Effects of Helicoverpa armigera Egg Age on Development, Reproduction, and Life Table Parameters of Trichogramma euproctidis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Experimental Procedure
2.3. Statistical Analysis
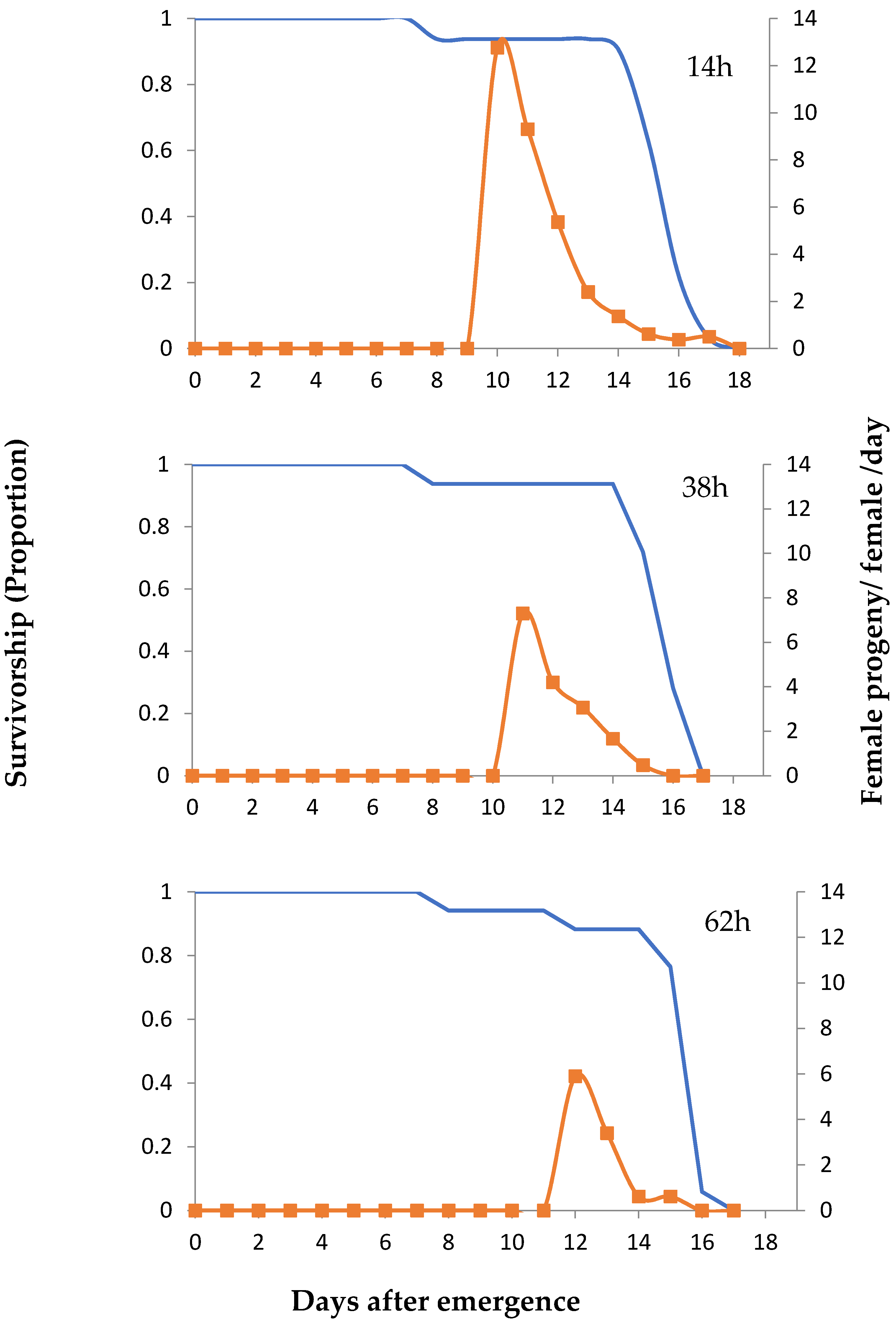
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behdad, B. Pests of Field Crops in Iran; Isfahan Neshat Press: Isfahan, Iran, 1982. [Google Scholar]
- Metcalf, R.A.; Metcalf, M.D. Destructive and Useful Insects: Their Habits and Control, 5th ed.; McGraw-Hill Inc.: New York, NY, USA, 1993. [Google Scholar]
- Mossadegh, M.S.; Kocheili, F. A Semi-Descriptive Checklist of Identified Species of Arthropods (Agricultural, Medical, …) and Other Pests from Khuzestan, Iran; Shahid Chamran University Press: Ahvaz, Iran, 2003. [Google Scholar]
- Khanjani, M. Field Crop Pests in Iran. (Insects and Mites); Bu-Ali Sina University Press: Hamedan, Iran, 2005. [Google Scholar]
- Fitt, G.P. Cotton pest management. Part 3. An Australian perspective. Annu. Rev. Entomol. 1994, 39, 543–562. [Google Scholar] [CrossRef]
- Xia, J.Y. Status and management of insecticide resistance of cotton insect pests in China (Mainland). ICAC Rec. 1993, 9, 10–14. [Google Scholar]
- Armes, N.J.; Jadhav, D.R.; DeSouza, K.R. A survey of insecticide resistance in Helicoverpa armigera in the Indian subcontinent. Bull. Entomol. Res. 1996, 86, 499–514. [Google Scholar] [CrossRef]
- Ahmad, M.; Arif, M.I.; Ahmad, Z. Patterns of resistance to organophosphate insecticides in field populations of Helicoverpa armigera in Pakistan. Pestic. Sci. 1999, 55, 626–632. [Google Scholar] [CrossRef]
- Ernst, G.; Dittrich, V. Comparative measurements of resistance to insecticides in three closely related old and new world bollworm species. Pestic. Sci. 1992, 34, 147–152. [Google Scholar] [CrossRef]
- Mossallanezhad, H.; Norouzi, M.; Mohammadbeigi, A. List of Important Plant Pests, Disease, Weeds and Recommended Pesticides; Educational Agricultural Publisher: Tehran, Iran, 2003. (In Persian) [Google Scholar]
- Buès, R.; Bouvier, J.C.; Boudinhon, L. Insecticide resistance and mechanisms of resistance to selected strains of Helicoverpa armigera (Lepidoptera: Noctuidae) in the south of France. Crop. Prot. 2005, 24, 814–820. [Google Scholar] [CrossRef]
- Cherry, A.; Cock, M.; van den Berg, H.; Kfir, R. Biological control of Helicoverpa armigera in Africa. In Biological Control in IPM Systems in Africa; Neuenschwander, P., Borgemeister, C., Langewald, J., Eds.; CAB International: Wallingford, UK, 2003; pp. 329–346. [Google Scholar]
- Smith, S.M. Biological control with Trichogramma: Advances, successes, and potential for their use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef]
- Tabone, T.; Bardon, C.; Desneux, N.; Wajnberg, E. Comparative assessment of parasitism of different Trichogramma species and strains on Plutella xylostella L. on greenhouse cauliflower. J. Pest. Sci. 2010, 83, 251–256. [Google Scholar] [CrossRef]
- Pizzol, J.; Desneux, N.; Wajnberg, E.; Thiery, D. Parasitoid and host egg ages have independent impact on various biological traits in a Trichogramma species. J. Pest. Sci. 2012, 85, 489–496. [Google Scholar] [CrossRef]
- Chailleux, A.; Desneux, N.; Seguret, J.; Khanh, H.D.T.; Maignet, P.; Tabone, E. Assessing European egg parasitoids as a mean of controlling the invasive South American tomato pinworm Tuta absoluta. PLoS ONE 2012, 7, e48068. [Google Scholar] [CrossRef] [PubMed]
- Rohi, L.; Pintureau, B. Reassessment of Trichogramma euproctidis (Girault, 1911) (Hymenoptera: Trichogrammatidae). Russian Entomol. J. 2003, 12, 373–379. [Google Scholar]
- Martel, V.; Darrouzet, E.; Boivin, G. Phenotypic plasticity in the reproductive traits of a parasitoid. J. Insect Physiol. 2011, 57, 682–687. [Google Scholar] [CrossRef]
- El-Arnaouty, S.A.; Pizzol, J.; Galal, H.H.; Kortam, M.N.; Afifi, A.I.; Beyssat, V.; Desneux, N.; Biondi, A.; Heika, I.H. Assessment of two Trichogramma species for the control of Tuta absoluta in North African tomato greenhouses. Afr. Entomol. 2014, 22, 801–809. [Google Scholar] [CrossRef]
- Adly, D.; Nouh, G.M. Impact of combine releases of the egg parasitoid, Trichogramma euproctidis (Girault) and the entomopathogenic nematode, Heterorhabditis bacteriophora to control Tuta absoluta (Meyrick) in tomato greenhouses in Egypt. Egypt. J. Pest. Control. 2019, 29, 91. [Google Scholar] [CrossRef]
- Pak, G.A. Behavioral variations among strains of Trichogramma spp.: A review of the literature on host-age selection. J. Appl. Entomol. 1986, 101, 55–64. [Google Scholar] [CrossRef]
- Tabebordbar, F.; Shishehbor, P.; Ebrahimi, E. Suitability of different egg ages of Ephestia kuehniella (Lep.: Pyralidae) for the development, reproduction and life table parameters of Trichogramma evanescens (Hym.: Trichogrammatidae). J. Crop. Prot. 2020, 9, 89–99. [Google Scholar]
- Calvin, D.D.; Losey, J.E.; Knapp, M.C.; Poston, F.L. Oviposition and development of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) in three age classes of southwestern corn borer eggs. Environ. Entomol. 1997, 26, 385–390. [Google Scholar] [CrossRef]
- Jarjees, E.; Merritt, D.J.; Gordh, G. Anatomy of the mouthparts and digestive tract during feeding in larvae of the parasitoid wasp Trichogramma australicum Girault (Hymenoptera: Trichogrammatidae). Int. J. Insect Morphol. Embryol. 1998, 27, 103–110. [Google Scholar] [CrossRef]
- Wu, Z.X.; Cohen, A.C.; Nordlund, D.A. The feeding behavior of Trichogramma brassicae: New evidence for selective ingestion of solid food. Entomol. Exp. Appl. 2000, 96, 1–8. [Google Scholar] [CrossRef]
- Jarjees, E.A.; Merritt, D.J. The effect of parasitization by Trichogramma australicum on Helicoverpa armigera host eggs and embryos. J. Invert. Pathol. 2004, 85, 1–8. [Google Scholar] [CrossRef]
- Romeis, J.; Shanower, T.G.; Zebitz, C.P.W. Trichogramma egg parasitism of Helicoverpa armigera on pigeonpea and sorghum in southern India. Entomol. Exp. Appl. 1999, 90, 69–81. [Google Scholar] [CrossRef]
- El-Heneidy, A.H.; El-Awady, S.M.; El-Dawwi, H.N. Control of the tomato fruit worm, Helicoverpa armigera (Hübner) by releasing the egg parasitoid, Trichogramma evanescens West. in tomato fields in southern Egypt. Egypt. J. Biol. Pest. Control. 2010, 20, 21–26. [Google Scholar]
- Laurentis, V.L.; Ramalho, D.G.; Santos, N.A.; Carvalho, V.F.P.; Vacari, A.M.; De Bortoli, S.A.; Veneziani, R.C.S.; Inácio, G.C.; Dami, B.G. Performance of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) on eggs of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Sci. Rep. 2019, 9, 1156. [Google Scholar] [CrossRef]
- Iranipour, S.; Vaez, N.; Ghanbalani, G.N.; Zakaria, R.A.; Jafarloo, M.M. Effect of host change on demographic fitness of the parasitoid, Trichogramma brassicae. J. Insect Sci. 2009, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Jarjees, E.A.; Merritt, D.J. Structure of the gut contents of Trichogramma australicum Girault (Hymenoptera: Trichogrammatidae) larvae fixed in situ in eggs of its host Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2003, 42, 203–209. [Google Scholar] [CrossRef]
- Pinto, J.D. Novel taxa of Trichogramma from the New World and Australia (Hymenoptera: Trichogrammatidae). J. N. Y. Entomol. Soc. 1992, 100, 621–633. [Google Scholar]
- Shorey, H.; Hale, R. Mass rearing of the larvae of nine noctuid species on a simple artificial medium. J. Econ. Entomol. 1965, 58, 522–524. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Ameri, M.; Rasekh, A.; Michaud, J.P.; Allahyari, H. Morphometric indicators for quality assessment in the aphid parasitoid, Lysiphlebus fabarum (Braconidae: Aphidiinae). Eur. J. Entomol. 2013, 110, 519–525. [Google Scholar] [CrossRef]
- SPSS. SPSS for Windows, Version 22.0; SPSS Institute Inc.: Chicago, IL, USA, 2018. [Google Scholar]
- Birch, L.C. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 7, 15–26. [Google Scholar] [CrossRef]
- Maia, A.H.N.; Alfredo, J.B.; Campanhola, C. Statistical influence on associated fertility life table parameters using Jackknife technique: Computational aspects. J. Econ. Entomol. 2000, 93, 511–518. [Google Scholar] [CrossRef]
- Godin, C.; Boivin, G. Effect of host age on parasitism and progeny allocation in Trichogrammatidae. Entomol. Exp. Appl. 2000, 97, 149–160. [Google Scholar] [CrossRef]
- Ruberson, J.R.; Kring, T.J. Parasitism of developing eggs by Trichogramma pretiosum (Hymenoptera: Trichogrammatidae): Host age preference and suitability. Biol. Control 1993, 3, 39–46. [Google Scholar] [CrossRef]
- Guang, L.Q.; Oloo, G.W. Host preference studies on Trichogramma sp. nr. mwanzai Schulten and Feijen (Hym: Trichogrammatidae) in Kenya. Insect Sci. Appl. 1990, 11, 757–763. [Google Scholar] [CrossRef]
- Miura, K.; Kobayashi, M. Effect of host-egg age on the parasitism of Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae), an egg parasitoid of the diamondback moth. Appl. Entomol. Zool. 1998, 33, 219–222. [Google Scholar] [CrossRef]
- Saour, G. Efficacy assessment of some Trichogramma species (Hymenoptera: Trichogrammatidae) in controlling the potato tuber moth Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae). J. Pest. Sci. 2004, 77, 229–234. [Google Scholar] [CrossRef]
- Stinguel, P.; Carvalho, J.R.; Pratissoli, D.; Zuim, V.; Mardgan, L. Efeito da idade dos ovos de Mocis latipes (Lepidoptera: Noctuidae) sobre o parasitismo de Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) com diferentes idades. Nucleus 2013, 100, 265–274. [Google Scholar] [CrossRef][Green Version]
- Oliveira, H.N.; Santana, D.R.S.; Bellon, P.P.; Oliveira, F.C. Age influence of Diatraea saccharalis (Lepidoptera: Crambidae) on the parasitism by Trichogramma galloi (Hymenoptera: Trichogrammatidae). Interciencia 2014, 39, 46–48. [Google Scholar]
- Tunçbilek, A.Ş.; Ayvaz, A. Influences of host age, sex ratio, population density, and photoperiod on parasitism by Trichogramma evanescens Westw. (Hym., Trichogrammatidae). J. Pest. Sci. 2003, 76, 176–180. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Umarova, T.Y.; Voinovich, N.D. The influence of previous host age on current host acceptance in Trichogramma. Entomol. Exp. Appl. 1997, 82, 153–157. [Google Scholar] [CrossRef]
- Takada, Y.; Kawamura, S.; Tanaka, T. Biological characteristic: Growth and development of the parasitoid Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae) on cabbage armyworm Mamestra brassicae (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2000, 35, 369–379. [Google Scholar] [CrossRef][Green Version]
- Moreno, F.; Perez-Moreno, I.; Marco, V. Effects of Lobesia botrana (Lepidoptera: Tortricidae) egg age, density, and Uv treatment on parasitism and development of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Environ. Entomol. 2009, 38, 1513–1520. [Google Scholar] [CrossRef]
- Paraiso, O.; Hight, S.D.; Kairo, M.T.K.; Bloem, S.; Carpenter, J.E.; Reitz, S. Laboratory biological parameters of Trichogramma fuentesi (Hymenoptera: Trichogrammatidae), an egg parasitoid of Cactoblastis cactorum (Lepidoptera: Pyralidae). Florida Entomol. 2012, 95, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.J.; Ren, B.Z.; Yuan, X.H.; Zang, L.S.; Ruan, C.C.; Sun, G.Z.; Shao, X.W. Effect of host-egg ages on host selection and suitability of four Chinese Trichogramma species, egg parasitoids of the rice striped stem borer, Chilo suppressalis. BioControl 2014, 59, 159–166. [Google Scholar] [CrossRef]
- Waage, J.K.; Ng, S.M. The reproductive strategy of a parasitic wasp. I. Optimal progeny and sex allocation in Trichogramma evanescens. J. Anim. Ecol. 1984, 53, 401–415. [Google Scholar] [CrossRef]
- van den Assem, J.; van Iersel, J.J.A.; Los-Den Hartog, R.L. Is being large more important for female than for male parasitic wasps? Behaviour 1989, 108, 160–195. [Google Scholar]
- Ali, A.; Choudhury, A.R.; Ahmad, Z.; Rahman, F.; Khan, F.R.; Ahmad, S.K. Some biological characteristics of Helicoverpa armigera on chickpea. Tunisian J. Plant. Prot. 2009, 4, 99–106. [Google Scholar]
- Zuim, V.; de Souza Rodrigues, H.; Pratissoli, D.; Freitas Bueno, C.O. Age and density of eggs of Helicoverpa armigera influence on Trichogramma pretiosum parasitism. Acta Scientiarum 2017, 39, 513–520. [Google Scholar] [CrossRef][Green Version]
- Bai, B.; Luck, R.F.; Foster, L.; Stephens, B.; Jansen, J.A.M. The effect of host size on quality attributes of the egg parasitoid, Trichogramma pretiosum. Entomol. Exp. Appl. 1992, 64, 37–48. [Google Scholar] [CrossRef]
- Hansen, L.S.; Jensen, K.M.V. Effect of temperature on parasitism and host-feeding of Trichogramma turkestanica (Hymenoptera: Trichogrammatidae) on Ephestia kuehniella (Lepidoptera: Pyralidae). J. Econ. Entomol. 2002, 95, 50–56. [Google Scholar] [CrossRef]
- Carey, J.R. Applied Demography for Biologists, with Special Emphasis on Insects; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
| Parameter | H. armigera Egg Age (h) | ||
|---|---|---|---|
| 14 | 38 | 62 | |
| Number of parasitized eggs | 30.80 ± 2.18 a | 15.0 ± 1.77 b | 11.60 ± 1.34 b |
| Female development time (d) | 10.07 ± 0.05 c | 11.57 ± 0.08 b | 12.07 ± 0.10 b |
| Male development time (d) | 10.28 ± 0.07 b | 11.26 ± 0.12 a | 11.41 ± 0.19 a |
| Survival rate (%) | 94.10 ± 1.38 a | 93.4 ± 1.79 a | 88.50 ± 2.49 a |
| Sex ratio (% female) | 73.70 ± 3.99 a | 64.70 ± 3.45 b | 59.80 ± 3.72 c |
| Size (mm) of right hind tibia (female) | 0.180 ± 0.056 a | 0.172 ± 0.085 b | 0.160 ± 0.080 c |
| Size (mm) of right hind tibia (male) | 0.170 ± 0.073 a | 0.160 ± 0.092 b | 0.150 ± 0.030 c |
| Number of parasitoids/host egg | 1.2 ± 0.45 a | 1.1 ± 0.36 ab | 1.03 ± 0.43 b |
| Parameters | H. armigera Egg Age (h) | ||
|---|---|---|---|
| 14 | 38 | 62 | |
| Oviposition period (d) | 5.19 ± 0.237 a | 4.46 ± 0.124 b | 3.62 ± 0.090 c |
| Female longevity (d) | 6.92 ± 0.159 a | 5.017 ± 0.122 b | 3.99 ± 0.70 c |
| Male longevity (d) | 4.59 ± 0.183 a | 2.86 ± 0.124 b | 1.829 ± 0.128 c |
| Diel fecundity | 10.83 ± 0.657 a | 6.84 ± 0.67 b | 5.32 ± 0.36 b |
| Total fecundity | 67.73 ± 4.33 a | 31.90 ± 3.73 b | 19.5 ± 2.91 c |
| Parameters | H. armigera Egg Age (h) | ||
|---|---|---|---|
| 14 | 38 | 62 | |
| GRR (offspring/Generation) | 32.6904 ± 0.1041 a | 16.7116 ± 0.0482 b | 10.5308 ± 0.274 c |
| R0 (offspring/Generation) | 30.1514 ± 0.091 a | 14.8696 ± 0.041 b | 9.2195 ± 0.023 c |
| r (d−1) | 0.3112 ± 0.00026 a | 0.2288 ± 0.00023 b | 0.1771 ± 0.0002 c |
| λ (d−1) | 1.3651 ± 0.0003 a | 1.2571 ± 0.0029 b | 1.1938 ± 0.0002 c |
| T (d) | 10.9437 ± 0.0016 c | 11.7974 ± 0.0028 b | 12.5409 ± 0.0012 a |
| DT (d) | 2.2271 ± 0.0018 c | 3.0296 ± 0.0031 b | 3.9136 ± 0.0044 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atashi, N.; Shishehbor, P.; Seraj, A.A.; Rasekh, A.; Hemmati, S.A.; Riddick, E.W. Effects of Helicoverpa armigera Egg Age on Development, Reproduction, and Life Table Parameters of Trichogramma euproctidis. Insects 2021, 12, 569. https://doi.org/10.3390/insects12070569
Atashi N, Shishehbor P, Seraj AA, Rasekh A, Hemmati SA, Riddick EW. Effects of Helicoverpa armigera Egg Age on Development, Reproduction, and Life Table Parameters of Trichogramma euproctidis. Insects. 2021; 12(7):569. https://doi.org/10.3390/insects12070569
Chicago/Turabian StyleAtashi, Nazanin, Parviz Shishehbor, Ali Asghar Seraj, Arash Rasekh, Seyed Ali Hemmati, and Eric W. Riddick. 2021. "Effects of Helicoverpa armigera Egg Age on Development, Reproduction, and Life Table Parameters of Trichogramma euproctidis" Insects 12, no. 7: 569. https://doi.org/10.3390/insects12070569
APA StyleAtashi, N., Shishehbor, P., Seraj, A. A., Rasekh, A., Hemmati, S. A., & Riddick, E. W. (2021). Effects of Helicoverpa armigera Egg Age on Development, Reproduction, and Life Table Parameters of Trichogramma euproctidis. Insects, 12(7), 569. https://doi.org/10.3390/insects12070569







