Estimation of the Recent Expansion Rate of Ruspolia nitidula (Orthoptera) on a Regional and Landscape Scale
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
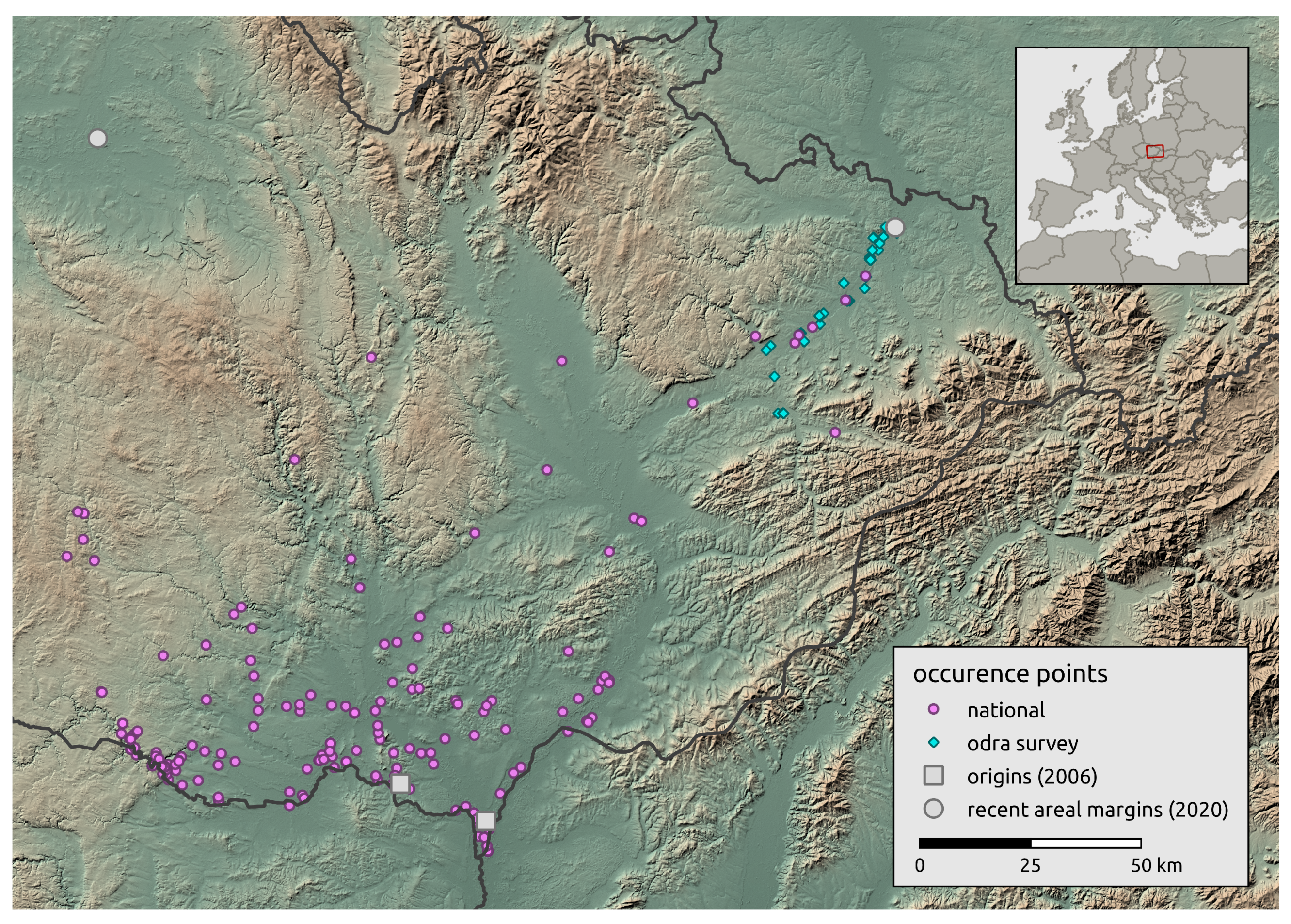
2.1. Model Species and Its Known Distribution
2.2. Landscape-Scale Occurrence Data
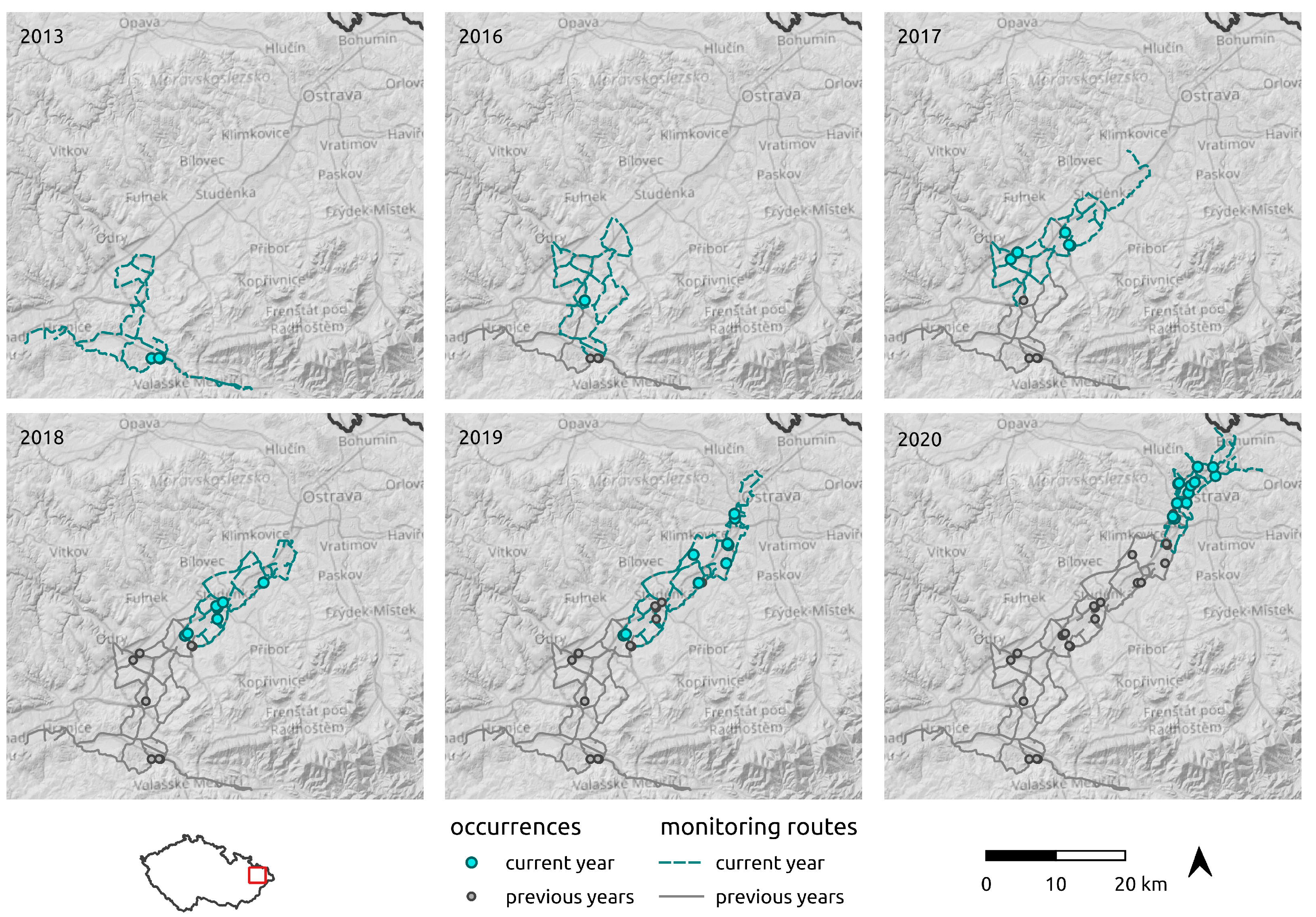
2.3. Regional-Scale Field Survey at the Areal Margin
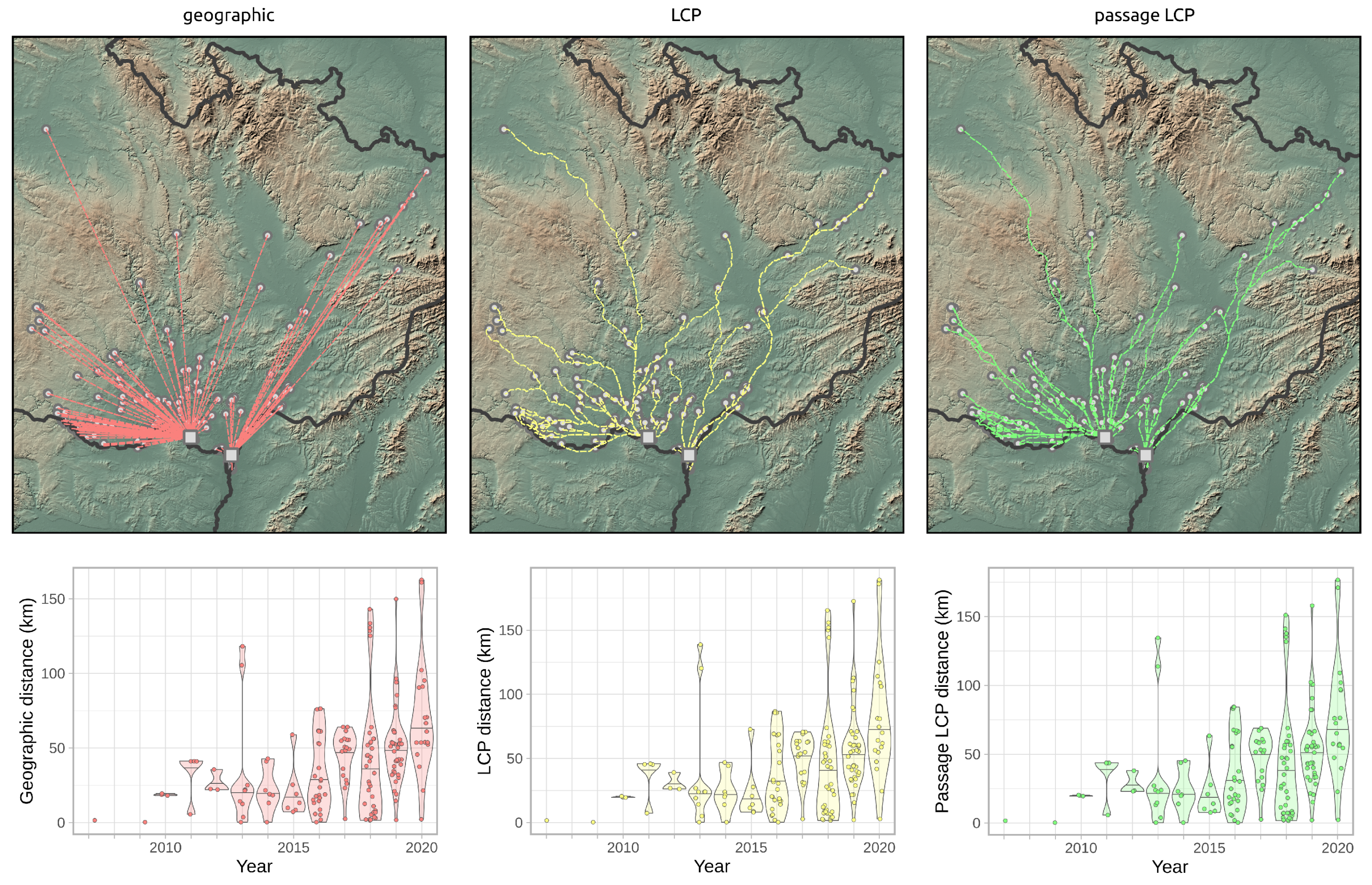
2.4. Dispersal Distances
2.5. Expansion Rate Analysis
3. Results
3.1. Habitat Suitability Model
3.2. Landscape Scale
3.3. Regional-Scale Survey in Odra Basin
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beckmann, B.C.; Purse, B.V.; Roy, D.B.; Roy, H.E.; Sutton, P.G.; Thomas, C.D. Two Species with an Unusual Combination of Traits Dominate Responses of British Grasshoppers and Crickets to Environmental Change. PLoS ONE 2015, 10, e0130488. [Google Scholar] [CrossRef] [Green Version]
- Mason, S.C.; Palmer, G.; Fox, R.; Gillings, S.; Hill, J.K.; Thomas, C.D.; Oliver, T.H. Geographical range margins of many taxonomic groups continue to shift polewards. Biol. J. Linn. Soc. 2015, 115, 586–597. [Google Scholar] [CrossRef]
- Löffler, F.; Poniatowski, D.; Fartmann, T. Orthoptera community shifts in response to land-use and climate change—Lessons from a long-term study across different grassland habitats. Biol. Conserv. 2019, 236, 315–323. [Google Scholar] [CrossRef]
- Menéndez, R.; González-Megías, A.; Jay-Robert, P.; Marquéz-Ferrando, R. Climate change and elevational range shifts: Evidence from dung beetles in two European mountain ranges. Glob. Ecol. Biogeogr. 2014, 23, 646–657. [Google Scholar] [CrossRef]
- Soroye, P.; Newbold, T.; Kerr, J. Climate change contributes to widespread declines among bumble bees across continents. Science 2020, 367, 685–688. [Google Scholar] [CrossRef]
- Lenoir, J.; Bertrand, R.; Comte, L.; Bourgeaud, L.; Hattab, T.; Murienne, J.; Grenouillet, G. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 2020, 4, 1044–1059. [Google Scholar] [CrossRef]
- Wilson, R.J.; Fox, R. Insect responses to global change offer signposts for biodiversity and conservation. Ecol. Entomol. 2021. [Google Scholar] [CrossRef]
- Didham, R.K.; Barbero, F.; Collins, C.M.; Forister, M.L.; Hassall, C.; Leather, S.R.; Packer, L.; Saunders, M.E.; Stewart, A.J.A. Spotlight on insects: Trends, threats and conservation challenges. Insect Conserv. Divers. 2020, 13, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Burton, J. The apparent influence of climatic change on recent changes of range by European insects (Lepidoptera, Orthoptera). Changes in ranges: Invertebrates on the move. In Proceedings of the 13th International Colloquium of the European Invertebrate Survey, Leiden, The Netherlands, 2–5 September 2001; Volume 2003, pp. 13–21. [Google Scholar]
- Fartmann, T.; Krämer, B.; Stelzner, F.; Poniatowski, D. Orthoptera as ecological indicators for succession in steppe grassland. Ecol. Indic. 2012, 20, 337–344. [Google Scholar] [CrossRef]
- Monnerat, C. Situation de Ruspolia nitidula (Scopoli, 1786) dans trois secteurs de Suisse occidentale: Les prémices d’une expansion? Bull. Romand Dentomol. 2003, 21, 33–47. [Google Scholar]
- Brodacki, M. First records of the Large Cone-head Bush-cricket Ruspolia nitidula (Scopoli, 1786)(Orthoptera, Tettigoniidae) in Poland. Naturalia 2015, 3, 123–126. [Google Scholar]
- Drukker, D.; van der Arend, I.; van Deijk, J.; Houkes, J.; Maas, D.; Schut, D. De grote spitskop Ruspolia nitidula in Nederland (Orthoptera). Ned. Faun. Meded. 2020, 54, 7–16. [Google Scholar]
- Braun, V.B.; Lederer, E.; Sackl, P.; Zechner, L. Verbreitung, Phänologie und Habitatansprüche der Großen Schiefkopfschrecke, Ruspolia nitidula Scopoli, 1786, in der Steiermark und im südlichen Burgenland. Abt. Zool. Landesmus 1995, 49, 57–87. [Google Scholar]
- Kočárek, P.; Holuša, J.; Vlk, R.; Marhoul, P. Rovnokřídlí (Insecta: Orthoptera) České Republiky; Academia: Praha, Czech Republic, 2013. [Google Scholar]
- Kleukers, R.; Decleer, K.; Haes, E.; Kolshorn, P.; Thomas, B. The recent expansion of Conocephalus discolor (Thunberg) (Orthoptera: Tettigoniidae) in western Europe. Entomol. Gaz. 1996, 47, 37–50. [Google Scholar]
- Fedor, P.J.; Majzlan, O. Distribution and infiltration of the tree Cricket Oecanthus pellucens (Scopoli, 1763) to unoriginal conditions in Slovakia. Bull. Soc. Nat. Luxemb. 2001, 102, 103–108. [Google Scholar]
- Kočárek, P.; Holuša, J.; Vlk, R.; Marhoul, P.; Zuna-Kratky, T. Recent expansions of bush-crickets Phaneroptera falcata and Phaneroptera nana (Orthoptera: Tettigoniidae) in the Czech Republic. Articulata 2008, 23, 67–75. [Google Scholar]
- Kenyeres, Z.; Takács, G.; Bauer, N. Response of orthopterans to macroclimate changes: A 15-year case study in Central European humid grasslands. J. Orthoptera Res. 2019, 28, 187–193. [Google Scholar] [CrossRef]
- Marini, L.; Bommarco, R.; Fontana, P.; Battisti, A. Disentangling effects of habitat diversity and area on orthopteran species with contrasting mobility. Biol. Conserv. 2010, 143, 2164–2171. [Google Scholar] [CrossRef]
- Penone, C.; Kerbiriou, C.; Julien, J.F.; Julliard, R.; Machon, N.; Le Viol, I. Urbanisation effect on Orthoptera: Which scale matters? Insect Conserv. Divers. 2013, 6, 319–327. [Google Scholar] [CrossRef]
- Detzel, P. Die Heuschrecken Baden-Württembergs; Verlag Eugen Ulmer GmbH & Co.: Stuttgart, Germany, 1998. [Google Scholar]
- Ingrisch, S.; Köhler, G. Die Heuschrecken Mitteleuropas; Westarp Wissenschaften: Magdeburg, Germany, 1998. [Google Scholar]
- Hassall, C.; Thompson, D.J. Accounting for recorder effort in the detection of range shifts from historical data. Methods Ecol. Evol. 2010, 1, 343–350. [Google Scholar] [CrossRef]
- Preuss, S.; Low, M.; Cassel-Lundhagen, A.; Berggren, Å. Evaluating range-expansion models for calculating nonnative species’ expansion rate. Ecol. Evol. 2014, 4, 2812–2822. [Google Scholar] [CrossRef]
- Etherington, T.R. Least-cost modelling and landscape ecology: Concepts, applications, and opportunities. Curr. Landsc. Ecol. Rep. 2016, 1, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.C.F.; Coulon, A.; Travis, J.M.J. Introducing a ‘stochastic movement simulator’ for estimating habitat connectivity. Methods Ecol. Evol. 2011, 2, 258–268. [Google Scholar] [CrossRef]
- Scharf, A.K.; Belant, J.L.; Beyer, D.E.; Wikelski, M.; Safi, K. Habitat suitability does not capture the essence of animal-defined corridors. Mov. Ecol. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Alexander, J.L.; Olimb, S.K.; Bly, K.L.S.; Restani, M. Use of least-cost path analysis to identify potential movement corridors of swift foxes in Montana. J. Mammal. 2016, 97, 891–898. [Google Scholar] [CrossRef] [Green Version]
- LaPoint, S.; Gallery, P.; Wikelski, M.; Kays, R. Animal behavior, cost-based corridor models, and real corridors. Landsc. Ecol. 2013, 28, 1615–1630. [Google Scholar] [CrossRef] [Green Version]
- LaRue, M.A.; Nielsen, C.K. Modelling potential dispersal corridors for cougars in midwestern North America using least-cost path methods. Ecol. Model. 2008, 212, 372–381. [Google Scholar] [CrossRef]
- Pullinger, M.G.; Johnson, C.J. Maintaining or restoring connectivity of modified landscapes: Evaluating the least-cost path model with multiple sources of ecological information. Landsc. Ecol. 2010, 25, 1547–1560. [Google Scholar] [CrossRef]
- Balbi, M.; Croci, S.; Petit, E.J.; Butet, A.; Georges, R.; Madec, L.; Caudal, J.P.; Ernoult, A. Least-cost path analysis for urban greenways planning: A test with moths and birds across two habitats and two cities. J. Appl. Ecol. 2021, 58, 632–643. [Google Scholar] [CrossRef]
- Mineur, F.; Davies, A.J.; Maggs, C.A.; Verlaque, M.; Johnson, M.P. Fronts, jumps and secondary introductions suggested as different invasion patterns in marine species, with an increase in spread rates over time. Proc. R. Soc. B Biol. Sci. 2010, 277, 2693–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuska, A. Ryjkowce (Coleoptera: Rhynchitidae, Apionidae, Nanophyidae, Curculionidae) terenow kserotermicznych Gornego Slaska. Nat. Silesiae Super. Cent. Dziedzictwa Przyr. Górnego Śląska 2001, 5, 61–77. [Google Scholar]
- Cieślak, E. Migration history of xerothermic plants on the area of the southern Poland, in terms of molecular data. Fragm. Florist. Geobot. Pol. 2015, 22, 3–13. [Google Scholar]
- Banaszak, J.; Twerd, L.; Sobieraj-Betlińska, A.; Kilińska, B. The Moravian Gate as route of migration of thermophilous bee species to Poland: Fact or myth? A case study in the “Góra Gipsowa” steppe reserve and other habitats near Kietrz. Pol. J. Entomol. 2017, 86, 141–164. [Google Scholar] [CrossRef]
- Parusel, J. Brama Morawska—Aspekty badawcze i turystyczne. In Brama Morawska—Aspekty Badawcze i Turystyczne; Jankowski, A.T., Ed.; Chapter Brama Morawska-Biogeograficzne Aspekty Badawcze; University of Silesia: Katowice, Poland, 1997; pp. 85–89. [Google Scholar]
- Sierka, W.; Sierka, E.; Fedor, P. Introduction to the probable movement of Thysanoptera through the Moravian Gate (Štramberk, Czech Republic). Acta Phytopathol. Entomol. Hung. 2008, 43, 367–372. [Google Scholar] [CrossRef]
- Kočárek, P.; Holuša, J.; Vidlička, L. Blattaria, Mantodea, Orthoptera & Dermaptera of the Czech and Slovak Republics; Kabourek: Zlín, Czech Republic, 2005. [Google Scholar]
- Krištín, A.; Kaňuch, P.; Sarossy, M. Did the northern range of distribution of two tropical orthopterans (Insecta) change recently. Pol. J. Ecol. 2007, 55, 297–304. [Google Scholar]
- Krištín, A.; Kaňuch, P.; Sarossy, M. Distribution and ecology of Ruspolia nitidula (Scopoli 1786) and Aiolopus thalassinus (Fabricius 1781)(Orthoptera) in Slovakia. Linzer Biol. Beitr. 2007, 39, 451–461. [Google Scholar]
- Mařan, J. Beitrag zur Kenntnis der Taxonomie, Okologie und der geographischen Verbreitung von Homorocoryphus nitidulus (Scop.) in der Tschechoslowakei (Orthoptera-Tettigonoidea). Acta Faun. Entomol. Mus. Nat. Pragae 1965, 11, 307–326. [Google Scholar]
- Holuša, J.; Kočárek, P.; Marhoul, P. First sightings of Ruspolia nitidula (Orthoptera: Tettigoniidae) and Mecostethus parapleurus (Orthoptera: Acrididae) after fifty years in the Czech Republic. Articulata 2007, 22, 47–51. [Google Scholar]
- AOPK ČR. Nálezová Databáze Ochrany Přírody. 2021. Available online: https://portal.nature.cz (accessed on 13 July 2021).
- Saerens, M.; Achbany, Y.; Fouss, F.; Yen, L. Randomized shortest-path problems: Two related models. Neural Comput. 2009, 21, 2363–2404. [Google Scholar] [CrossRef]
- van Etten, J.; Hijmans, R.J. A Geospatial Modelling Approach Integrating Archaeobotany and Genetics to Trace the Origin and Dispersal of Domesticated Plants. PLoS ONE 2010, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.; Dudík, M.; Schapire, R. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.3). 2020. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 13 July 2021).
- Naimi, B.; Araújo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. dismo: Species Distribution Modeling. R Package Version 1.1-4. 2017. Available online: https://CRAN.R-project.org/package=dismo (accessed on 13 July 2021).
- Global Administrative Areas. GADM Database of Global Administrative Areas. Version 3.6. 2018. Available online: www.gadm.org (accessed on 13 July 2021).
- Amatulli, G.; McInerney, D.; Sethi, T.; Strobl, P.; Domisch, S. Geomorpho90m, empirical evaluation and accuracy assessment of global high-resolution geomorphometric layers. Sci. Data 2020, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- © European Union, Copernicus Land Monitoring Service; European Environment Agency (EEA): København, Denmark, 2021.
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling. Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Marhoul, P.; Kočárek, P. Biological Library—BioLib. Chapter Mapa Rozšíření Ruspolia nitidula v České Republice. 2021. Available online: https://www.biolib.cz/cz/taxonmap/id377/ (accessed on 13 July 2021).
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Boyce, M.S.; Vernier, P.R.; Nielsen, S.E.; Schmiegelow, F.K. Evaluating resource selection functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef] [Green Version]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Ferro, C.A.; Stephenson, D.B. Extremal dependence indices: Improved verification measures for deterministic forecasts of rare binary events. Weather Forecast. 2011, 26, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Wunderlich, R.F.; Lin, Y.P.; Anthony, J.; Petway, J.R. Two alternative evaluation metrics to replace the true skill statistic in the assessment of species distribution models. Nat. Conserv. 2019, 35, 97–116. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 3.4-10. 2021. Available online: https://CRAN.R-project.org/package=raster (accessed on 13 July 2021).
- van Etten, J. R Package gdistance: Distances and Routes on Geographical Grids. J. Stat. Softw. Artic. 2017, 76, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2016, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. QGIS Association, 2021. Available online: https://qgis.org/en/site/getinvolved/faq/index.html#how-to-cite-qgis (accessed on 13 July 2021).
- Simmons, A.; Thomas, C. Changes in Dispersal during Species’ Range Expansions. Am. Nat. 2004, 164, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Travis, J.M.; Dytham, C. Dispersal evolution during invasions. Evol. Ecol. Res. 2002, 4, 1119–1129. [Google Scholar]
- Wagner, D.L.; Fox, R.; Salcido, D.M.; Dyer, L.A. A window to the world of global insect declines: Moth biodiversity trends are complex and heterogeneous. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Fahrner, S.; Aukema, B.H. Correlates of spread rates for introduced insects. Glob. Ecol. Biogeogr. 2018, 27, 734–743. [Google Scholar] [CrossRef]
- Zuna-Kratky, T.; Landmann, A.; Illich, I.; Zechner, L.; Essl, F.; Lechner, K.; Ortner, A.; Weißmair, W.; Wöss, G. Die Heuschrecken Österreichs; Biologiezentrum: Linz, Austria, 2017. [Google Scholar]
- Zuna-Kratky, T.; Karner-Ranner, E.; Lederer, E.; Braun, B.; Berg, H.M.; Denner, M.; Bieringer, G.; Ranner, A.; Zechner, L. Verbreitungsatlas der Heuschrecken und Fangschrecken Ostösterreichs; Naturhistorischen Museums Wien: Wien, Austria, 2009. [Google Scholar]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Hochkirch, A.; Damerau, M. Rapid range expansion of a wing-dimorphic bush-cricket after the 2003 climatic anomaly. Biol. J. Linn. Soc. 2009, 97, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Walker, T.J.; Nickle, D.A. Introduction and Spread of Pest Mole Crickets: Scapteriscus vicinus and S. acletus Reexamined1. Ann. Entomol. Soc. Am. 1981, 74, 158–163. [Google Scholar] [CrossRef]
- Coulon, A.; Aben, J.; Palmer, S.C.F.; Stevens, V.M.; Callens, T.; Strubbe, D.; Lens, L.; Matthysen, E.; Baguette, M.; Travis, J.M.J. A stochastic movement simulator improves estimates of landscape connectivity. Ecology 2015, 96, 2203–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milanesi, P.; Holderegger, R.; Caniglia, R.; Fabbri, E.; Randi, E. Different habitat suitability models yield different least-cost path distances for landscape genetic analysis. Basic Appl. Ecol. 2016, 17, 61–71. [Google Scholar] [CrossRef]
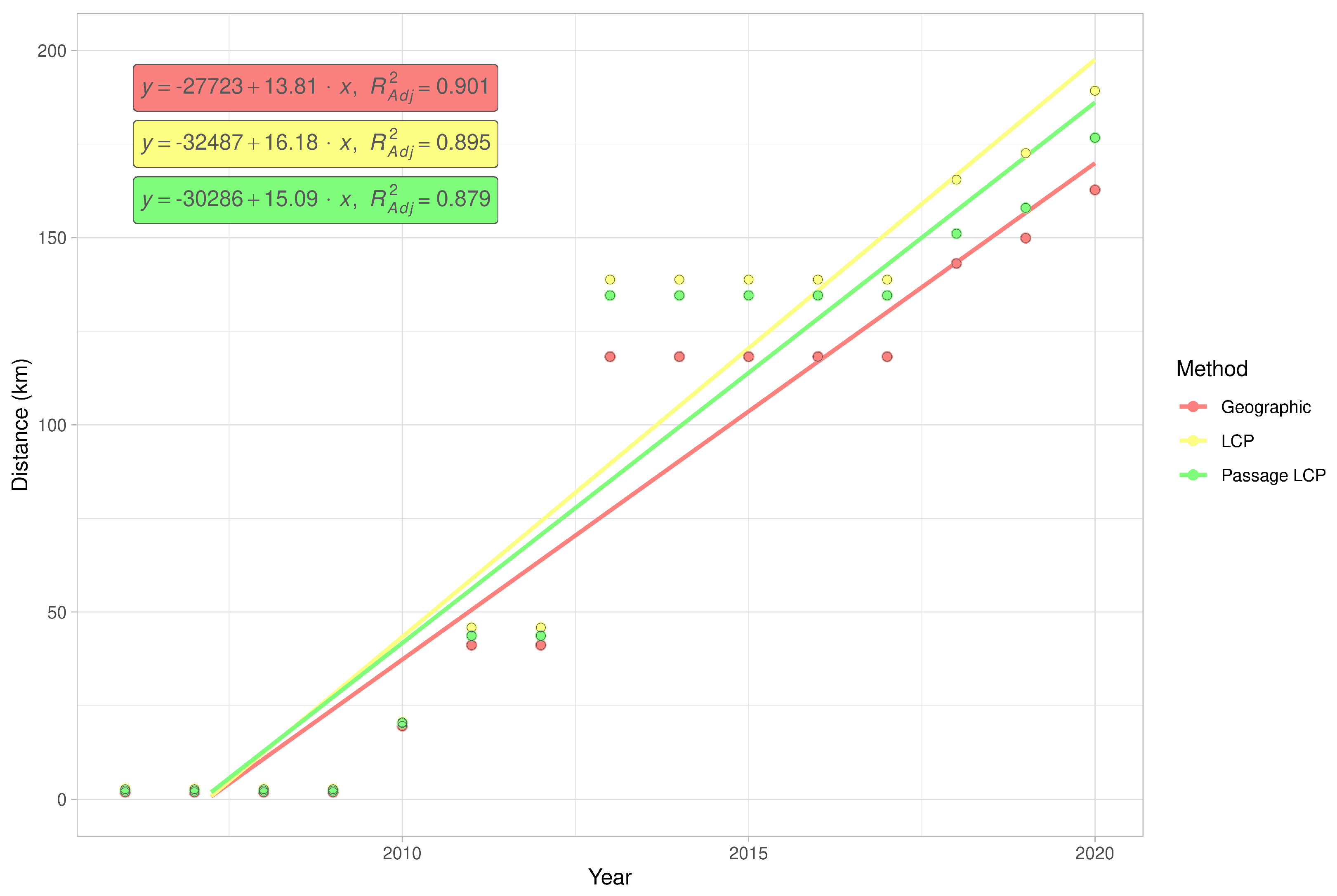
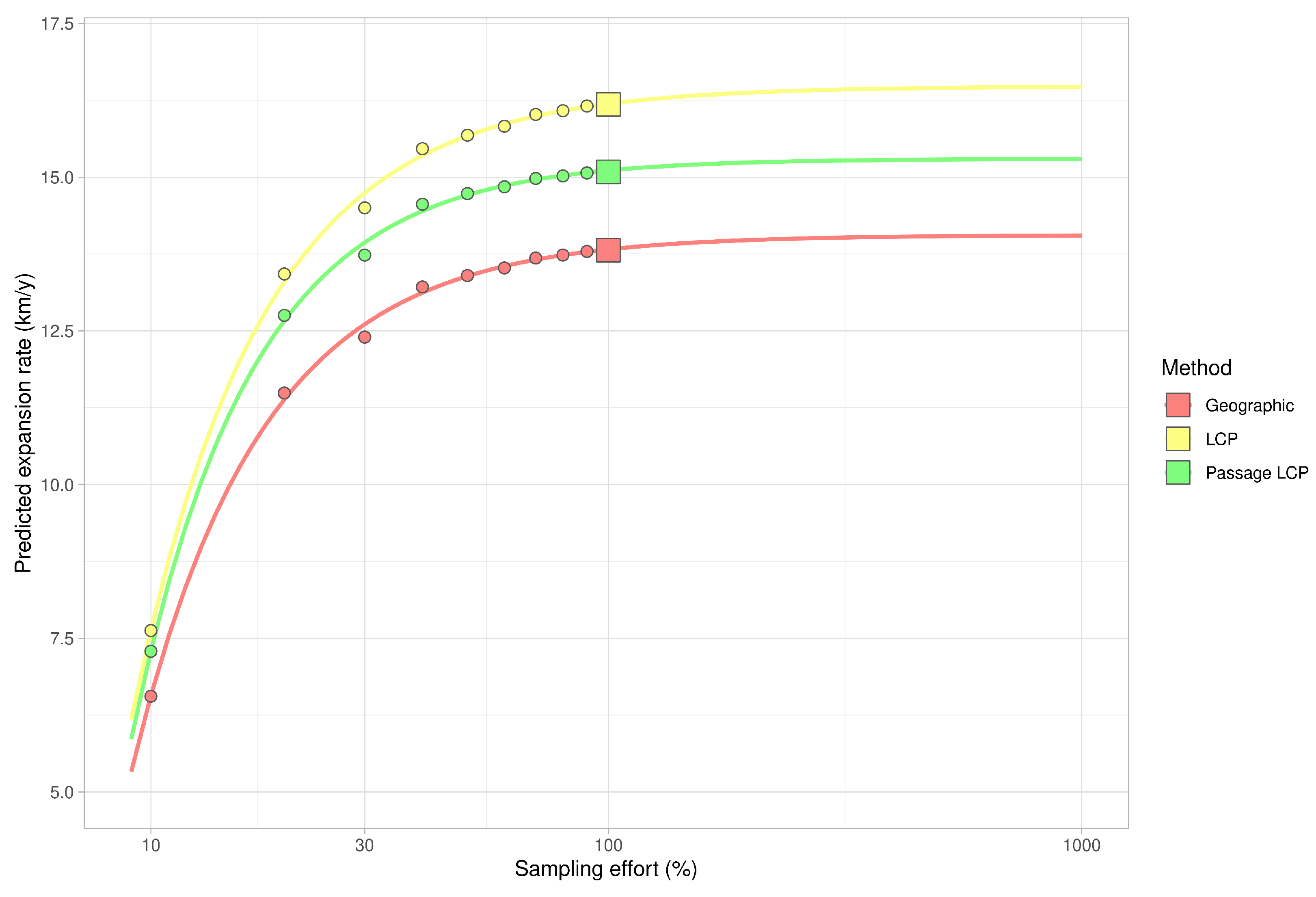
| Method | km/y | Adjusted R2 | F | CI 2.5 | CI 97.5 | p-Value |
|---|---|---|---|---|---|---|
| Geographic | 13.81 | 0.90 | 119.11 | 11.05 | 16.57 | <0.001 |
| LCP | 16.18 | 0.90 | 112.31 | 12.86 | 19.51 | <0.001 |
| Passage LCP | 15.09 | 0.88 | 95.07 | 11.71 | 18.46 | <0.001 |
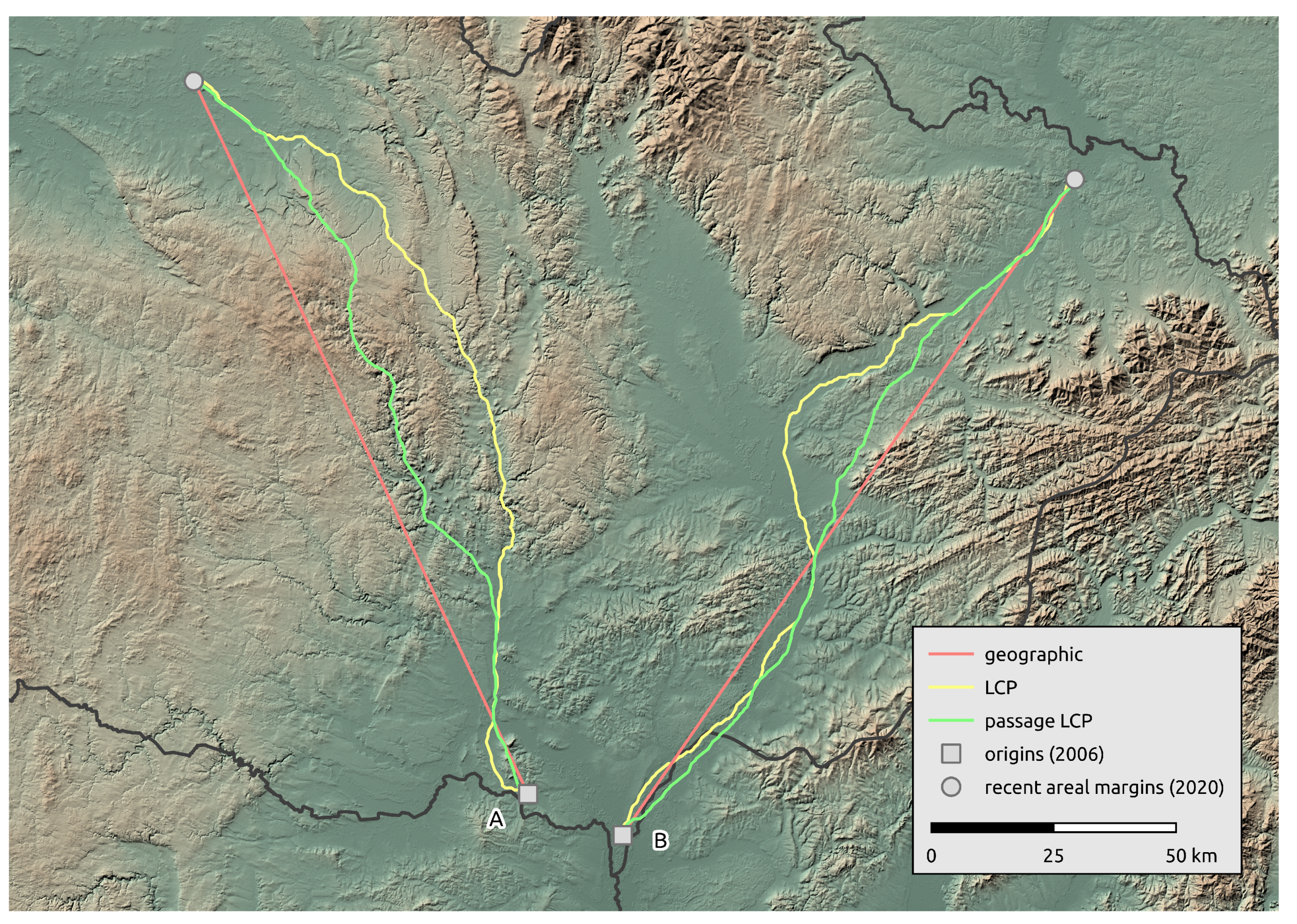
| Origin | Method | km | km/y |
|---|---|---|---|
| Geographic | 161.0 | 11.5 | |
| A | LCP | 189.2 | 13.5 |
| Passage LCP | 176.7 | 12.6 | |
| Geographic | 162.7 | 11.6 | |
| B | LCP | 186.1 | 13.3 |
| Passage LCP | 171.1 | 12.2 |
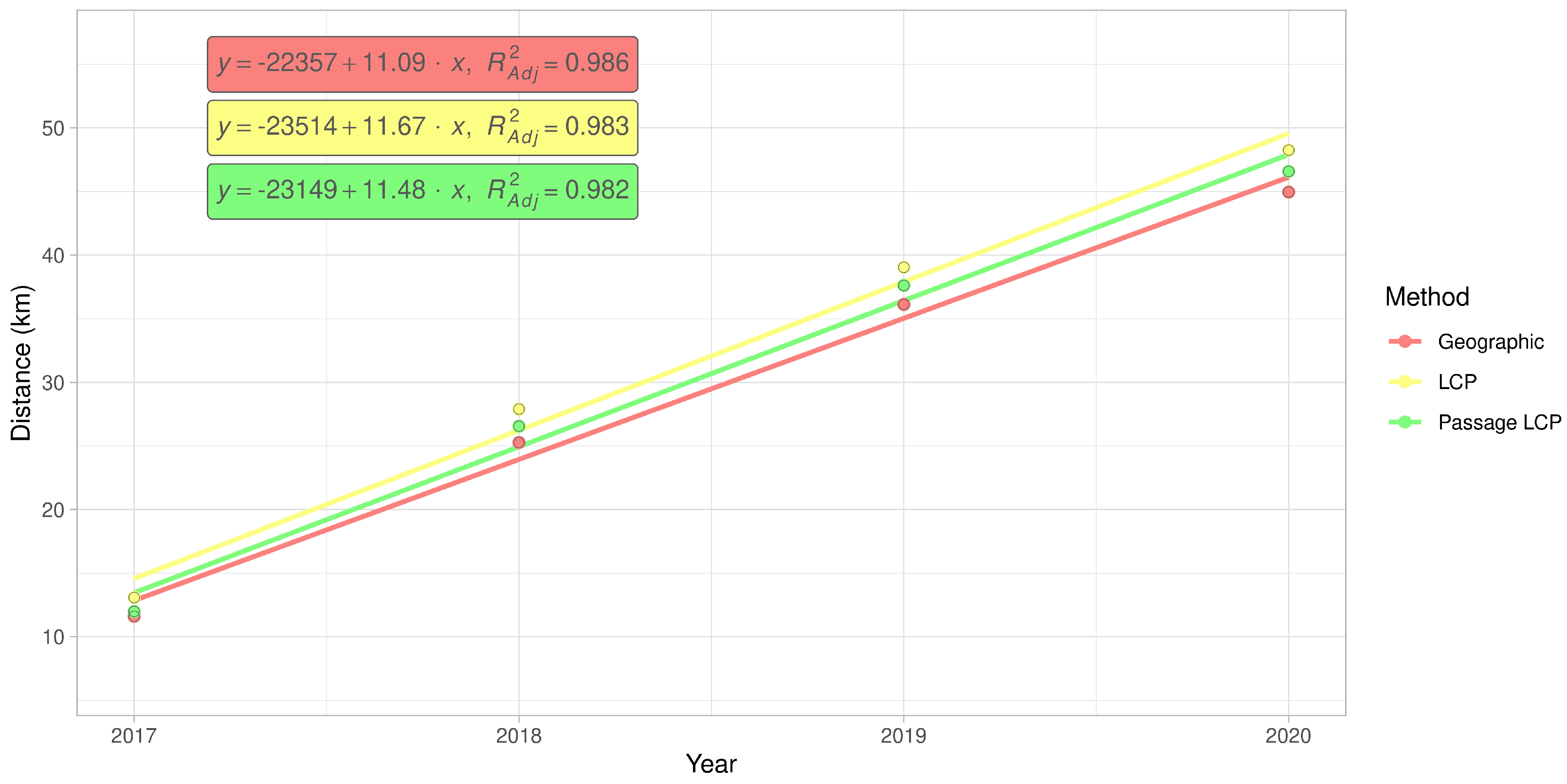
| Method | km/y | Adjusted R2 | F | CI 2.5 | CI 97.5 | p-Value |
|---|---|---|---|---|---|---|
| Geographic | 11.09 | 0.99 | 209.64 | 7.80 | 14.39 | 0.005 |
| LCP | 11.67 | 0.98 | 169.61 | 7.81 | 15.52 | 0.006 |
| Passage LCP | 11.48 | 0.98 | 165.83 | 7.65 | 15.32 | 0.006 |
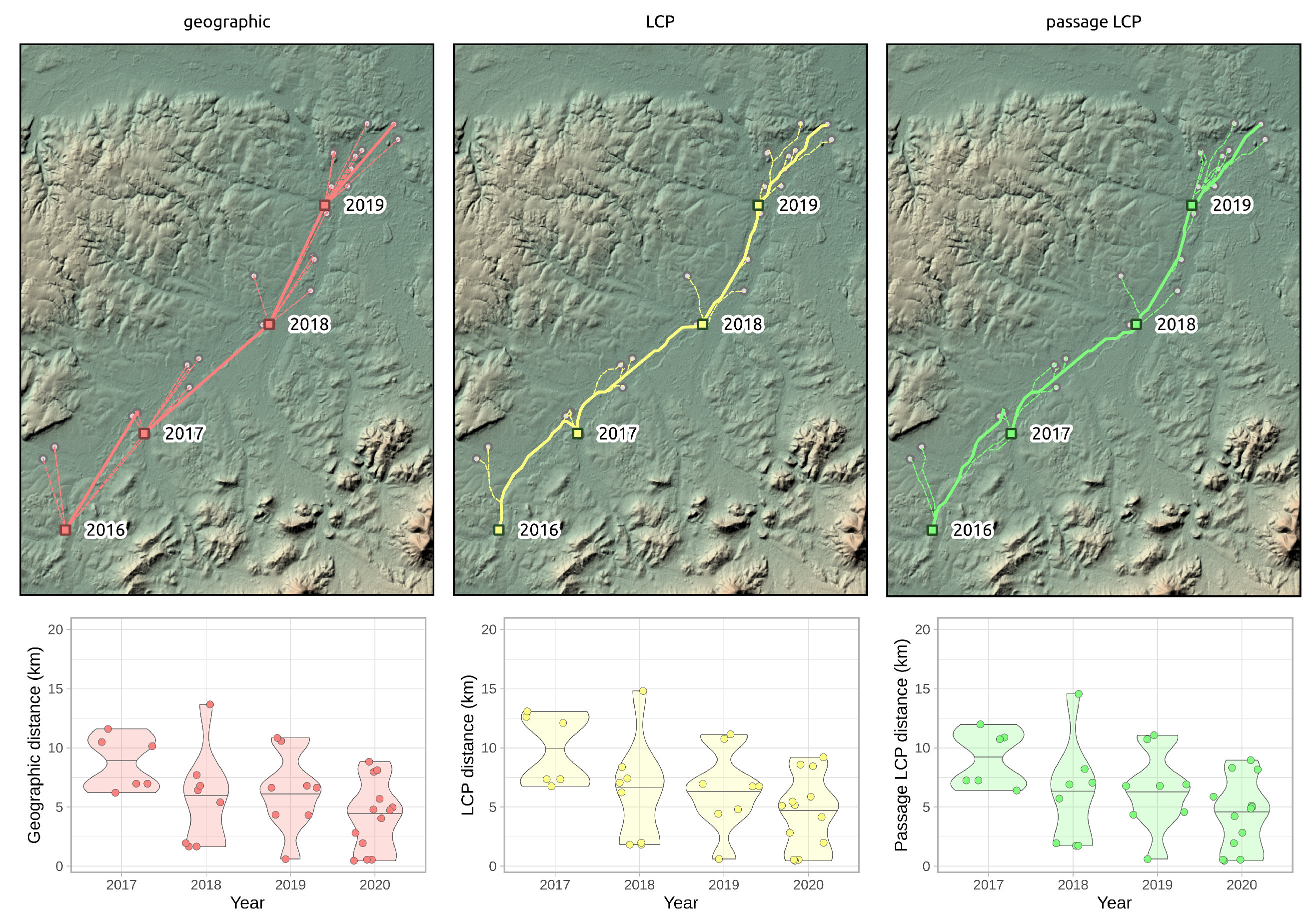
| Method | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 | Mean ± SD |
|---|---|---|---|---|---|
| Geographic | 11.60 | 13.67 | 10.85 | 8.83 | 11.24 ± 2.00 |
| LCP | 13.08 | 14.82 | 11.14 | 9.21 | 12.06 ± 2.42 |
| Passage LCP | 11.99 | 14.57 | 11.06 | 8.96 | 11.64 ± 2.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaláb, O.; Pyszko, P.; Kočárek, P. Estimation of the Recent Expansion Rate of Ruspolia nitidula (Orthoptera) on a Regional and Landscape Scale. Insects 2021, 12, 639. https://doi.org/10.3390/insects12070639
Kaláb O, Pyszko P, Kočárek P. Estimation of the Recent Expansion Rate of Ruspolia nitidula (Orthoptera) on a Regional and Landscape Scale. Insects. 2021; 12(7):639. https://doi.org/10.3390/insects12070639
Chicago/Turabian StyleKaláb, Oto, Petr Pyszko, and Petr Kočárek. 2021. "Estimation of the Recent Expansion Rate of Ruspolia nitidula (Orthoptera) on a Regional and Landscape Scale" Insects 12, no. 7: 639. https://doi.org/10.3390/insects12070639
APA StyleKaláb, O., Pyszko, P., & Kočárek, P. (2021). Estimation of the Recent Expansion Rate of Ruspolia nitidula (Orthoptera) on a Regional and Landscape Scale. Insects, 12(7), 639. https://doi.org/10.3390/insects12070639






