09 (±0.02) a |
| Forficulidae | 18.48 (±0.53) b | 16.62 (±0.56) a | 18.0 (±0.54) ab |
| Araneae | 1.04 (±0.08) a | 0.95 (±0.79) a | 1.11 (±0.09) a |
| Other arthropods | 1.24 (±0.10) a | 1.07 (±0.09) a | 1.23 (±0.10) a |
| Proportion of damaged apple | 0.51 (±0.05) a | 0.47 (±0.06) a | 0.63 (±0.04) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laffon, L.; Bischoff, A.; Gautier, H.; Gilles, F.; Gomez, L.; Lescourret, F.; Franck, P. Conservation Biological Control of Codling Moth (Cydia pomonella): Effects of Two Aromatic Plants, Basil (Ocimum basilicum) and French Marigolds (Tagetes patula). Insects 2022, 13, 908. https://doi.org/10.3390/insects13100908
Laffon L, Bischoff A, Gautier H, Gilles F, Gomez L, Lescourret F, Franck P. Conservation Biological Control of Codling Moth (Cydia pomonella): Effects of Two Aromatic Plants, Basil (Ocimum basilicum) and French Marigolds (Tagetes patula). Insects. 2022; 13(10):908. https://doi.org/10.3390/insects13100908
Chicago/Turabian StyleLaffon, Ludivine, Armin Bischoff, Hélène Gautier, Florent Gilles, Laurent Gomez, Françoise Lescourret, and Pierre Franck. 2022. "Conservation Biological Control of Codling Moth (Cydia pomonella): Effects of Two Aromatic Plants, Basil (Ocimum basilicum) and French Marigolds (Tagetes patula)" Insects 13, no. 10: 908. https://doi.org/10.3390/insects13100908
APA StyleLaffon, L., Bischoff, A., Gautier, H., Gilles, F., Gomez, L., Lescourret, F., & Franck, P. (2022). Conservation Biological Control of Codling Moth (Cydia pomonella): Effects of Two Aromatic Plants, Basil (Ocimum basilicum) and French Marigolds (Tagetes patula). Insects, 13(10), 908. https://doi.org/10.3390/insects13100908








