Effects of Long-Term Exposure to Cadmium on Development, Reproduction and Antioxidant Enzymes of Aleuroglyphus ovatus (Acari: Acaridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mites Culture
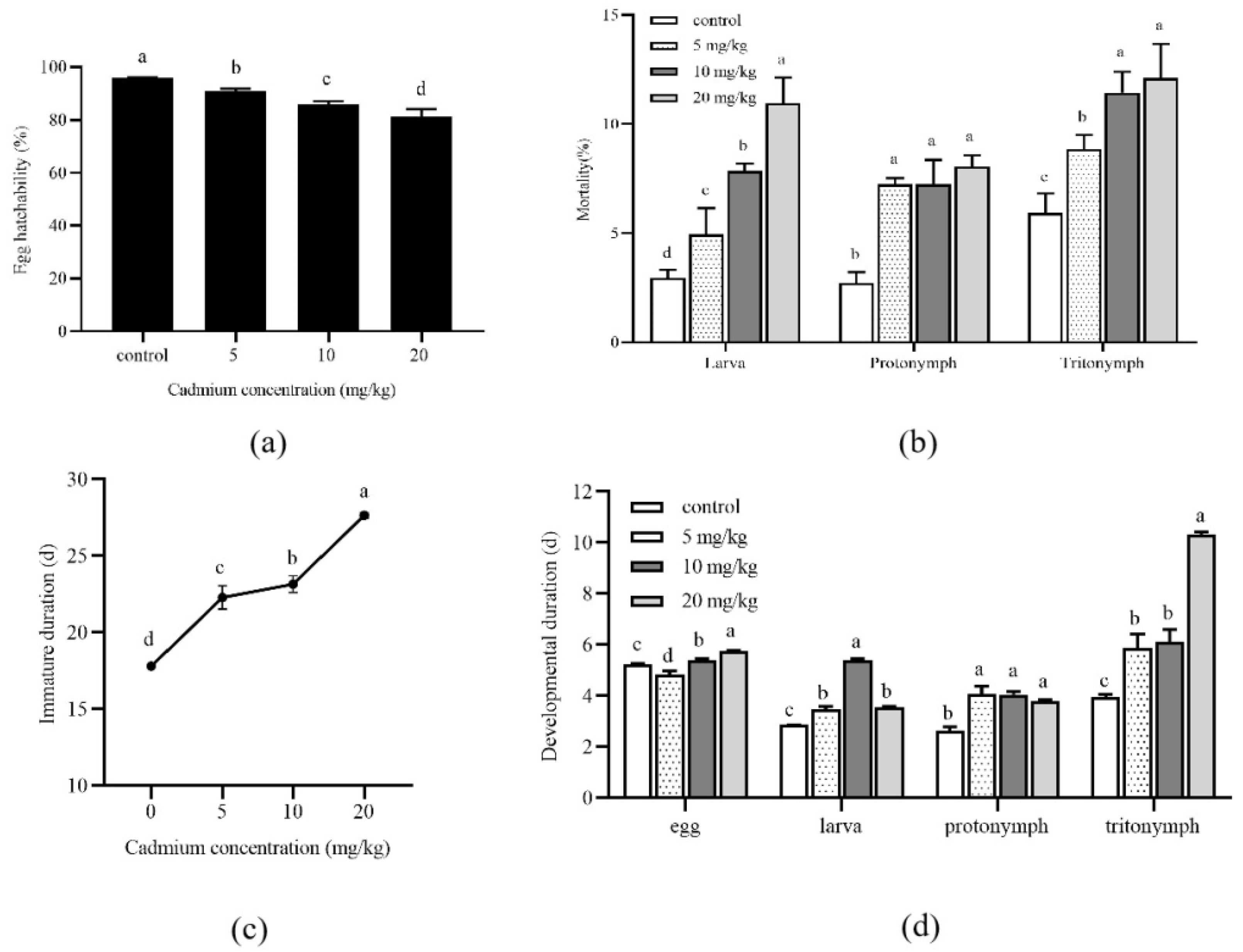
2.2. Developmental Durations
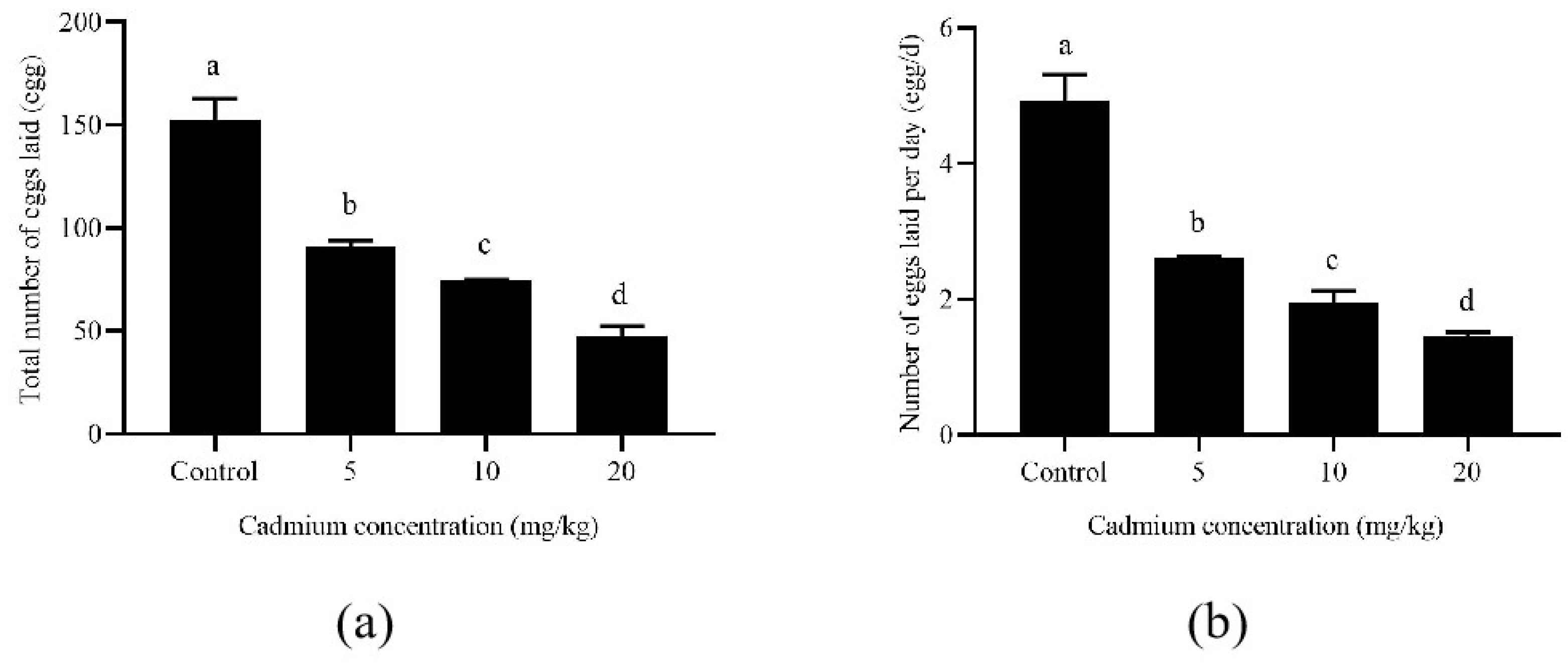
2.3. Reproductive Evaluation
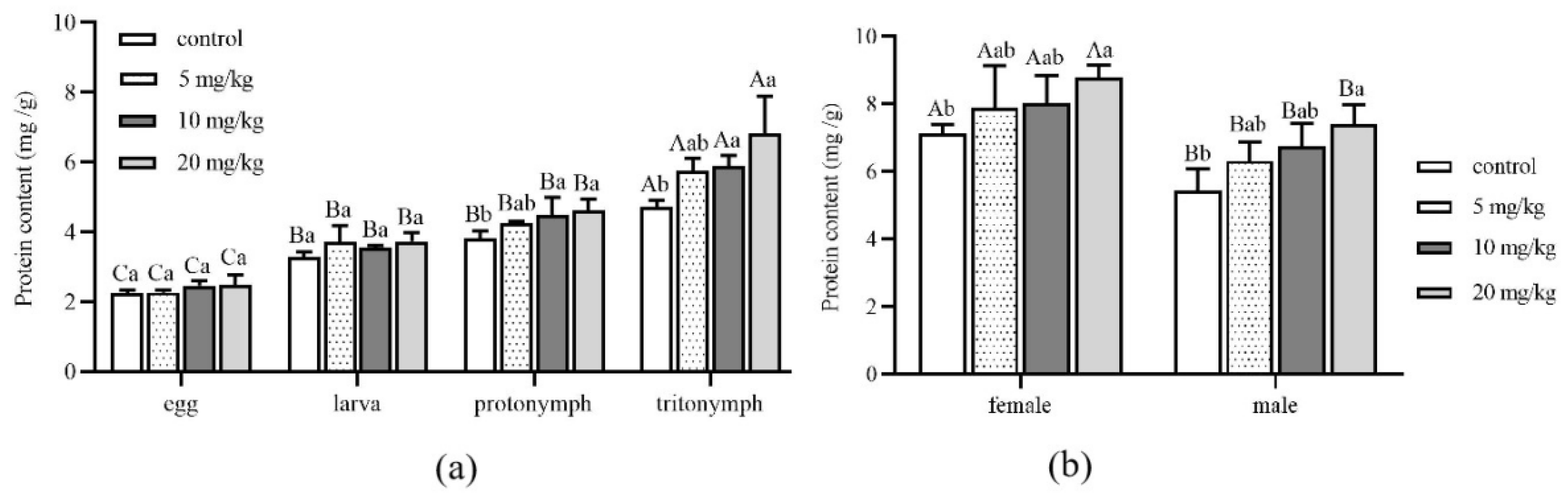
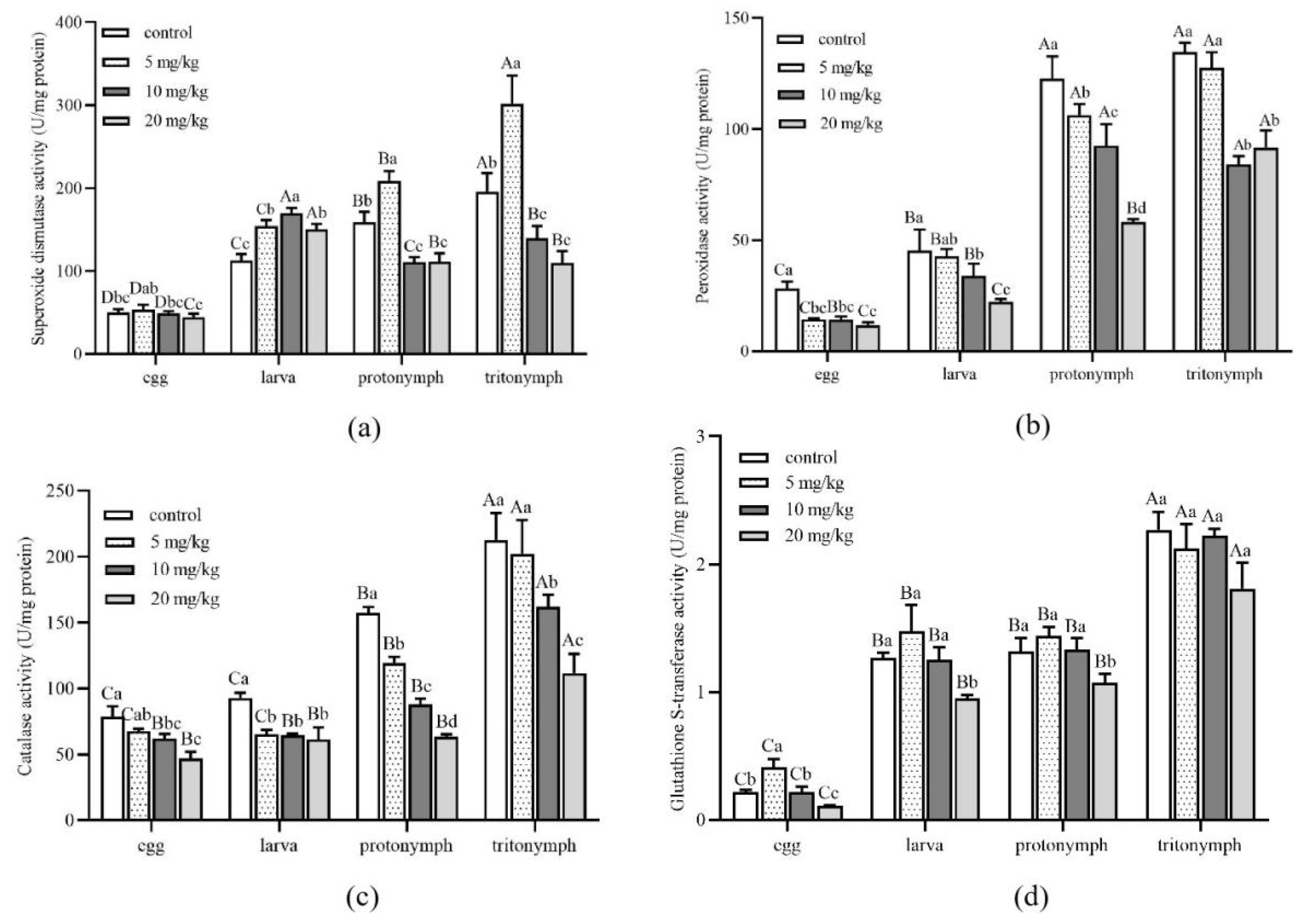
2.4. Determination of Protein Content and Enzyme Activity
2.5. Statistical Analysis
3. Results
3.1. Effects of Cadmium on the Development
3.2. Reproductive Evaluation
3.3. Effects of Cadmium on Protein Content
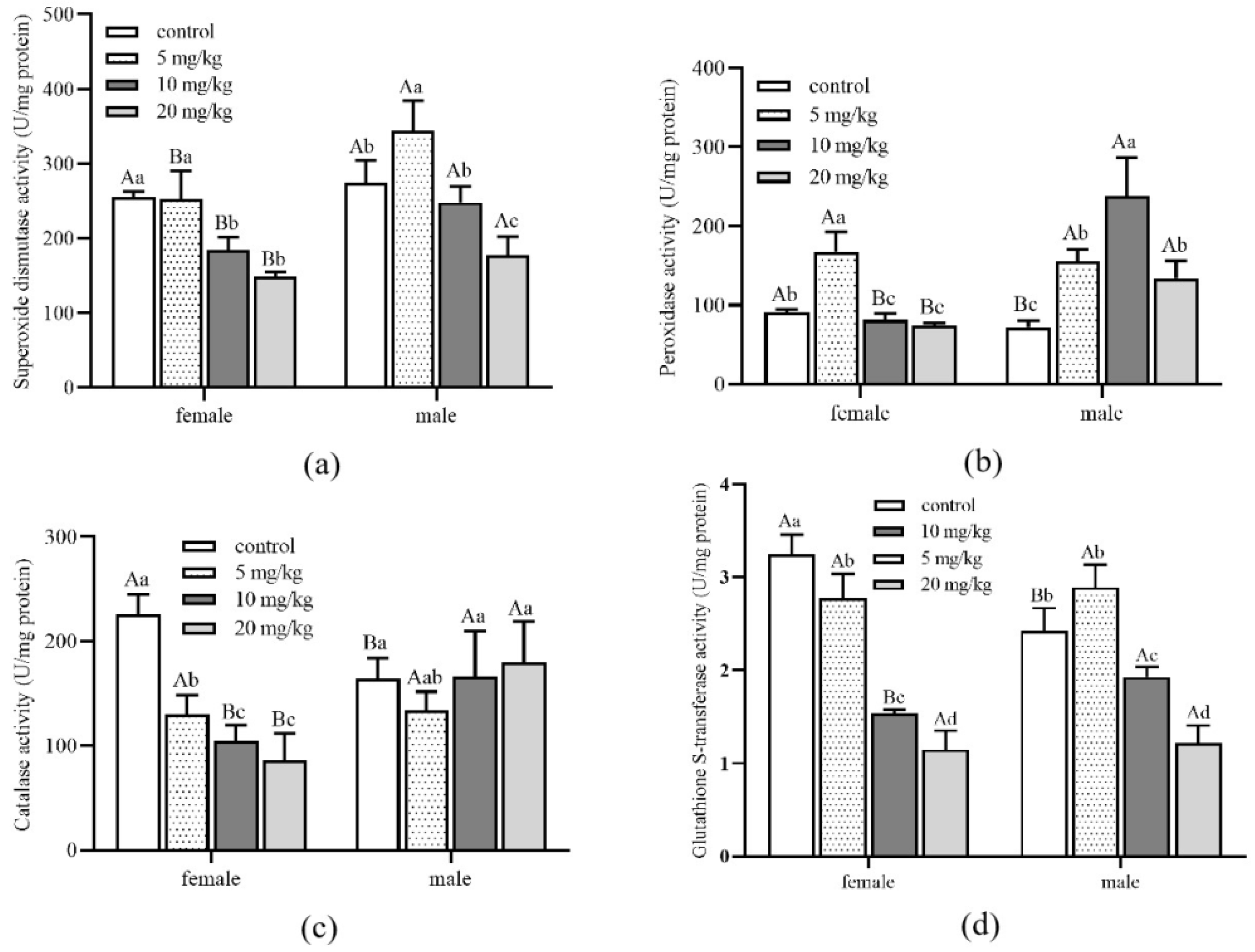
3.4. Effects of Cadmium on Activities of Detoxification Enzyme
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium: Mitigation strategies to reduce dietary exposure. J. Food Sci. 2020, 85, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-Ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. Int. 2016, 23, 17859–17879. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.; Rafiq, M.T.; Li, T.; Liu, D.; He, Z.; Stoffella, P.J.; Sun, K.; Xiaoe, Y. Uptake of cadmium by rice grown on contaminated soils and its bioavailability/toxicity in human cell lines (Caco-2/HL-7702). J. Agric. Food Chem. 2015, 63, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yang, Y.; Gu, J.; Zhao, J. Distribution and geochemical speciation of heavy metals in sediments from coastal area suffered rapid urbanization, a case study of Shantou Bay, China. Mar. Pollut. Bull. 2013, 68, 140–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Feng, Q.; Shao, M.; Yuan, F.; Liu, F. Polydatin attenuates cadmium-induced oxidative stress via stimulating SOD activity and regulating mitochondrial function in Musca domestica larvae. Chemosphere 2020, 248, 1–9. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.; Wang, W.; Lei, C.; Zhu, F. Influences of chromium and cadmium on the development of black soldier fly larvae. Environ. Sci. Pollut. Res. Int. 2017, 24, 8637–8644. [Google Scholar] [CrossRef]
- Yu, L.; Chen, X.; Wei, Y.; Ding, Y.; Wang, Q.; Wang, S.; Tang, B.; Wang, S. Effects of long-term cadmium exposure on trehalose metabolism, growth, and development of Aedes albopictus (Diptera: Culicidae). Ecotoxicol. Environ. Saf. 2020, 204, 1–9. [Google Scholar] [CrossRef]
- Cervera, A.; Maymó, A.C.; Sendra, M.; Martínez-Pardo, R.; Garcerá, M.D. Cadmium effects on development and reproduction of Oncopeltus fasciatus (Heteroptera: Lygaeidae). J. Insect Physiol. 2004, 50, 737–749. [Google Scholar] [CrossRef]
- Jeanrenaud, A.C.S.N.; Brooke, B.D.; Oliver, S.V. Second generation effects of larval metal pollutant exposure on reproduction, longevity and insecticide tolerance in the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Parasit. Vectors 2020, 13, 4–15. [Google Scholar] [CrossRef]
- Olivares-Castro, G.; Cáceres-Jensen, L.; Guerrero-Bosagna, C.; Villagra, C. Insect epigenetic mechanisms facing anthropogenic-derived contamination, an overview. Insects 2021, 12, 780. [Google Scholar] [CrossRef]
- Jiang, D.; Yan, S. Effects of Cd, Zn, or Pb Stress in Populus alba berolinensis on the antioxidant, detoxifying, and digestive enzymes of Lymantria dispar. Environ. Entomol. 2018, 47, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Vukašinović, E.L.; Čelić, T.V.; Kojić, D.; Franeta, F.; Milić, S.; Ninkov, J.; Blagojević, D.; Purać, J. The effect of long term exposure to cadmium on Ostrinia nubilalis growth, development, survival rate and oxidative status. Chemosphere 2020, 243, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, T.V.; Kojić, D.; Orčić, S.; Batinić, D.; Vukašinović, E.; Blagojević, D.P.; Purać, J. The impact of sublethal concentrations of Cu, Pb and Cd on honey bee redox status, superoxide dismutase and catalase in laboratory conditions. Chemosphere 2016, 164, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, Y.; Peng, X.; Bo, L.; Wang, Z.; Song, Q. Characterization of cadmium-responsive transcription factors in wolf spider Pardosa pseudoannulata. Chemosphere 2021, 268, 1–8. [Google Scholar] [CrossRef]
- Li, C.P.; Cui, Y.B.; Wang, J.; Yang, Q.G.; Tian, Y. Acaroid mite, intestinal and urinary acariasis. World. J. Gastroenterol. 2003, 9, 874–877. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.; Han, T.; Ding, Y.; Sun, J.; Wang, F.; Zhu, C. Heavy metal pollution in a soil-rice system in the Yangtze river region of China. Int. J. Environ. Res. Public Health 2015, 13, 63–79. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Liao, X.; Li, X.; Zheng, S.; Zhao, F. Phytoexclusion of heavy metals using low heavy metal accumulating cultivars: A green technology. J. Hazard Mater. 2021, 413, 1–15. [Google Scholar] [CrossRef]
- Román-Ochoa, Y.; Choque-Delgado, G.T.; Tejada, T.R.; Yucra, H.R.; Durand, A.E.; Hamaker, B.R. Heavy metal contamination and health risk assessment in grains and grain-based processed food in Arequipa region of Peru. Chemosphere 2021, 274, 63–79. [Google Scholar] [CrossRef]
- Merrington, G.; Winder, L.; Green, I. The bioavailability of cadmium and zinc from soils amended with sewage sludge to winter wheat and subsequently to the grain aphid Sitobion avenae. Sci. Total. Environ. 1997, 205, 245–254. [Google Scholar] [CrossRef]
- Warchałowska-Sliwa, E.; Niklińska, M.; Görlich, A.; Michailova, P.; Pyza, E. Heavy metal accumulation, heat shock protein expression and cytogenetic changes in Tetrix tenuicornis (L.) (Tetrigidae, Orthoptera) from polluted areas. Environ. Pollut. 2005, 133, 373–381. [Google Scholar] [CrossRef]
- Tylko, G.; Banach, Z.; Borowska, J.; Niklinska, M.; Pyza, E. Elemental changes in the brain, muscle, and gut cells of the housefly, Musca domestica, exposed to heavy metals. Microsc. Res. Tech. 2005, 66, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Hu, X.; Yang, P.; Sun, L.; Gu, W.; Zhang, M. The effects of cadmium on the development of Drosophila and its transgenerational inheritance effects. Toxicology 2021, 462, 1–10. [Google Scholar] [CrossRef]
- Hu, X.; Fu, W.; Yang, X.; Mu, Y.; Gu, W.; Zhang, M. Effects of cadmium on fecundity and defence ability of Drosophila melanogaster. Ecotoxicol. Environ. Saf. 2019, 171, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, W.; Guo, J.; Xiao, R.; Jiang, F.; Zheng, L.; Zhang, G. Effects of nickel exposure on testicular function, oxidative stress, and male reproductive dysfunction in Spodoptera litura Fabricius. Chemosphere 2016, 148, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Khatun, S.; Rajak, P.; Dutta, M.; Roy, S. Sodium fluoride adversely affects ovarian development and reproduction in Drosophila melanogaster. Chemosphere 2017, 186, 51–61. [Google Scholar] [CrossRef]
- Płachetka-Bożek, A.; Kafel, A.; Augustyniak, M. Reproduction and development of Spodoptera exigua from cadmium and control strains under differentiated cadmium stress. Ecotoxicol. Environ. Saf. 2018, 166, 138–145. [Google Scholar] [CrossRef]
- Iskender, E.; Tamer, K.; Mustafa, C.; Osman, D.; Yeter, C.H. Changes in antioxidative enzyme activity, glycogen, lipid, protein, and malondialdehyde content in cadmium-treated Galleria mellonella Larvae. Ann. Entomol. Soc. Am. 2013, 106, 371–377. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Kafel, A.; Kandziora-Ciupa, M.; Gospodarek, J.; Zawisza-Raszka, A. Accumulation of heavy metals and antioxidant responses in Vicia faba plants grown on monometallic contaminated soil. Environ. Sci. Pollut. Res. Int. 2013, 20, 1124–1134. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef]
- Coskun, M.; Kayis, T.; Yilmaz, M.; Dursun, O.; Emre, I. Copper and zinc impact on stress biomarkers and growth parameters in a model organism, Galleria mellonella larvae. Biometals 2021, 34, 1263–1273. [Google Scholar] [CrossRef]
- Tang, T.; Huang, D.W.; Zhou, C.Q.; Li, X.; Xie, Q.J.; Liu, F.S. Molecular cloning and expression patterns of copper/zinc superoxide dismutase and manganese superoxide dismutase in Musca domestica. Gene 2012, 505, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Lee, J.W.; Seo, J.S.; Shin, K.H.; Rhee, J.S.; Lee, J.S. Modulated expression and enzymatic activity of the monogonont rotifer Brachionus koreanus Cu/Zn- and Mn-superoxide dismutase (SOD) in response to environmental biocides. Chemosphere 2015, 120, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Shih, C.M.; Huang, C.J.; Lin, C.M.; Chou, C.M.; Tsai, M.L.; Liu, T.P.; Chiu, J.F.; Chen, C.T. Effects of cadmium on structure and enzymatic activity of Cu, Zn-SOD and oxidative status in neural cells. J. Cell Biochem. 2006, 98, 577–589. [Google Scholar] [CrossRef]
- Ahmad, S.; Pristos, C.A.; Bowen, S.M.; Heisler, C.R.; Blomquist, G.J.; Pardini, R.S. Subcellular distribution and activities of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase in the southern armyworm, Spodoptera eridania. Arch. Insect Biochem. Physiol. 1988, 7, 173–186. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Song, D.N.; Wu, H.H.; Yang, H.M.; Zhang, J.Z.; Li, L.J.; Ma, E.B.; Guo, Y.P. Effect of dietary cadmium on the activity of glutathione S-transferase and carboxylesterase in different developmental stages of the Oxya chinensis (Orthoptera: Acridoidea). Environ. Entomol. 2014, 43, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Wilczek, G.; Kramarz, P.; Babczyńska, A. Activity of carboxylesterase and glutathione S-transferase in different life-stages of carabid beetle (Poecilus cupreus) exposed to toxic metal concentrations. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 134, 501–512. [Google Scholar] [CrossRef]
| Duration (d) (Mean ± SD) | Cadmium Concentration (mg/kg) | |||
|---|---|---|---|---|
| 0 | 5 | 10 | 20 | |
| Pre-oviposition period | 2.45 ± 0.13 c | 4.52 ± 0.21 b | 5.15 ± 0.10 a | 5.62 ± 0.09 a |
| Oviposition period | 31.34 ± 1.94 bc | 34.62 ± 0.44 ac | 37.64 ± 1.48 a | 32.36 ± 0.79 bc |
| After the oviposition period | 10.90 ± 1.19 a | 6.95 ± 0.48 cd | 9.09 ± 0.44a b | 8.55 ± 0.05 bc |
| Lifespan of female adult | 44.69 ± 2.71 bc | 46.08 ± 0.55 ab | 50.76 ± 1.37 a | 46.01 ± 1.34 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xiong, W.; Yang, S.; Ai, H.; Zou, Z.; Xia, B. Effects of Long-Term Exposure to Cadmium on Development, Reproduction and Antioxidant Enzymes of Aleuroglyphus ovatus (Acari: Acaridae). Insects 2022, 13, 895. https://doi.org/10.3390/insects13100895
Zhang Y, Xiong W, Yang S, Ai H, Zou Z, Xia B. Effects of Long-Term Exposure to Cadmium on Development, Reproduction and Antioxidant Enzymes of Aleuroglyphus ovatus (Acari: Acaridae). Insects. 2022; 13(10):895. https://doi.org/10.3390/insects13100895
Chicago/Turabian StyleZhang, Yu, Wenhui Xiong, Shan Yang, Hui Ai, Zhiwen Zou, and Bin Xia. 2022. "Effects of Long-Term Exposure to Cadmium on Development, Reproduction and Antioxidant Enzymes of Aleuroglyphus ovatus (Acari: Acaridae)" Insects 13, no. 10: 895. https://doi.org/10.3390/insects13100895
APA StyleZhang, Y., Xiong, W., Yang, S., Ai, H., Zou, Z., & Xia, B. (2022). Effects of Long-Term Exposure to Cadmium on Development, Reproduction and Antioxidant Enzymes of Aleuroglyphus ovatus (Acari: Acaridae). Insects, 13(10), 895. https://doi.org/10.3390/insects13100895






