The Handsome Cross Grasshopper Oedaleus decorus (Germar, 1825) (Orthoptera: Acrididae) as a Neglected Pest in the South-Eastern Part of West Siberian Plain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Territory
2.2. Field Studies
- SE Aleksandrovskij settlement (Novosibirskaya Oblast (Novosibirsk Region), 53.67° N 78.25° E, northern steppe, in 2003 the local control model plot was moved about 100 m southwards, because the main part of the area was ploughed).
- SW Yarovoe town (Altai Krai (Altaj Region), 52.85° N 78.57° E, dry steppe (actually very old crested wheatgrass field)).
- E Ust-Volchikha settlement (Altai Krai (Altaj Region), 51.93° N 80.28° E, dry steppe).
2.3. Data Analysis
3. Results
3.1. Taxonomical Notes
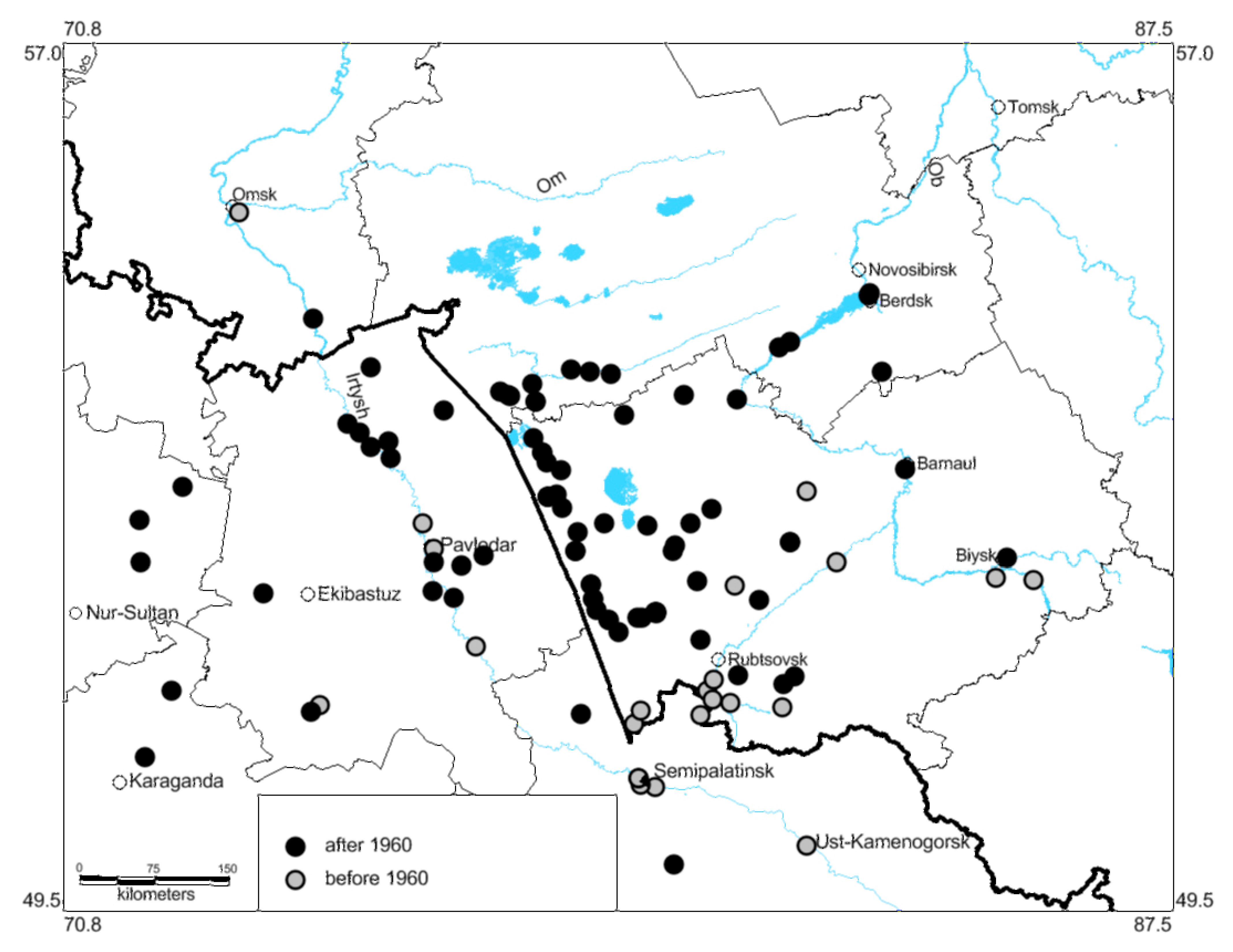
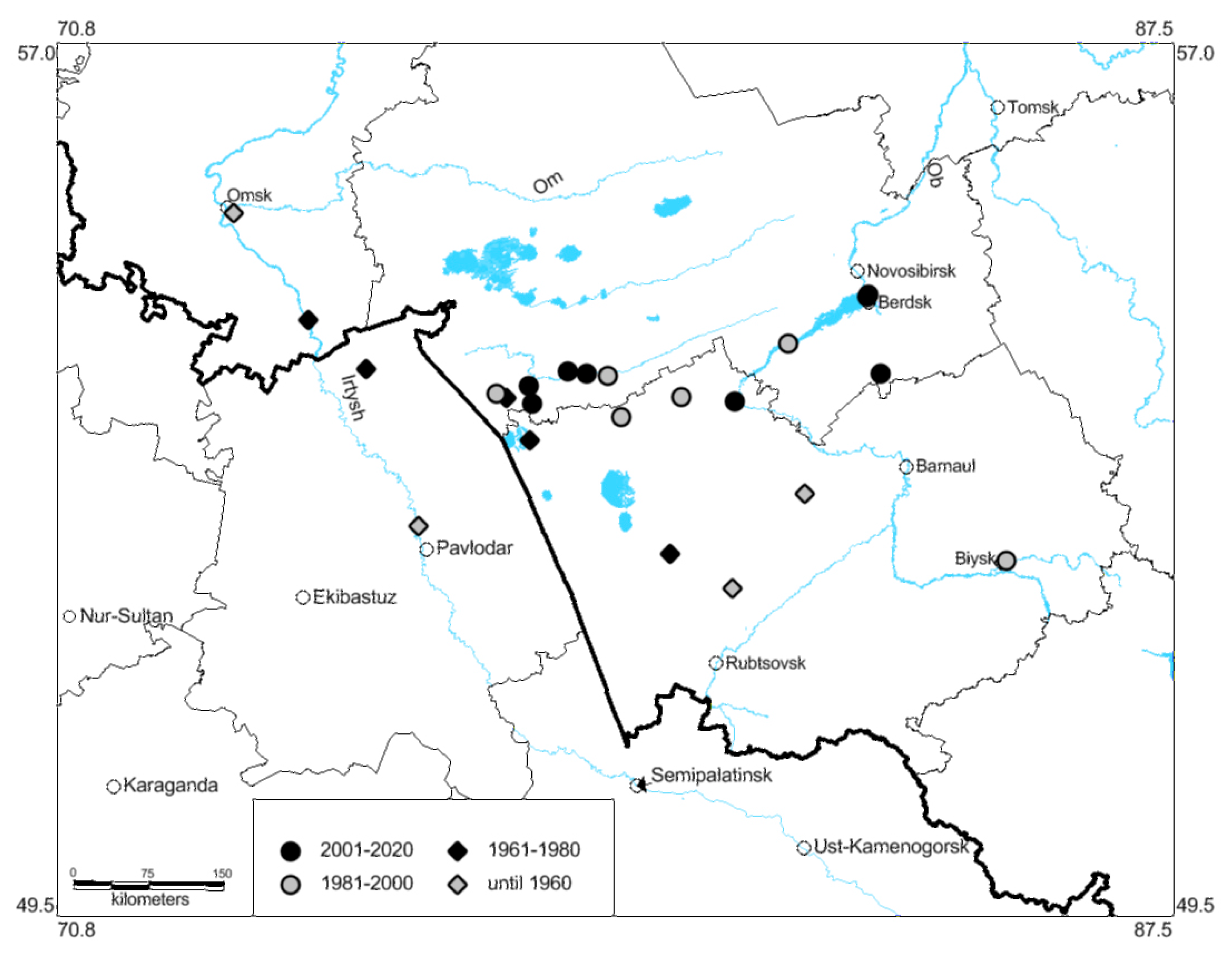
3.2. Shifts in Species Distribution
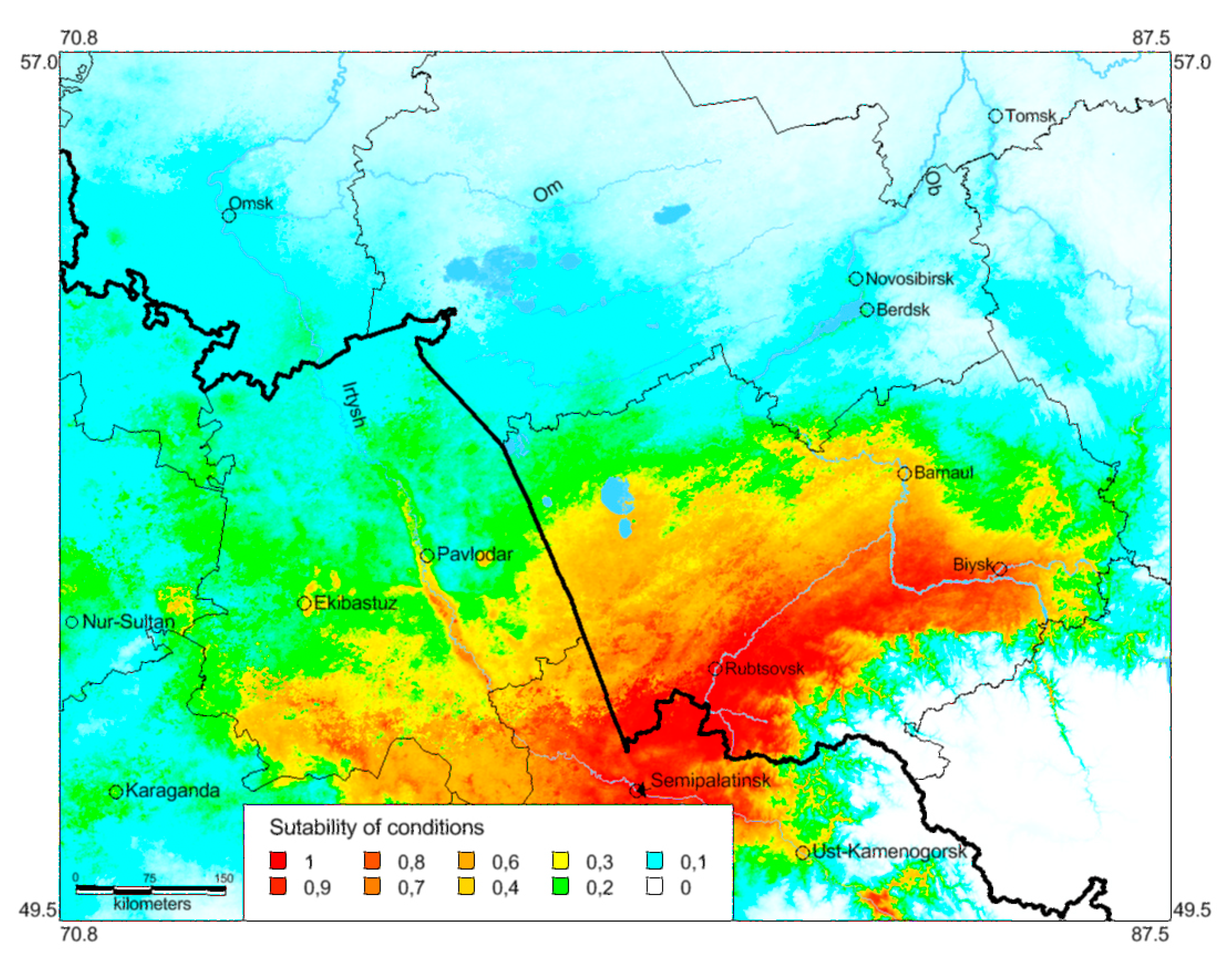
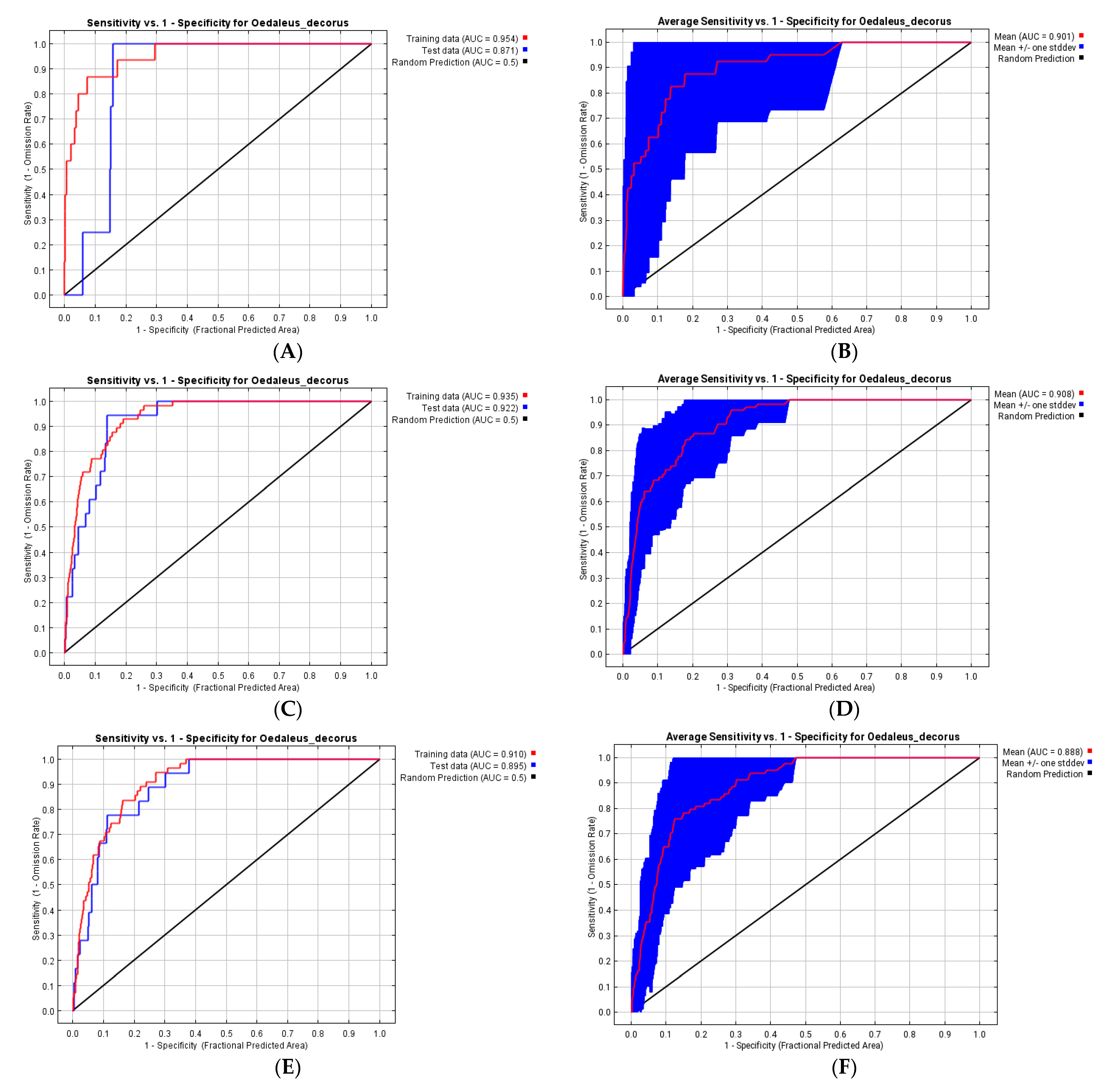
3.3. Ecological Models of the Species Distribution
3.4. Peculiarities of the Long-Term Dynamics of Species Populations
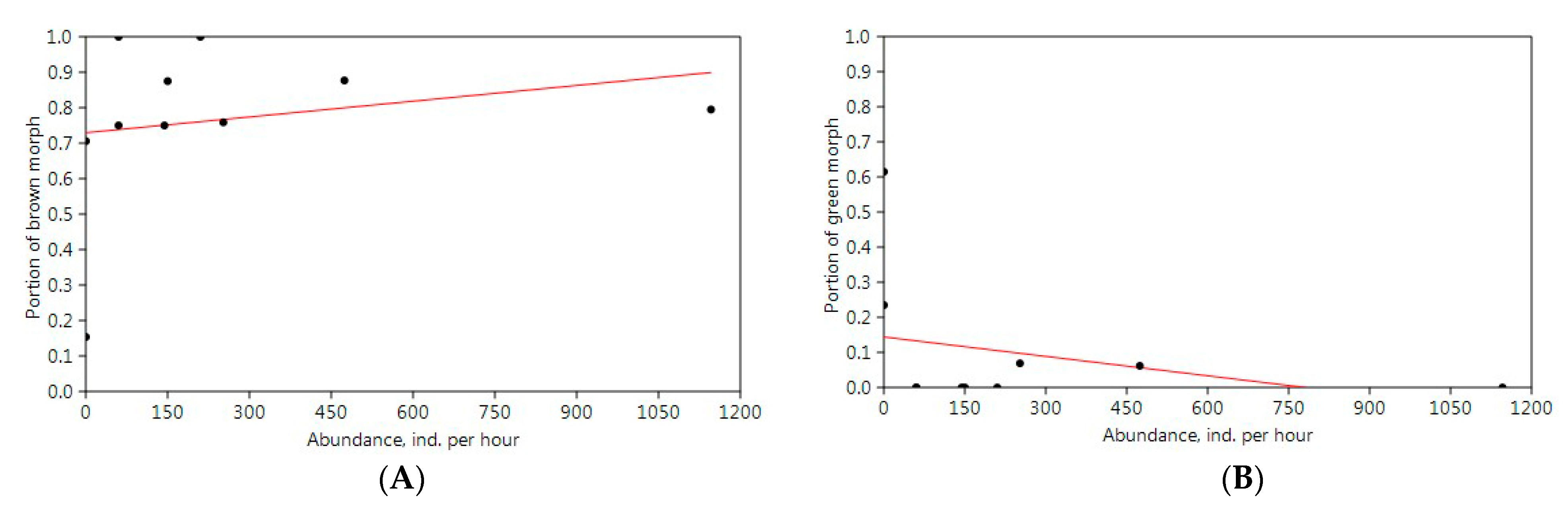
3.5. Frequency of the Color Morphs during the Species Outbreak
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popova, K.V.; Baturina, N.S.; Molodtsov, V.V.; Yefremova, O.V.; Zharkov, V.D.; Sergeev, M.G. The handsome cross grasshopper Oedaleus decorus (Germ.) (Orthoptera: Acrididae) as a newly emerging pest in the south-eastern part of West Siberian Plain. In Proceedings of the 1st International Electronic Conference on Entomology, online, 1–15 July 2021. [Google Scholar]
- Popova, K.V.; Molodtsov, V.V.; Efremova, O.V.; Sergeev, M.G. Grasshoppers in steppe areas of the south-eastern West Siberian Plain: Centennial transformations of biodiversity. IOP Conf. Ser. Earth Environ. Sci. 2021, 817, 012088. [Google Scholar] [CrossRef]
- Ritchie, J.M. A taxonomic revision of the genus Oedaleus Fieber (Orthoptera: Acrididae). Bull. Br. Mus. (Nat. Hist.). Entomol. 1981, 42, 83–183. [Google Scholar]
- Sergeev, M.G. Patterns of Orthoptera Distribution in North Asia; Nauka Publ.: Novosibirsk, Russia, 1986; p. 237. (In Russian) [Google Scholar]
- Storozhenko, S.Y. Locusts and Grasshoppers Pests of U.S.S.R.; Orthopterists’ Society: Ste-Anne-de-Bellevue, QC, Canada, 1991; p. 89. [Google Scholar]
- Childebaev, M.K.; Storozhenko, S.Y. An annotated list of brachycerous orthopterous insects (Orthoptera: Caelifera) occurring in Kazakhstan. Tethys Entomol. Res. 2001, 3, 5–48. [Google Scholar]
- Chogsomzhav, L. Acridoidea and Tettigonioidea of the Mongolian People’s Republic. Nasek. Mong. 1972, 1, 151–198. (In Russian) [Google Scholar]
- Mistshenko, L.L. Orthopteroid insects (Orthopteroidea) collected by the entomological expedition of the Zoological Institute of Academy Sciences of USSR in the Mongolian People’s Republic in 1967. Entomol. Obozr. 1968, 47, 482–498. (In Russian) [Google Scholar]
- Vorontsovsky, P.A. Data to knowledge of the Acridoidea fauna of Orenburg Region. Izv. Orenb. Stantsii Zastshity Rastenii 1928, 1, 5–25. (In Russian) [Google Scholar]
- Ivanov, E.N. About biology and ecology of Oedaleus decorus Germ. In Grasshoppers of Middle Asia; Nevsky, V.P., Ed.; SaOgiz: Tashkent, Uzbekistan, 1934; pp. 113–123. (In Russian) [Google Scholar]
- Pravdin, F.N. Ecological Geography of Insects of Middle Asia; Nauka Publ.: Moscow, Russia, 1978; p. 271. (In Russian) [Google Scholar]
- Gangwere, S.K.; Morales-Agacino, E. Food selection and feeding behaviour in Iberian Orthopteroidea. An. Inst. Nac. Investig. Agrar. Ser. Protección Veg. 1973, 3, 251–344. [Google Scholar]
- Mistshenko, L.L. Orthoptera (Saltatoria). In Insects and Mites—Pests of Agriculture Crops; Kryzhanovsky, O.L., Danzig, E.M., Eds.; Nauka Publ.: Leningrad, Russia, 1972; Volume 1, pp. 16–115. (In Russian) [Google Scholar]
- Stebaev, I.V.; Pshentsyna, L.B. Food selectivity of dominant grasshopper species in the steppes and the flood-plain meadows near the Irtysh River based on the botanical analysis of their feces. In Issues of Ecology: Species, Population, Community; Stebaev, I.V., Ed.; Novosibirsk State University: Novosibirsk, Russia, 1978; pp. 18–59. (In Russian) [Google Scholar]
- Pshentsyna, L.B. Trophic specialization of grasshoppers in a habitat and their influence on vegetation. In Issues of Ecology: Behavior and Ecology of Insects Associated with Agrobiogeocoenoses; Stebaev, I.V., Ed.; Novosibirsk State University: Novosibirsk, Russia, 1981; pp. 66–84. (In Russian) [Google Scholar]
- Kang, L.; Gan, Y.; Li, S. The structural adaptation on mandibles and food specificity in grasshoppers on Inner Mongolian grasslands. J. Orthoptera Res. 1999, 6, 257–269. [Google Scholar] [CrossRef]
- Zaim, A.; Petit, D.; Elghadraoui, L. Dietary diversification and variations in the number of labrum sensilla in grasshoppers: Which came first? J. Biosci. (Indian Acad. Sci.) 2013, 38, 339–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecoq, M.; Zhang, L. (Eds.) Encyclopedia of Pest Orthoptera of the World; China Agricultural University Press: Beijing, China, 2019; p. 310. [Google Scholar]
- Predtechensky, S.A.; Zhdanov, S.P.; Popova, A.A. Pest locusts in the USSR. Tr. Po Zastshite Rastenij. Ser. Entomol. 1935, 18, 1–168. (In Russian) [Google Scholar]
- Gupta, V.K. The Locust and Grasshopper Agricultural Manual; Centre for Overseas Pest Research: London, UK, 1982; p. 690. [Google Scholar]
- Latchininsky, A.V.; Sergeev, M.G.; Childebaev, M.K.; Chernijakhovskij, M.E.; Lockwood, J.A.; Kambulin, V.E.; Gapparov, F.A. The Grasshoppers of Kazakhstan, Middle Asia and Adjacent Territories; Association for Applied Acridology International & University of Wyoming: Laramie, WY, USA, 2002; p. vi + 387. (In Russian) [Google Scholar]
- Chen, Y. The Main Acridoids and Ecological Management of Locust Plagues in China; Science Press: Beijing, China, 2005; p. viii + 361. (In Chinese) [Google Scholar]
- Le Gall, M.; Overson, R.; Cease, A. A global review on locusts (Orthoptera: Acrididae) and their interactions with livestock grazing practices. Front. Ecol. Evol. 2019, 7, 263. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, Y.; Wang, G.; Lowry, A.; Huang, W.; Dong, Y.; Shang, S.; Luke, B. The effects of vegetation type on Oedaleus decorus asiaticus (Orthoptera: Acrididae) oviposition and hatching success. Environ. Entomol. 2021, 50, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Berezhkov, R.P. The Grasshoppers of West Siberia; Tomsk University Publ.: Tomsk, Russia, 1956; p. 174. (In Russian) [Google Scholar]
- Ul’anov, L.N. Development of Siberian virgin lands relative to evolution of the region. In Economic Development of Siberia and Increasing of Its Population (18th–20th Centuries); Okladnikov, A.P., Ed.; Novosibirsk State University: Novosibirsk, Russia, 1979; pp. 59–69. (In Russian) [Google Scholar]
- Smelansky, I.E.; Tishkov, A.A. The steppe biome in Russia: Ecosystem services, conservation status, and actual challenges. In Eurasian Steppes. Ecological Problems and Livelihoods in a Changing World; Plant and Vegetation; Werger, M.J.A., van Staalduinen, M.A., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2012; Volume 6, pp. 45–101. [Google Scholar] [CrossRef]
- Sergeev, M.G. Distribution patterns of grasshoppers and their kin over the Eurasian Steppes. Insects 2021, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Isachenko, A.G. Landscapes of the USSR; Leningrad University Publ.: Leningrad, Russia, 1985; p. 320. (In Russian) [Google Scholar]
- Gause, G.F. Studies on the ecology of the Orthoptera. Ecology 1930, 11, 307–325. [Google Scholar] [CrossRef]
- Sergeev, M.G.; Denisova, O.V.; Vanjkova, I.A. How do spatial population structures affect acridid management? In Grasshoppers and Grassland Health: Managing Grasshopper Outbreaks without Risking Environmental Disaster; Lockwood, J.A., Latchininsky, A.V., Sergeev, M.G., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 71–87. [Google Scholar] [CrossRef]
- Sergeev, M.G.; Van’kova, I.A. Dynamics of the Italian locust Calliptamus italicus L. population in the southeast of the West Siberian Plain. Contemp. Probl. Ecol. 2008, 1, 204–209. [Google Scholar] [CrossRef]
- Sergeev, M.G. Ups and downs of the Italian locust (Calliptamus italicus L.) populations in the Siberian steppes: On the horns of dilemmas. Agronomy 2021, 11, 746. [Google Scholar] [CrossRef]
- Zubowsky, N. Zur Acridiodea-Fauna de asiatischen Russlands. Annuaire du Musée Zoologique de l’Académie Impériale des Sciences de St.-Pétersbourg 1898, 1, 68–110. [Google Scholar]
- Tarbinskij, S.P. Materials concerning the orthopterous fauna of the Province of Altai. Rev. Russ. Entom. 1925, 19, 176–195. (In Russian) [Google Scholar]
- Wnukowskij, W. Zur Fauna der Orthopteren and Dermapteren des Besirks Kamenj (südwestrliches Sibirien, früheres Gouvernement Tomsk). Mitt. Münch. Ent. Ges. 1926, 5, 87–92. [Google Scholar]
- Bey-Bienko, G.Y. A review of Dermaptera and Orthoptera fauna of Omsk Region. Tr. Po Zastshite Rasteniy Seriya Entomol. 1930, 1, 161–177. (In Russian) [Google Scholar]
- Bey-Bienko, G.Y. Materials concerning the Orthoptera of the District of Semipalatinsk. News West-Sib. Geogr. Soc. 1930, 7, 189–214. (In Russian) [Google Scholar]
- Bey-Bienko, G.Y. Materials concerning the fauna of Orthoptera of Altaj and adjacent steppes. Proc. Sib. Agric. Acad. 1925, 5, 37–56. (In Russian) [Google Scholar]
- Lavrov, S.D. Orthoptera (Saltatoria) of the vicinities of Siberian Agricultural Academy. Proc. Sib. Agric. Acad. 1924, 3, 85–88. (In Russian) [Google Scholar]
- Bey-Bienko, G.Y. The zonal and ecological distribution of Acrididae in West Siberian and Zaisan Plains. Tr. Po Zastshite Rasteniy Seriya Entomol. 1930, 1, 51–90. (In Russian) [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, B. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent. Lessons Conserv. 2017, 3, 108–135. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent (accessed on 17 October 2021).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- WorldClim. Available online: https://worldclim.org (accessed on 19 September 2021).
- Seferian, R. CNRM-CERFACS CNRM-ESM2-1 Model Output Prepared for CMIP6 CMIP Amip. Version 20211010. Earth System Grid Federation. 2018. Available online: https://doi.org/10.22033/ESGF/CMIP6.3924 (accessed on 10 October 2021).
- Séférian, R.; Nabat, P.; Michou, M.; Saint-Martin, D.; Voldoire, A.; Colin, J.; Decharme, B.; Delire, C.; Berthet, S.; Chevallier, M.; et al. Evaluation of CNRM Earth-System model, CNRM-ESM2-1: Role of Earth system processes in present-day and future climate. J. Adv. Modeling Earth Syst. 2019, 11, 4182–4227. [Google Scholar] [CrossRef] [Green Version]
- Tatebe, H.; Watanabe, M. MIROC MIROC6 Model Output Prepared for CMIP6 CMIP Historical. Version 20211010. Earth System Grid Federation. 2018. Available online: https://doi.org/10.22033/ESGF/CMIP6.5603 (accessed on 10 October 2021).
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Abe, M.; Saito, F.; et al. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef] [Green Version]
- Meinshausen, M.; Nicholls, Z.R.J.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N.; et al. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernándex, I.C.; Baca-González, V. MaxEnts’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef]
- Cease, A.J.; Hao, S.; Kang, L.; Elser, J.J.; Harrison, J.F. Are color or high rearing density related to migratory polyphenism in the band-winged grasshopper, Oedaleus asiaticus? J. Insect Physiol. 2010, 56, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Bey-Bienko, G.Y. New and little-known Orthoptera found in the USSR. Zap. Leningr. Selskokhozjastvennogo Inst. 1941, 4, 147–159. (In Russian) [Google Scholar]
- Ma, Y.; Li, H.; Kang, L. The Grassland Insects of Inner Mongolia; Tianze Eldonejo: Beijing, China, 1991; p. xiv + 467. (In Chinese) [Google Scholar]
- Zheng, Z.; Xia, K. (Eds.) Fauna Sinica. Insecta. Vol. 10. Orthoptera. Acridoidea. Oedipodidae and Arcypteridae; Science Press: Beijing, China, 1998; p. xv + 616. (In Chinese) [Google Scholar]
- Sergeev, M.G.; Storozhenko, S.Y.; Benediktov, A.A. An annotated check-list of Orthoptera of Tuva and adjacent regions. Part 3. Suboder Caelifera (Acrididae: Gomphocerinae: Gomphocerini; Locustinae). Far East. Entomol. 2020, 402, 1–36. [Google Scholar] [CrossRef]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. 2021. Available online: http://Orthoptera.SpeciesFile.org (accessed on 5 June 2021).
- Fries, M.; Chapco, W.; Contreras, D. A molecular phylogenetic analysis of the Oedipodinae and their intercontinental relationships. J. Orthoptera Res. 2007, 16, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Kindler, E.; Arlettaz, R.; Heckel, G. Deep phylogeographic divergence and cytonuclear discordance in the grasshopper Oedaleus decorus. Mol. Phylogenet. Evol. 2012, 65, 695–704. [Google Scholar] [CrossRef]
- Schmid, S.; Neuenschwander, S.; Pitteloud, C.; Heckel, G.; Pajkovic, M.; Arlettaz, R.; Alvarez, N. Spatial and temporal genetic dynamics of the grasshopper Oedaleus decorus revealed by museum genomics. Ecol. Evol. 2018, 8, 1480–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergeev, M.G. New data about Orthoptera of the Novosibirsk vicinities. Euroasian Entomol. J. 2004, 3, 173–174. (In Russian) [Google Scholar]
- Zubowsky, N. Beitrag zur Kenntnis der sibirischen Acridiodeen. Horae Soc. Entomol. Ross. 1900, 34, 1–23. [Google Scholar]
- Fan, X.; Duan, Q.; Shen, C.; Wu, Y.; Xing, C. Global surface air temperatures in CMIP6: Historical performance and future changes. Environ. Res. Lett. 2020, 15, 104056. [Google Scholar] [CrossRef]
- Wilson, J.B. Guilds, functional types and ecological groups. Oikos 1999, 86, 507–522. [Google Scholar] [CrossRef]
- Sobolev, N.N. Some features of the cryptic behavior as illustrated by different color forms of Acrida oxycephala and Oedaleus decorus. Zool. Zhurnal 1990, 69, 149–151. (In Russian) [Google Scholar]
- Preuss, S.; Cassel-Lundhagen, A.; Berggren, Å. Modelling the distribution of the invasive Roesel’s bushcricket (Metrioptera roeselii) in a fragmented landscape. NeoBiota 2011, 11, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Poniatowski, D.; Beckmann, C.; Löffler, F.; Münsch, T.; Helbing, F.; Samways, M.J.; Fartmann, T. Relative impacts of land-use and climate change on grasshopper range shifts have changed over time. Glob. Ecol. Biogeogr. 2020, 29, 2190–2202. [Google Scholar] [CrossRef]
- Dey, L.-S.; Husemann, M.; Hochkirch, A.; Simões, M.V.P. Species distribution modelling sheds light on the widespread distribution of Sphingonotus (Sphingonotus) rubescens (Orthoptera: Acrididae: Oedipodinae). Biol. J. Linn. Soc. 2021, 132, 912–924. [Google Scholar] [CrossRef]
- Olfert, O.; Weiss, R.M.; Kriticos, D. Application of general circulation models to assess the potential impact of climate change on potential distribution and relative abundance of Melanoplus sanguinipes (Fabricius) (Orthoptera: Acrididae) in North America. Psyche 2011, 2011, 980372. [Google Scholar] [CrossRef] [Green Version]
- Kimathi, E.; Tonnang, H.E.Z.; Subramanian, S.; Cressman, K.; Abdel-Rahman, E.-M.; Tesfayohannes, M.; Niassy, S.; Torto, B.; Dubois, T.; Tanga, C.M.; et al. Prediction of breeding regions for the desert locust Schistocerca gregaria in East Africa. Sci. Rep. 2020, 10, 11937. [Google Scholar] [CrossRef]
- Saha, A.; Rahman, S.; Alam, S. Modeling current and future potential distributions of desert locust Schistocerca gregaria (Forskål) under climate change scenarios using MaxEnt. J. Asia-Pac. Biodivers. 2021, 14, 399–409. [Google Scholar] [CrossRef]
- Uvarov, B.P. Grasshoppers of the European Part of the USSR and West Siberia; Novaya Derevnja: Moscow, Russia, 1925; p. 120. (In Russian) [Google Scholar]
- Bey-Bienko, G.Y.; Mistshenko, L.L. Locusts and Grasshoppers of the USSR and Adjacent Countries; USSR Academy of Science Publ.: Moscow/Leningrad, Russia, 1951; Volumes 1–2, p. 667. (In Russian) [Google Scholar]
- Kuřavová, K. The grasshopper Oedaleus decorus in the Czech Republic (Orthoptera: Acrididae). Klapalekiana 2015, 51, 55–60. [Google Scholar]
- Mikhailenko, A.P.; Bolshakov, L.V. New findings of Oedaleus decorus (Germar, 1817) (Orthoptera: Acrididae) in the forest-steppe of central European Russia. Eversmannia 2014, 39, 45. (In Russian) [Google Scholar]
- Kopysov, V.A. Orthoptera. In Fauna of the Kirov Oblast; Shernin, A.I., Ed.; Kirov State Teacher Institute: Kirov, Russia, 1974; Volume 2, pp. 31–47. (In Russian) [Google Scholar]
- Antsiferov, A.L.; Efimova, A.A.; Krinitsyn, I.G.; Maltseva, O.V.; Sitnikov, K.S. New data about insect fauna of forest meadows of the Manurov division of the Kologrivski Forest Reserve with an analysis of its general composition. Vestn. Kostroma State Univ. 2014, 20, 66–70. (In Russian) [Google Scholar]
- Karmazina, I.O.; Shulaev, N.V. Orthopterous insects (Orthoptera) from the central part of the Volga–Kama Region: Faunistic barriers and zoogeographical zoning. Proc. Kazan Univ. Nat. Sci. 2014, 156, 110–126. (In Russian) [Google Scholar]
- Sergeev, M.G. Metapopulations of locusts and grasshoppers: Spatial structures, their dynamics and early warning systems. In New Strategies in Locust Control; Krall, S., Peveling, R., Ba Diallo, D., Eds.; Birkhauser: Basel, Switzerland, 1997; pp. 75–80. [Google Scholar] [CrossRef]
- Qin, X.; Wu, H.; Huang, X.; Lock, T.L.; Kallenbach, R.L.; Ma, J.; Ali, M.P.; Tu, X.; Cao, G.; Wang, G.; et al. Plant composition changes in a small-scale community have a large effect on the performance of an economically important grassland pest. BMC Ecol. 2019, 19, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cease, A.J.; Harrison, J.F.; Hao, S.; Niren, D.C.; Zhang, G.; Kang, L.; Elser, J.J. Nutritional imbalance suppresses migratory phenotypes of the Mongolian locust (Oedaleus asiaticus). R. Soc. Open Sci. 2017, 4, 161039. [Google Scholar] [CrossRef] [PubMed] [Green Version]


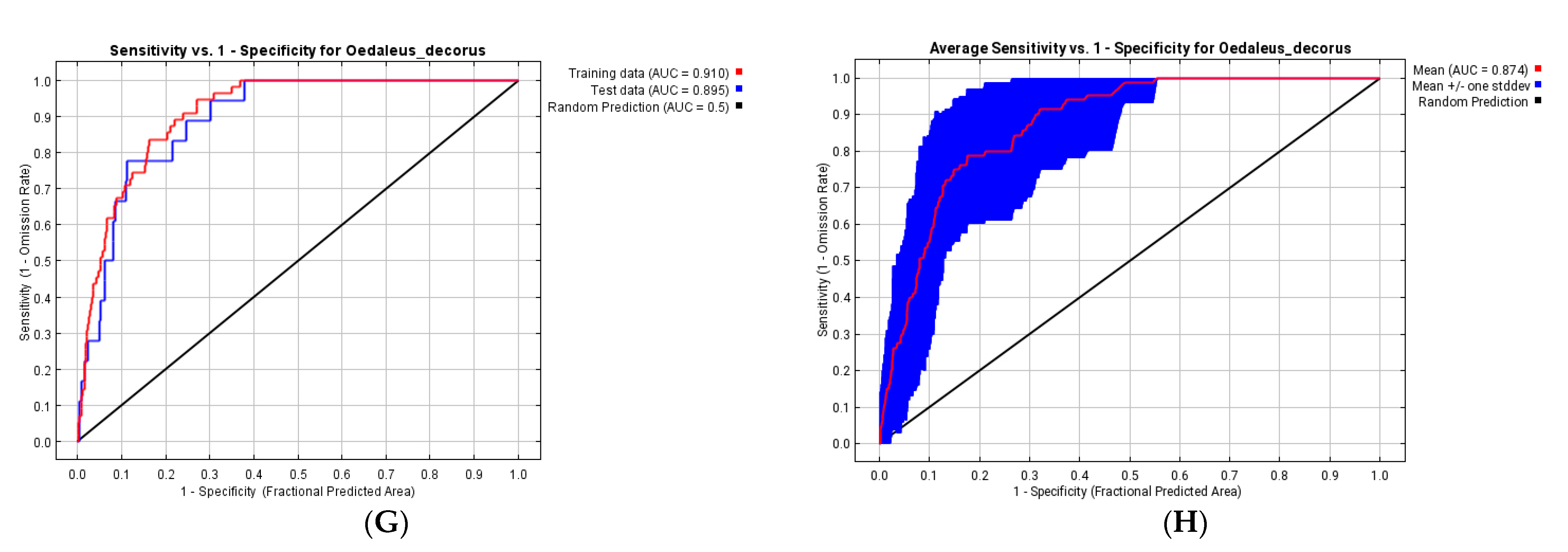
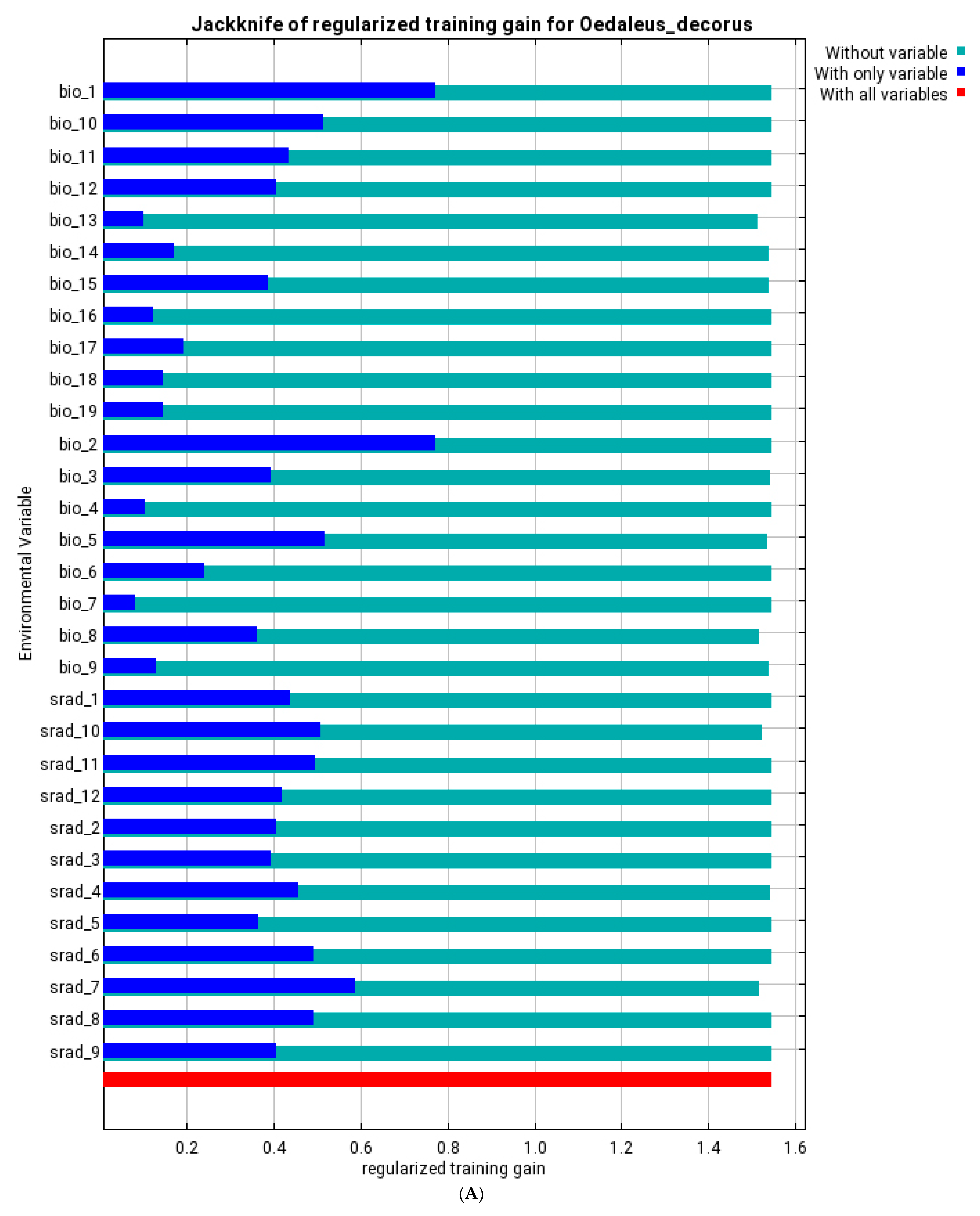
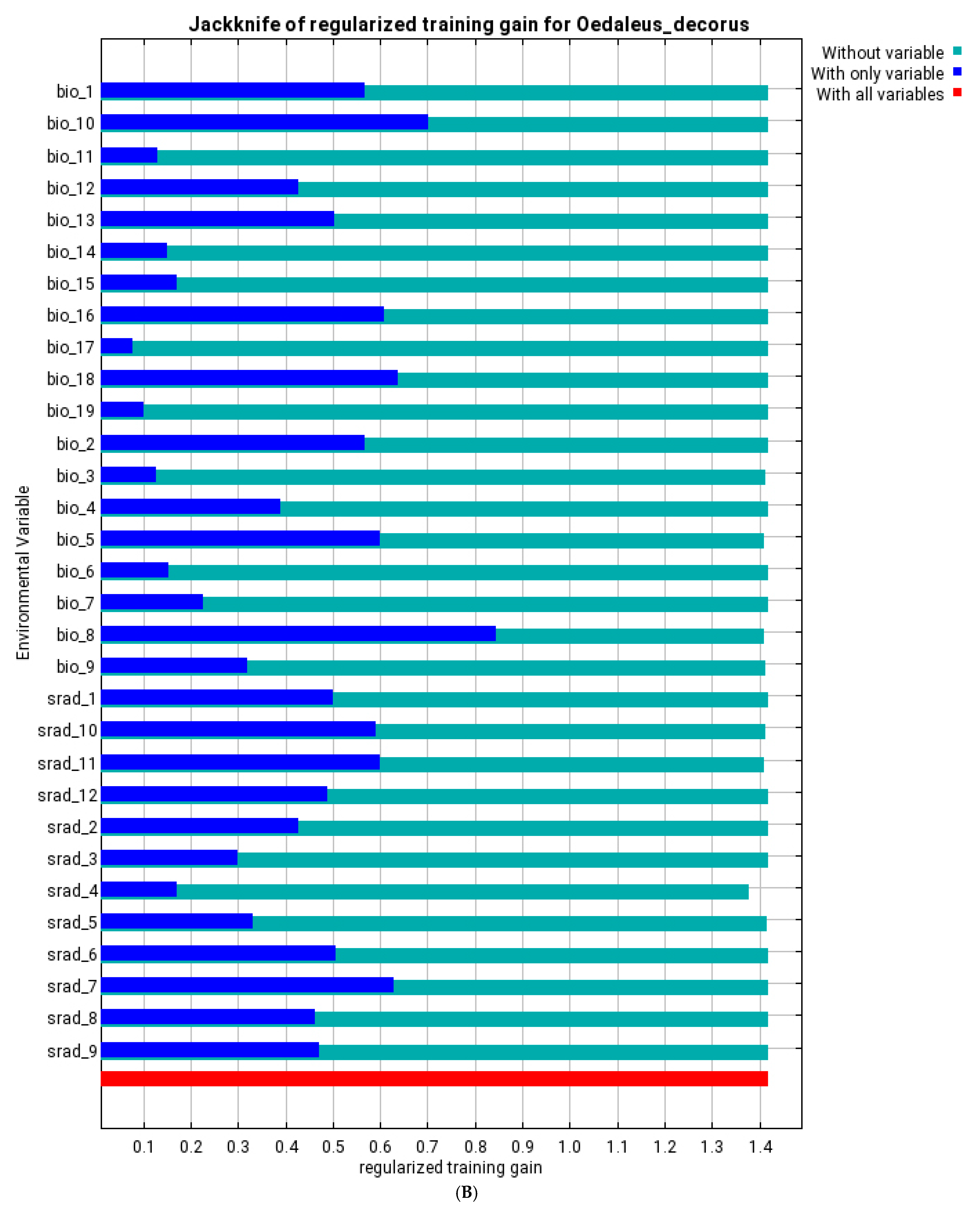
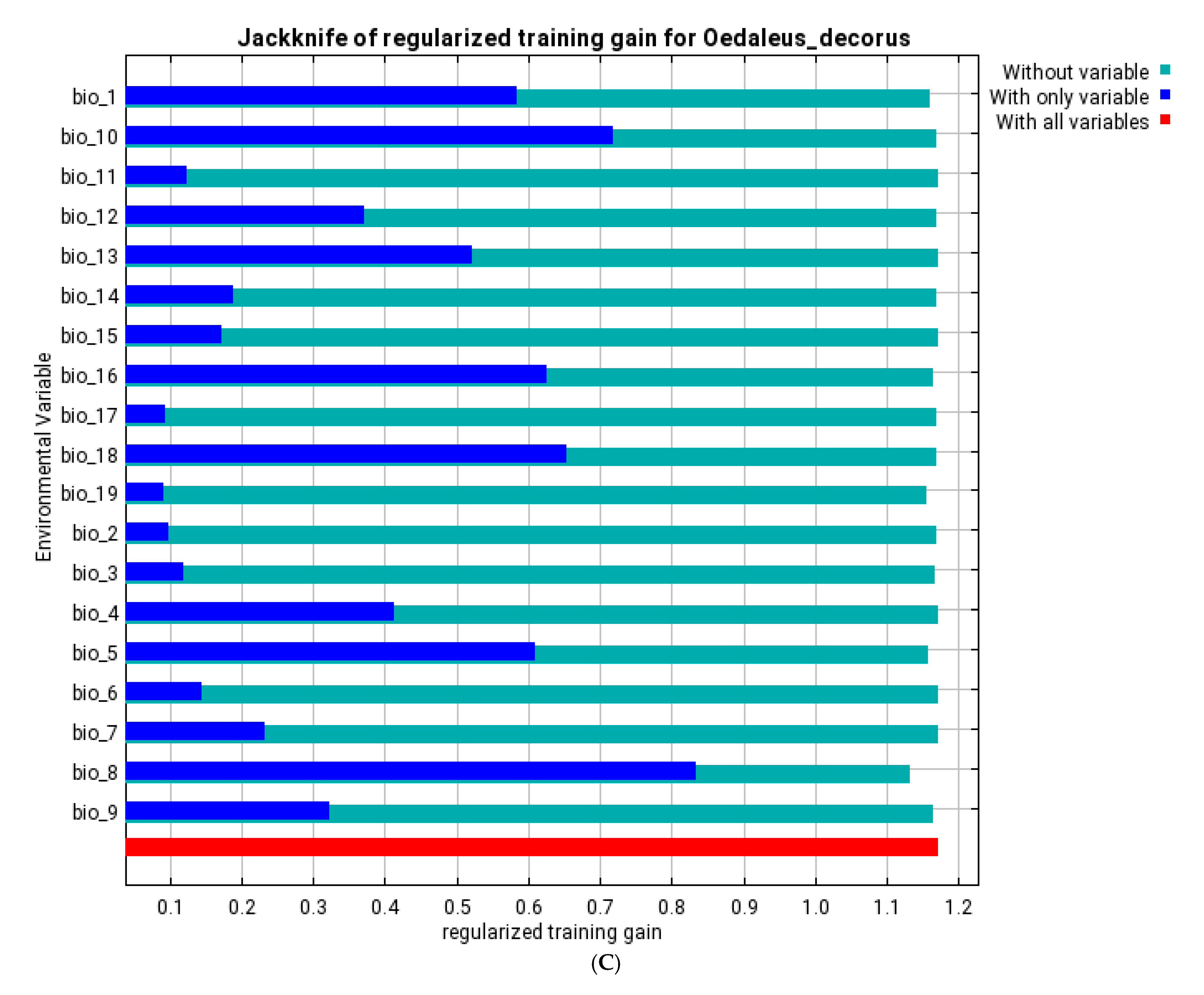

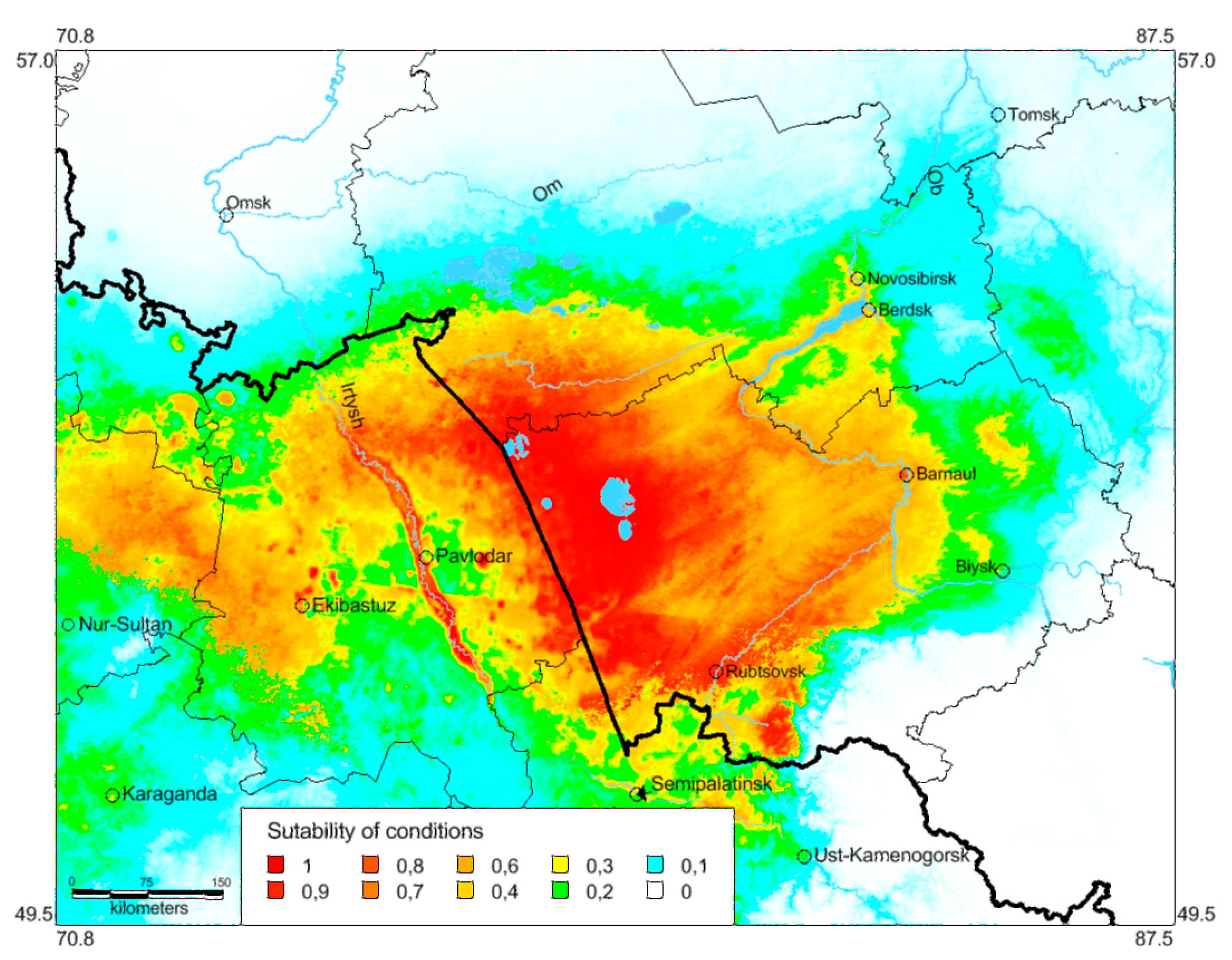
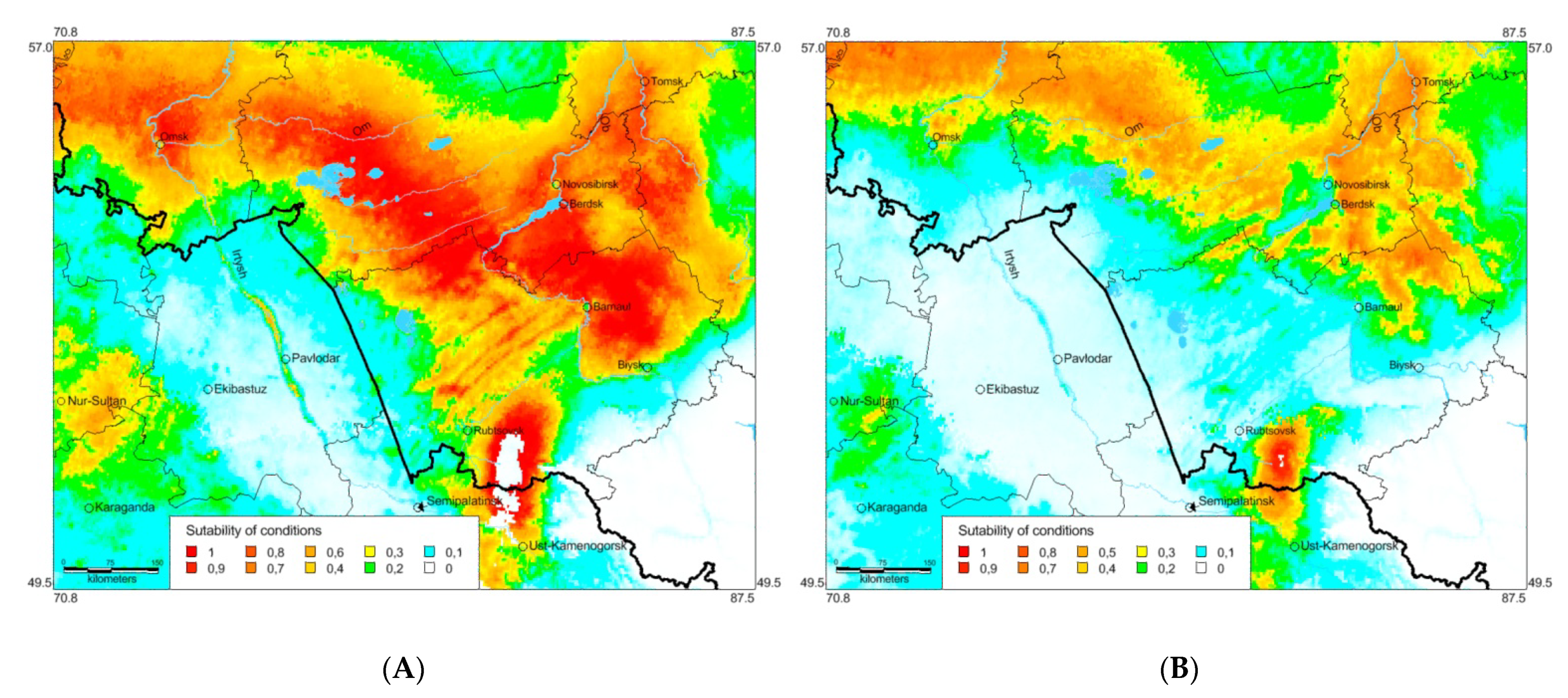

| Variable | Variable Explanation | Distribution Until 1960 | Distribution 1961–2021 | CNRM-ESM2-1 | MIROC6 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Percent Contribution | Permutation Importance | Percent Contribution | Permutation Importance | Percent Contribution | Permutation Importance | Percent Contribution | Permutation Importance | ||
| bio_1 | annual mean temperature | 10.9 | 12.4 | 0.4 | 1.3 | 5.1 | 17.1 | 3.2 | 18.4 |
| bio_2 | mean diurnal range (mean of monthly (max temp—min temp)) | 2.7 | 25.7 | 0.1 | 0.9 | 0.3 | 0.5 | 0.2 | 1.2 |
| bio_3 | isothermality (bio2/bio7) (×100) | 3.5 | 1.5 | 1.8 | 1.1 | 0.2 | 0.1 | 0.6 | 0.2 |
| bio_4 | temperature seasonality (standard deviation ×100) | 0.1 | 0 | 0.2 | 0 | 0.1 | 0 | 0.1 | 0.1 |
| bio_5 | max temperature of warmest month | 1.4 | 2.1 | 5.5 | 4.5 | 7.6 | 11 | 9.9 | 13.9 |
| bio_6 | min temperature of coldest month | 0 | 1.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| bio_7 | temperature annual range (bio5-bio6) | 0.2 | 1.4 | 0.1 | 1 | 0.1 | 0.1 | 0.1 | 0.1 |
| bio_8 | mean temperature of wettest quarter | 1 | 0.3 | 31.3 | 17.9 | 57.9 | 26.9 | 60.4 | 21.3 |
| bio_9 | mean temperature of driest quarter | 0.7 | 2.4 | 0.4 | 0.3 | 3.8 | 0.1 | 3.5 | 0.4 |
| bio_10 | mean temperature of warmest quarter | 0.3 | 0 | 0.2 | 0.4 | 6.4 | 1.2 | 6.2 | 0.3 |
| bio_11 | mean temperature of coldest quarter | 0 | 0 | 0 | 0 | 0 | 0.2 | 0 | 0.1 |
| bio_12 | annual precipitation | 0.4 | 0.1 | 0 | 0.1 | 0.2 | 4.8 | 0.2 | 1.9 |
| bio_13 | precipitation of wettest month | 8.7 | 8.4 | 2.4 | 0 | 2.5 | 0.1 | 2.1 | 0.3 |
| bio_14 | precipitation of driest month | 2.4 | 1.8 | 0.1 | 0.5 | 0.5 | 2.4 | 0.5 | 3.1 |
| bio_15 | precipitation seasonality (coefficient of variation) | 0.8 | 0.6 | 0.6 | 0.8 | 0.2 | 1.8 | 0.4 | 3.1 |
| bio_16 | precipitation of wettest quarter | 0.1 | 0 | 2.2 | 5.9 | 3.6 | 19.2 | 3.3 | 18.9 |
| bio_17 | precipitation of driest quarter | 4.1 | 0.1 | 0 | 0.1 | 1.4 | 0.9 | 1.7 | 0.9 |
| bio_18 | precipitation of warmest quarter | 0 | 0 | 2.4 | 1.4 | 5.9 | 0.9 | 4.3 | 1.1 |
| bio_19 | precipitation of coldest quarter | 0.5 | 0.1 | 0.8 | 0.3 | 4.1 | 12.6 | 3.3 | 14.8 |
| srad_1 | solar radiation, January | 0 | 0 | 0.1 | 0.4 | n.a. | n.a. | n.a. | n.a. |
| srad_2 | solar radiation, February | 0 | 0 | 0 | 0 | n.a. | n.a. | n.a. | n.a. |
| srad_3 | solar radiation, March | 0 | 0 | 0 | 0 | n.a. | n.a. | n.a. | n.a. |
| srad_4 | solar radiation, April | 11.9 | 0.6 | 4.7 | 25.3 | n.a. | n.a. | n.a. | n.a. |
| srad_5 | solar radiation, May | 0 | 0 | 0.1 | 1.7 | n.a. | n.a. | n.a. | n.a. |
| srad_6 | solar radiation, June | 0.3 | 0 | 0.3 | 0 | n.a. | n.a. | n.a. | n.a. |
| srad_7 | solar radiation, July | 36.9 | 30.4 | 25 | 0.4 | n.a. | n.a. | n.a. | n.a. |
| srad_8 | solar radiation, August | 0 | 0 | 0.8 | 0 | n.a. | n.a. | n.a. | n.a. |
| srad_9 | solar radiation, September | 0 | 0 | 0 | n.a. | n.a. | n.a. | n.a. | |
| srad_10 | solar radiation, October | 12.8 | 11.1 | 13.4 | 22.9 | n.a. | n.a. | n.a. | n.a. |
| srad_11 | solar radiation, November | 0.4 | 0 | 7.1 | 12.7 | n.a. | n.a. | n.a. | n.a. |
| srad_12 | solar radiation, December | 0 | 0 | 0 | 0 | n.a. | n.a. | n.a. | n.a. |
| Year | Aleksandrovskij | Yarovoe | Ust-Volchikha |
|---|---|---|---|
| 2000 | 1146/2682 | 252/582 | 474/144 |
| 2001 | 210/246 | 54/450 | 24/234 |
| 2002 | 6/0 | 0/24 | 72/42 |
| 2003 | 6/+ | 0/4 | 6/6 |
| 2004 | 0/+ | 0/4 | 0/+ |
| 2005 | 0/24 | 54/6 | 0/0 |
| 2006 | 0/18 | 60/30 | 6/0 |
| 2007 | 18/84 | 150/60 | 20/+ |
| 2008 | 60/78 | 144/6 | 40/0 |
| 2015 | +/+ | 4/0 | 204/480 |
| 2018 | +/6 | ? | 17.2/17.1 |
| Spearman’s correlation | 0.586 (p = 0.058) | 0.609 (p = 0.063) | 0.518 (p = 0.102) |
| Year | Aleksandrovskij | Yarovoe | Ust-Volchikha |
|---|---|---|---|
| 2004 | 0/0.32 ± 0.32 | 0.32 ± 0.16/+ | 0/0.32 ± 0.32 |
| 2005 | 0.43 ± 0.30/0.32 ± 0.23 | 0.13 ± 0.13/0.38 ± 0.22 | 0/+ |
| 2006 | 0/+ | 0.53 ± 0.24/0.21 ± 0.15 | 0.16 ± 0.16/0.16 ± 0.16 |
| 2007 | 0.48 ± 0.32/0.32 ± 0.23 | 0.96 ± 0.48/0.64 ± 0.32 | 0.16 ± 0.16/+ |
| 2008 | 0.42 ± 1.06/0.48 ± 0.27 | 0.27 ± 0.18/0.51 ± 0.25 | 0.32 ± 0.32/ 0 |
| 2015 | +/0 | 0/0 | 0.64 ± 0.64/ 1.28 ± 0.89 |
| 2018 | 0/+ | ? | 0.32 ± 0.32/0.32 ± 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, K.V.; Baturina, N.S.; Molodtsov, V.V.; Yefremova, O.V.; Zharkov, V.D.; Sergeev, M.G. The Handsome Cross Grasshopper Oedaleus decorus (Germar, 1825) (Orthoptera: Acrididae) as a Neglected Pest in the South-Eastern Part of West Siberian Plain. Insects 2022, 13, 49. https://doi.org/10.3390/insects13010049
Popova KV, Baturina NS, Molodtsov VV, Yefremova OV, Zharkov VD, Sergeev MG. The Handsome Cross Grasshopper Oedaleus decorus (Germar, 1825) (Orthoptera: Acrididae) as a Neglected Pest in the South-Eastern Part of West Siberian Plain. Insects. 2022; 13(1):49. https://doi.org/10.3390/insects13010049
Chicago/Turabian StylePopova, Kristina V., Natalya S. Baturina, Vladimir V. Molodtsov, Oxana V. Yefremova, Vasily D. Zharkov, and Michael G. Sergeev. 2022. "The Handsome Cross Grasshopper Oedaleus decorus (Germar, 1825) (Orthoptera: Acrididae) as a Neglected Pest in the South-Eastern Part of West Siberian Plain" Insects 13, no. 1: 49. https://doi.org/10.3390/insects13010049
APA StylePopova, K. V., Baturina, N. S., Molodtsov, V. V., Yefremova, O. V., Zharkov, V. D., & Sergeev, M. G. (2022). The Handsome Cross Grasshopper Oedaleus decorus (Germar, 1825) (Orthoptera: Acrididae) as a Neglected Pest in the South-Eastern Part of West Siberian Plain. Insects, 13(1), 49. https://doi.org/10.3390/insects13010049







