Can Plant Lectins Help to Elucidate Insect Lectin-Mediated Immune Response?
Abstract
Simple Summary
Abstract
1. Introduction
2. Insect Innate Immunity
3. Insect Lectins
| Lectin Families | Insect Species | Gene/Protein a | Lectin Functions | Experiment Verification | Predicted by GO/Homology | References | |
|---|---|---|---|---|---|---|---|
| RNA b | Protein c | ||||||
| CTL | Aedes aegypti | AaeCTLs; CTL-20; mosGCTL-7 | Pathogen recognition; interacts with phosphatase; reduces exogenous toxin toxicity | + | + | [9,67,68,69] | |
| Tribolium castaneum | TcCTL6, TcCTL3 | Responds to pathogen infection; regulates AMP expression | + | [70,71] | |||
| Spodoptera litura | SliCTLs | Responds to pathogen infection | + | [21] | |||
| Mythimna separata | EPL | Promotes encapsulation | + | [72] | |||
| Ostrinia furnacalis | OfCTLs, OfIMLs | + | [73] | ||||
| Spodoptera exigua | Se-LLs, Se-BLLs | Responds to virus infection | + | [74] | |||
| Thitarodes xiaojinensis | CTL-S, CTL-X, IMLs | Responds to pathogen infection | + | [75] | |||
| Helicoverpa armigera | Ha-lectin, HaCTL | Regulates ecdysone and juvenile hormone signaling; regulates AMP expression; promotes phagocytosis | + | [76] | |||
| Drosophila melanogaster | Slf, DL2-3 | Organizes the cuticle layers; enhances encapsulation | + | [77,78] | |||
| Antheraea pernyi | Ap-CT | Binds PAMPs; activates PO | + | ||||
| Bombyx mori | BmIML, BmMBP, CTL-S3, BmEL-1, 2, 3 | Recognizes PAMPs; activates PO; promotes melanization; | + | ||||
| Hyphantria cunea | Hdd15 | + | |||||
| Periplaneta americana | LPS-BP | Responds to E. coli | + | ||||
| Heliothis virescens | MBL | + | Reviewed by [9] | ||||
| Manduca sexta | MsIML-1, 2, 3, 4 | Responds to pathogens; binds PAMPs; activates PO; enhances encapsulation | + | ||||
| Anopheles gambiae | AgamCTLs | Responds to pathogens | |||||
| Nilaparvata lugens | n.d. | ||||||
| Plutella xylostella | n.d. | ||||||
| Apis mellifera | n.d. | ||||||
| Acyrthosiphon pisum | n.d. | ||||||
| Chitinase like | Acyrthosiphon pisum | AcypiCht1 (IDGF homologue) | Expresses in bacteriocyte and midgut | + | [41] | ||
| Anopheles gambiae | AgIDGF2, AgIDGF4 | Expresses in different developmental stages and tissues | + | [79] | |||
| Bombyx mori | BmIDGF | Expresses in eggs, hemocytes, fat body, and silk gland | + | [80,81] | |||
| Drosophila melanogaster | IDGF1-6 | Participates in would healing and wing development | + | + | [38,39,82] | ||
| Nilaparvata lugens | NlIDGF | Expresses in female reproductive organs and fat body | + | [42] | |||
| Tribolium castaneum | TcIDGF2, 4 | Acts in adult eclosion | + | [83] | |||
| Plutella xylostella | PxIDGF | n.d. | + | [84] | |||
| Manduca sexta | MsIDGF1 | n.d. | + | [85] | |||
| Bemisia tabaci | BtIDGF1-3 | Highly abundant in adults | + | [86] | |||
| Galectin | Drosophila melanogaster | Dmgal | Expresses in hemocytes and in different developmental stages | + | [59,87] | ||
| Phlebotomus papatasi | PpGalec | Strong expression in adult female; binds pathogen | [61] | ||||
| Anopheles gambiae | Agalectin, GALE6-8 | Expresses in salivary gland; Responds to viral infection | + | + | [52,88] | ||
| Bombyx mori | BmGalectin-4 | Responds to bacteria in fertilized eggs; binds bacteria | + | [89] | |||
| Aedes aegypti | galectin-6, galectin-14 | Reduces exogenous toxin toxicity | + | [57,58] | |||
| Anopheles darlingi | n.d. | ||||||
| Anopheles stephensi | n.d. | ||||||
| Culex quinquefasciatus | n.d. | ||||||
| Drosophila ananassae | n.d. | ||||||
| Drosophila mojavensis | n.d. | ||||||
| Drosophila pseudoobscura | n.d. | ||||||
| Drosophila virilis | n.d. | + | Predicted by [87] | ||||
| Drosophila willistoni | n.d. | ||||||
| Drosophila yakuba | n.d. | ||||||
| Glossina morsitans | n.d. | ||||||
| Malus domestica | n.d. | ||||||
| malectin | Aedes aegypti | n.d. | + | [27,28] | |||
| Drosophila melanogaster | n.d. | + | |||||
| Calnexin/calreticulin | Bombyx mori | Calr/Canx; BmCNX | Responds to ER stress | + | + | [30,90] | |
| Drosophila melanogaster | Cnx | Regulates the function of sodium channel paralytic | + | [32] | |||
| F-type lectin | Drosophila melanogaster | Furrowed | Functions in planar cell polarity | + | [37] | ||
| Anopheles gambiae | n.d. | Reviewed by [36] | |||||
| I-type (immuno-globulin fold) | Drosophila melanogaster | hemolin | n.d. | + | Reviewed by [91] | ||
| Manduca sexta | HEM | Recognizes PAMPs; promotes nodulation, hemocyte aggregation, and phagocytosis | [63] | ||||
| Spodoptera exigua | SeHem | Acts as opsonin; regulates phagocytic activities and encapsulation | + | [62] | |||
| Plodia interpunctella | PiHem | Function related to gut bacteria | + | [92] | |||
| Bombyx mori | Hemolin | n.d. | + | [93] | |||
| Actias selene | As-HEM | Mediates immune response | + | [94] | |||
| Antheraea pernyi | Hemolin | Regulates innate immunity | + | [95] | |||
| L-type | Drosophila melanogaster | ERGIC-53 homolog | n.d. | [48], reviewed by [96] | |||
| Bombyx mori | ERGIC-53 | Responds to ER stress | + | [50] | |||
| R-type (ricin B type) | Drosophila melanogaster | lectin domain of GalNAc Transferase | Binds glycopeptides | + | [97], reviewed by [65] | ||
4. Endogenous Insect Lectins as Immune Modulators
4.1. Pathogen Recognition
4.2. Lectin-Induced Cellular Immunity
4.2.1. Phagocytosis
4.2.2. Encapsulation
4.3. Lectin-Induced AMP Expression
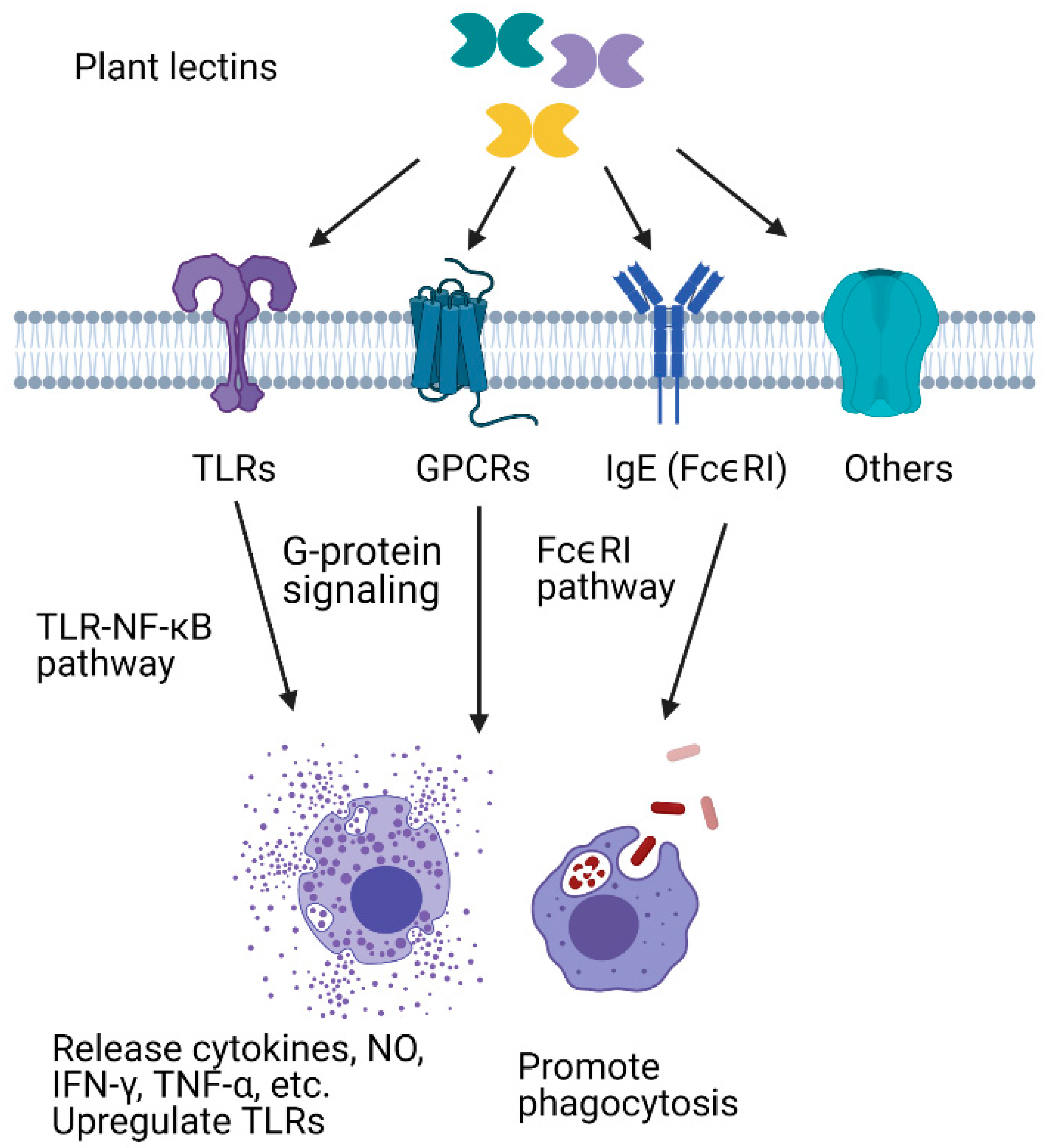
5. Plant Lectins as Exogenous Immune Modulators
5.1. Immunomodulatory Effects of Plant Lectins in Mammalian Cells
5.1.1. Plant Lectins Interact with Immune-Related Proteins and Enhance Pathogen Recognition
5.1.2. Plant Lectins Enhance Phagocytosis
5.1.3. Plant Lectins Promote the Release of Cytokines and Other Effectors
5.2. Plant Lectins as a Tool to Study Insect Immune Responses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peumans, W.J.; Van Damme, E.J.M. Lectins as plant defense proteins. Plant Physiol. 1995, 109, 347–352. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Lectins: Cell-agglutinating and sugar-specific proteins. Science 1972, 177, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Esch, L.; Schaffrath, U. An update on jacalin-like lectins and their role in plant defense. Int. J. Mol. Sci. 2017, 18, 1592. [Google Scholar] [CrossRef]
- Mukherjee, S.; Zheng, H.; Derebe, M.G.; Callenberg, K.M.; Partch, C.L.; Rollins, D.; Propheter, D.C.; Rizo, J.; Grabe, M.; Jiang, Q.-X. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 2014, 505, 103–107. [Google Scholar] [CrossRef]
- Hartmann, M.; Lindhorst, T.K. The bacterial lectin FimH, a target for drug discovery–carbohydrate inhibitors of type 1 fimbriae-mediated bacterial adhesion. Eur. J. Org. Chem. 2011, 2011, 3583–3609. [Google Scholar] [CrossRef]
- Caramelo, J.J.; Parodi, A.J. Getting in and out from calnexin/calreticulin cycles. J. Biol. Chem. 2008, 283, 10221. [Google Scholar] [CrossRef]
- Cha, S.-K.; Ortega, B.; Kurosu, H.; Rosenblatt, K.P.; Kuro-o, M.; Huang, C.-L. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. USA 2008, 105, 9805–9810. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, K.; Van Damme, E.J.M. Protein-carbohydrate interactions as part of plant defense and animal immunity. Molecules 2015, 20, 9029–9053. [Google Scholar] [CrossRef]
- Xia, X.; You, M.; Rao, X.-J.; Yu, X.-Q. Insect C-type lectins in innate immunity. Dev. Comp. Immunol. 2018, 83, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Blow, F.; Douglas, A.E. The hemolymph microbiome of insects. J. Insect Physiol. 2019, 115, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S. The role of the immune system beyond the fight against infection. Immunol. Cardiovasc. Homeost. Pathol. 2017, 1003, 3–14. [Google Scholar]
- Ali Mohammadie Kojour, M.; Han, Y.S.; Jo, Y.H. An overview of insect innate immunity. Entomol. Res. 2020, 50, 282–291. [Google Scholar] [CrossRef]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Müller, U.; Vogel, P.; Alber, G.; Schaub, G.A. The innate immune system of mammals and insects. Trends Innate Immun. 2008, 15, 21–44. [Google Scholar]
- Vlisidou, I.; Wood, W. Drosophila blood cells and their role in immune responses. FEBS J. 2015, 282, 1368–1382. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Vigneron, A.; Broderick, N.A.; Wu, Y.; Sun, J.S.; Carlson, J.R.; Aksoy, S.; Weiss, B.L. Symbiont-induced odorant binding proteins mediate insect host hematopoiesis. eLife 2017, 6, e19535. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Drickamer, K.; Schnaar, R.L. Discovery and Classification of Glycan-Binding Proteins. In Essentials of GlycoBiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; Chapter 28. [Google Scholar]
- Nagae, M.; Yamaguchi, Y. Structural aspects of carbohydrate recognition mechanisms of C-type lectins. C-Type Lectins Immune Homeost. 2019, 147–176. [Google Scholar] [CrossRef]
- Dodd, R.B.; Drickamer, K. Lectin-like proteins in model organisms: Implications for evolution of carbohydrate-binding activity. Glycobiology 2001, 11, 71R–79R. [Google Scholar] [CrossRef]
- Lu, Y.; Su, F.; Zhu, K.; Zhu, M.; Li, Q.; Hu, Q.; Zhang, J.; Zhang, R.; Yu, X.-Q. Comparative genomic analysis of C-type lectin-domain genes in seven holometabolous insect species. Insect Biochem. Mol. Biol. 2020, 126, 103451. [Google Scholar] [CrossRef]
- Cummings, R.D.; McEver, R.P. C-Type Lectins In: Varki, A. Essentials Glycobiol. 2017, 4, 435–452. [Google Scholar]
- Qin, S.-Y.; Hu, D.; Matsumoto, K.; Takeda, K.; Matsumoto, N.; Yamaguchi, Y.; Yamamoto, K. Malectin forms a complex with ribophorin I for enhanced association with misfolded glycoproteins. J. Biol. Chem. 2012, 287, 38080–38089. [Google Scholar] [CrossRef]
- Braakman, I.; Hebert, D.N. Protein folding in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5, a013201. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Wang, B.J.; Liu, M.; Jiang, K.Y.; Wang, L. The first identification of a malectin gene (CfMal) in scallop Chlamys farreri: Sequence features and expression profiles. Invertebr. Surviv. J. 2019, 16, 25–33. [Google Scholar]
- Sellaththurai, S.; Shanaka, K.; Liyanage, D.S.; Yang, H.; Priyathilaka, T.T.; Lee, J. Molecular and functional insights into a novel teleost malectin from big-belly seahorse Hippocampus Abdominalis. Fish Shellfish Immunol. 2020, 99, 483–494. [Google Scholar] [CrossRef]
- Schallus, T.; Jaeckh, C.; Fehér, K.; Palma, A.S.; Liu, Y.; Simpson, J.C.; Mackeen, M.; Stier, G.; Gibson, T.J.; Feizi, T. Malectin: A novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Mol. Biol. Cell 2008, 19, 3404–3414. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.S.; Liu, Y.; Muhle-Goll, C.; Butters, T.D.; Zhang, Y.; Childs, R.; Chai, W.; Feizi, T. Multifaceted approaches including neoglycolipid oligosaccharide microarrays to ligand discovery for malectin. Methods Enzymol. 2010, 478, 265–286. [Google Scholar]
- Kim, S.-R.; Lee, K.-S.; Kim, I.; Kang, S.-W.; Nho, S.-K.; Sohn, H.-D.; Jin, B.-R. Molecular cloning of a cDNA encoding putative calreticulin from the silkworm, Bombyx mori. Int. J. Ind. Entomol. 2003, 6, 93–97. [Google Scholar]
- Lee, K.R.; Kim, S.-W.; Kim, Y.K.; Kwon, K.; Choi, J.-S.; Yu, K.; Kwon, O.-Y. Silkworm hemolymph down-regulates the expression of endoplasmic reticulum chaperones under radiation-irradiation. Int. J. Mol. Sci. 2011, 12, 4456–4464. [Google Scholar] [CrossRef]
- Rosenbaum, E.E.; Hardie, R.C.; Colley, N.J. Calnexin is essential for rhodopsin maturation, Ca2+ regulation, and photoreceptor cell survival. Neuron 2006, 49, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, C.; Yu, T.-M.; Ou, J.; Rui, M.; Zhai, Y.; He, Y.; Xue, L.; Ho, M.S. Molecular chaperone calnexin regulates the function of Drosophila sodium channel paralytic. Front. Mol. Neurosci. 2017, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Ramya, T.N.C. Nature-inspired engineering of an F-type lectin for increased binding strength. Glycobiology 2018, 28, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, R.; Khatri, I.; Subramanian, S.; Ramya, T.N.C. Prevalence of the F-type lectin domain. Glycobiology 2015, 25, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Odom-Crespo, E.W. F-Type Lectins: Biochemical, Genetic, and Topological Characterization of a Novel Lectin Family in Lower Vertebrates. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2004. [Google Scholar]
- Vasta, G.R.; Amzel, L.M.; Bianchet, M.A.; Cammarata, M.; Feng, C.; Saito, K. F-type lectins: A highly diversified family of fucose-binding proteins with a unique sequence motif and structural fold, involved in self/non-self-recognition. Front. Immunol. 2017, 8, 1648. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.-L.; Mlodzik, M. The Drosophila selectin furrowed mediates intercellular planar cell polarity interactions via frizzled stabilization. Dev. Cell 2013, 26, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, L.; Broz, V.; Arefin, B.; Maaroufi, H.O.; Hurychova, J.; Strnad, H.; Zurovec, M.; Theopold, U. The Drosophila chitinase-like protein IDGF3 is involved in protection against nematodes and in wound healing. J. Innate Immun. 2016, 8, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Broz, V.; Kucerova, L.; Rouhova, L.; Fleischmannova, J.; Strnad, H.; Bryant, P.J.; Zurovec, M. Drosophila imaginal disc growth factor 2 is a trophic factor involved in energy balance, detoxification, and innate immunity. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Ganbaatar, O.; Cao, B.; Zhang, Y.; Bao, D.; Bao, W.; Wuriyanghan, H. Knockdown of Mythimna separata chitinase genes via bacterial expression and oral delivery of RNAi effectors. BMC Biotechnol. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Nakabachi, A.; Shigenobu, S.; Miyagishima, S. Chitinase-like proteins encoded in the genome of the pea aphid, Acyrthosiphon pisum. Insect Mol. Biol. 2010, 19, 175–185. [Google Scholar] [CrossRef]
- Xi, Y.; Pan, P.; Ye, Y.; Yu, B.; Xu, H.; Zhang, C. Chitinase-like gene family in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2015, 24, 29–40. [Google Scholar] [CrossRef]
- Zhu, Q.; Arakane, Y.; Banerjee, D.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Domain organization and phylogenetic analysis of the chitinase-like family of proteins in three species of insects. Insect Biochem. Mol. Biol. 2008, 38, 452–466. [Google Scholar] [CrossRef]
- Liu, T.; Guo, X.; Bu, Y.; Zhou, Y.; Duan, Y.; Yang, Q. Structural and biochemical insights into an insect gut-specific chitinase with antifungal activity. Insect Biochem. Mol. Biol. 2020, 119, 103326. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, L.; Zhou, Y.; Jiang, X.; Duan, Y.; Yang, Q. Structure, catalysis, and inhibition of OfChi-h, the Lepidoptera-exclusive insect chitinase. J. Biol. Chem. 2017, 292, 2080–2088. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, T.; Huang, W.; Luo, P.; Huo, D.; Yun, L.; Hu, C.; Cai, Y. A new L-type lectin (LvLTLC1) from the shrimp Litopenaeus vannamei facilitates the clearance of Vibrio harveyi. Fish Shellfish Immunol. 2018, 73, 185–191. [Google Scholar] [CrossRef]
- Satoh, T.; Kato, K. Recombinant expression and purification of animal intracellular L-type lectins. In Lectin Purification and Analysis; Springer: Berlin/Heidelberg, Germany, 2020; pp. 21–28. [Google Scholar]
- Appenzeller, C.; Andersson, H.; Kappeler, F.; Hauri, H.-P. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat. Cell Biol. 1999, 1, 330–334. [Google Scholar] [CrossRef]
- Brown, N.H.; Gregory, S.L.; Rickoll, W.L.; Fessler, L.I.; Prout, M.; White, R.A.H.; Fristrom, J.W. Talin is essential for integrin function in Drosophila. Dev. Cell 2002, 3, 569–579. [Google Scholar] [CrossRef]
- Mao, T.; Cheng, X.; Fang, Y.; Li, M.; Lu, Z.; Qu, J.; Chen, J.; Wang, H.; Li, F.; Li, B. Induction of ER stress, antioxidant and detoxification response by sublethal doses of chlorantraniliprole in the silk gland of silkworm, Bombyx mori. Pestic. Biochem. Physiol. 2020, 170, 104685. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Alvarez, L.; Ortega, E. The many roles of galectin-3, a multifaceted molecule, in innate immune responses against pathogens. Mediat. Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef] [PubMed]
- Francischetti, I.M.B.; Ma, D.; Andersen, J.F.; Ribeiro, J.M.C. Evidence for a lectin specific for sulfated glycans in the salivary gland of the malaria vector, Anopheles gambiae. PLoS ONE 2014, 9, e107295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cerliani, J.P.; Stowell, S.R.; Mascanfroni, I.D.; Arthur, C.M.; Cummings, R.D.; Rabinovich, G.A. Expanding the universe of cytokines and pattern recognition receptors: Galectins and glycans in innate immunity. J. Clin. Immunol. 2011, 31, 10–21. [Google Scholar] [CrossRef]
- Cummings, R.D.; Liu, F.T.; Vasta, G.R. Galectins. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; Chapter 36. [Google Scholar]
- Rabinovich, G.A.; Van Kooyk, Y.; Cobb, B.A. Glycobiology of immune responses. Ann. N. Y. Acad. Sci. 2012, 1253, 1–15. [Google Scholar] [CrossRef]
- Chiu, Y.-P.; Sun, Y.-C.; Qiu, D.-C.; Lin, Y.-H.; Chen, Y.-Q.; Kuo, J.-C.; Huang, J. Liquid-liquid phase separation and extracellular multivalent interactions in the tale of galectin-3. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Hu, X.-H.; Wu, S.-Q.; Batool, K.; Chowdhury, M.; Lin, Y.; Zhang, J.; Gill, S.S.; Guan, X.; Yu, X.-Q. Aedes aegypti galectin competes with Cry11Aa for binding to ALP1 to modulate Cry toxicity. J. Agric. Food Chem. 2018, 66, 13435–13443. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, H.; Xu, J.; Zhao, G.; Huang, X.; Liu, J.; Batool, K.; Wu, C.; Wu, S.; Huang, E. Function of Aedes aegypti galectin-6 in modulation of Cry11Aa toxicity. Pestic. Biochem. Physiol. 2020, 162, 96–104. [Google Scholar] [CrossRef]
- Pace, K.E.; Baum, L.G. Insect galectins: Roles in immunity and development. Glycoconj. J. 2002, 19, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Pace, K.E.; Lebestky, T.; Hummel, T.; Arnoux, P.; Kwan, K.; Baum, L.G. Characterization of a novel Drosophila melanogaster galectin: Expression in developing immune, neural, and muscle tissues. J. Biol. Chem. 2002, 277, 13091–13098. [Google Scholar] [CrossRef]
- Kamhawi, S.; Ramalho-Ortigao, M.; Pham, V.M.; Kumar, S.; Lawyer, P.G.; Turco, S.J.; Barillas-Mury, C.; Sacks, D.L.; Valenzuela, J.G. A role for insect galectins in parasite survival. Cell 2004, 119, 329–341. [Google Scholar] [CrossRef]
- Jung, J.; Sajjadian, S.M.; Kim, Y. Hemolin, an immunoglobulin-like peptide, opsonizes nonself targets for phagocytosis and encapsulation in Spodoptera exigua, a lepidopteran insect. J. Asia. Pac. Entomol. 2019, 22, 947–956. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Gökçen, F.; Felföldi, G.; Millichap, P.J.; Trenczek, T.E.; Ffrench-Constant, R.H.; Reynolds, S.E. The immunoglobulin family protein Hemolin mediates cellular immune responses to bacteria in the insect Manduca sexta. Cell. Microbiol. 2007, 9, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-D.; Gastinel, L.N.; Vaughn, D.E.; Faye, I.; Poon, P.; Bjorkman, P.J. Crystal structure of hemolin: A horseshoe shape with implications for homophilic adhesion. Science 1998, 281, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D.L.; Schnaar, R. R-Type Lectins. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; Chapter 31. [Google Scholar]
- Hagen, F.K.; Nehrke, K. cDNA Cloning and expression of a family of UDP-N-acetyl-D-galactosamine: Polypeptide N-acetylgalactosaminyltransferase sequence homologs from Caenorhabditis elegans. J. Biol. Chem. 1998, 273, 8268–8277. [Google Scholar] [CrossRef] [PubMed]
- Adelman, Z.N.; Myles, K.M. The C-type lectin domain gene family in Aedes aegypti and their role in arbovirus infection. Viruses 2018, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Batool, K.; Alam, I.; Zhao, G.; Wang, J.; Xu, J.; Yu, X.; Huang, E.; Guan, X.; Zhang, L. C-Type lectin-20 interacts with ALP1 receptor to reduce cry toxicity in Aedes aegypti. Toxins 2018, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Qian, Y.; Jung, Y.-S.; Zhou, B.; Cao, R.; Shen, T.; Shao, D.; Wei, J.; Ma, Z.; Chen, P. mosGCTL-7, a C-type lectin protein, mediates Japanese encephalitis virus infection in mosquitoes. J. Virol. 2017, 91, e01348-16. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Feng, F.; Li, J.; Mao, J.; Ning, M.; Song, X.; Xie, J.; Tang, J.; Li, B. AC-type lectin with a single carbohydrate-recognition domain involved in the innate immune response of Tribolium castaneum. Insect Mol. Biol. 2019, 28, 649–661. [Google Scholar] [CrossRef]
- Bi, J.; Ning, M.; Li, J.; Zhang, P.; Wang, L.; Xu, S.; Zhong, Y.; Wang, Z.; Song, Q.; Li, B. AC-type lectin with dual-CRD from Tribolium castaneum is induced in response to bacterial challenge. Pest Manag. Sci. 2020, 76, 3965–3974. [Google Scholar] [CrossRef]
- Ishihara, T.; Maruyama, Y.; Furukawa, S. Gene expression and molecular characterization of a novel C-type lectin, encapsulation promoting lectin (EPL), in the rice armyworm, Mythimna separata. Insect Biochem. Mol. Biol. 2017, 89, 51–57. [Google Scholar] [CrossRef]
- Shen, D.; Wang, L.; Ji, J.; Liu, Q.; An, C. Identification and characterization of C-type lectins in Ostrinia furnacalis (Lepidoptera: Pyralidae). J. Insect Sci. 2018, 18, 18. [Google Scholar] [CrossRef]
- Gasmi, L.; Jakubowska, A.K.; Ferré, J.; Ogliastro, M.; Herrero, S. Characterization of two groups of Spodoptera exigua Hübner (Lepidoptera: Noctuidae) C-type lectins and insights into their role in defense against the densovirus JcDV. Arch. Insect Biochem. Physiol. 2018, 97, e21432. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, J.; Zhang, H.; Zhou, G.; Ni, R.; Zhao, Y.; Qin, Q.; Zou, Z. Comparative analysis of C-type lectin domain proteins in the ghost moth, Thitarodes xiaojinensis (Lepidoptera: Hepialidae). Insect Sci. 2019, 26, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, G.; Zhuo, X.; Liu, Y.; Tang, L.; Liu, X.; Wang, J. C-type lectin-mediated microbial homeostasis is critical for Helicoverpa armigera larval growth and development. PLoS Pathog. 2020, 16, e1008901. [Google Scholar] [CrossRef] [PubMed]
- Zuber, R.; Shaik, K.S.; Meyer, F.; Ho, H.-N.; Speidel, A.; Gehring, N.; Bartoszewski, S.; Schwarz, H.; Moussian, B. The putative C-type lectin Schlaff ensures epidermal barrier compactness in Drosophila. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Ao, J.; Ling, E.; Yu, X.Q. Drosophila C-type lectins enhance cellular encapsulation. Mol. Immunol. 2007, 44, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Ma, E.; Zhu, K.Y. Comparative genomic analysis of chitinase and chitinase-like genes in the African malaria mosquito (Anopheles gambiae). PLoS ONE 2011, 6, e19899. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, K.; Xia, H.; Yao, Q.; Gao, L.; Lü, P.; He, Y.; Wang, L. Molecular cloning, expression and characterization of BmIDGF gene from Bombyx mori. Z. Naturforsch. C 2010, 65, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lü, P.; Wang, Y.; Yin, L.; Ma, H.; Ma, G.; Chen, K.; He, Y. In silico identification of novel chitinase-like proteins in the silkworm, Bombyx mori, genome. J. Insect Sci. 2012, 12, 150. [Google Scholar] [CrossRef]
- Pesch, Y.-Y.; Riedel, D.; Patil, K.R.; Loch, G.; Behr, M. Chitinases and Imaginal disc growth factors organize the extracellular matrix formation at barrier tissues in insects. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Muthukrishnan, S. Insect chitinase and chitinase-like proteins. Cell. Mol. life Sci. 2010, 67, 201–216. [Google Scholar] [CrossRef]
- Liao, Z.H.; Kuo, T.C.; Kao, C.H.; Chou, T.M.; Kao, Y.H.; Huang, R.N. Identification of the chitinase genes from the diamondback moth, Plutella xylostella. Bull. Entomol. Res. 2016, 106, 769. [Google Scholar] [CrossRef]
- Tetreau, G.; Cao, X.; Chen, Y.-R.; Muthukrishnan, S.; Jiang, H.; Blissard, G.W.; Kanost, M.R.; Wang, P. Overview of chitin metabolism enzymes in Manduca sexta: Identification, domain organization, phylogenetic analysis and gene expression. Insect Biochem. Mol. Biol. 2015, 62, 114–126. [Google Scholar] [CrossRef]
- Peng, Z.; Ren, J.; Su, Q.; Zeng, Y.; Tian, L.; Wang, S.; Wu, Q.; Liang, P.; Xie, W.; Zhang, Y. Genome-wide identification and analysis of chitinase-like gene family in Bemisia tabaci (Hemiptera: Aleyrodidae). Insects 2021, 12, 254. [Google Scholar] [CrossRef]
- Sackton, T.B.; Lazzaro, B.P.; Clark, A.G. Rapid expansion of immune-related gene families in the house fly, Musca domestica. Mol. Biol. Evol. 2017, 34, 857–872. [Google Scholar] [CrossRef]
- Waldock, J.; Olson, K.E.; Christophides, G.K. Anopheles gambiae antiviral immune response to systemic O’nyong-nyong infection. PLoS Negl. Trop. Dis. 2012, 6, e1565. [Google Scholar] [CrossRef]
- Rao, X.-J.; Wu, P.; Shahzad, T.; Liu, S.; Chen, L.; Yang, Y.-F.; Shi, Q.; Yu, X.-Q. Characterization of a dual-CRD galectin in the silkworm Bombyx mori. Dev. Comp. Immunol. 2016, 60, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Kusakabe, T.; Xu, J.; Li, Z.; Shirai, S.; Mon, H.; Morokuma, D.; Lee, J.M. Roles of silkworm endoplasmic reticulum chaperones in the secretion of recombinant proteins expressed by baculovirus system. Mol. Cell. Biochem. 2015, 409, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I.; Cytryńska, M.; Zdybicka-Barabas, A.; Kordaczuk, J. Insect Defense Proteins and Peptides. Vertebr. Invertebr. Respir. Proteins Lipoproteins Other Body Fluid Proteins 2020, 81–121. [Google Scholar] [CrossRef]
- Orozco-Flores, A.A.; Valadez-Lira, J.A.; Oppert, B.; Gomez-Flores, R.; Tamez-Guerra, R.; Rodríguez-Padilla, C.; Tamez-Guerra, P. Regulation by gut bacteria of immune response, Bacillus thuringiensis susceptibility and hemolin expression in Plodia interpunctella. J. Insect Physiol. 2017, 98, 275–283. [Google Scholar] [CrossRef]
- Aathmanathan, V.S.; Jothi, N.; Prajapati, V.K.; Krishnan, M. Investigation of immunogenic properties of Hemolin from silkworm, Bombyx mori as carrier protein: An immunoinformatic approach. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Qian, C.; Wang, F.; Zhu, B.; Wang, L.; Wei, G.; Sun, Y.; Li, S.; Liu, C. Identification of a hemolin protein from Actias selene mediates immune response to pathogens. Int. Immunopharmacol. 2017, 42, 74–80. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, L.; Sun, Y.; Wang, L.; Qian, C.; Wei, G.; Zhu, B.-J.; Liu, C.-L. Gene expression patterns in response to pathogen challenge and interaction with hemolin suggest that the Yippee protein of Antheraea pernyi is involved in the innate immune response. J. Invertebr. Pathol. 2016, 138, 10–17. [Google Scholar] [CrossRef]
- Hauri, H.-P.; Appenzeller, C.; Kuhn, F.; Nufer, O. Lectins and traffic in the secretory pathway. FEBS Lett. 2000, 476, 32–37. [Google Scholar] [CrossRef]
- Pedersen, J.W.; Bennett, E.P.; Katrine, T.-B.S.; Meldal, M.; Holmér, A.P.; Blixt, O.; Cló, E.; Levery, S.B.; Clausen, H.; Wandall, H.H. Lectin domains of polypeptide GalNAc transferases exhibit glycopeptide binding specificity. J. Biol. Chem. 2011, 286, 32684–32696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, M.; Liu, X.; Xia, H.; Chen, K. Peptidoglycan recognition proteins in insect immunity. Mol. Immunol. 2019, 106, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhuo, X.-R.; Tang, L.; Liu, X.-S.; Wang, Y.-F.; Wang, G.-X.; Yu, X.-Q.; Wang, J.-L. C-type lectin interacting with β-integrin enhances hemocytic encapsulation in the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 2017, 86, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Ling, E.; Yu, X.Q. Cellular encapsulation and melanization are enhanced by immulectins, pattern recognition receptors from the tobacco hornworm Manduca sexta. Dev. Comp. Immunol. 2006, 30, 289–299. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, J.-L.; Cheng, Y.; Wang, J.-X.; Zou, Z. Pattern recognition receptors from lepidopteran insects and their biological functions. Dev. Comp. Immunol. 2020, 108, 103688. [Google Scholar] [CrossRef]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Melcarne, C.; Lemaitre, B.; Kurant, E. Phagocytosis in Drosophila: From molecules and cellular machinery to physiology. Insect Biochem. Mol. Biol. 2019, 109, 1–12. [Google Scholar] [CrossRef]
- Tian, Y.-Y.; Liu, Y.; Zhao, X.-F.; Wang, J.-X. Characterization of a C-type lectin from the cotton bollworm, Helicoverpa armigera. Dev. Comp. Immunol. 2009, 33, 772–779. [Google Scholar] [CrossRef]
- Nauta, A.J.; Raaschou-Jensen, N.; Roos, A.; Daha, M.R.; Madsen, H.O.; Borrias-Essers, M.C.; Ryder, L.P.; Koch, C.; Garred, P. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur. J. Immunol. 2003, 33, 2853–2863. [Google Scholar] [CrossRef]
- Wang, X.-W.; Zhao, X.-F.; Wang, J.-X. C-type lectin binds to β-integrin to promote hemocytic phagocytosis in an invertebrate. J. Biol. Chem. 2014, 289, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, S.-C.; Li, Y.-L.; Li, J.; Liu, H.-P. Hemocyte-mediated phagocytosis in crustaceans. Front. Immunol. 2020, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Rizki, T.M.; Rizki, R.M. Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev. Comp. Immunol. 1992, 16, 103–110. [Google Scholar] [CrossRef]
- Fauvarque, M.-O.; Williams, M.J. Drosophila cellular immunity: A story of migration and adhesion. J. Cell Sci. 2011, 124, 1373–1382. [Google Scholar] [CrossRef]
- Yu, D.-H.; Qu, C.-K.; Henegariu, O.; Lu, X.; Feng, G.-S. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 1998, 273, 21125–21131. [Google Scholar] [CrossRef]
- Cheng, A.; Bal, G.S.; Kennedy, B.P.; Tremblay, M.L. Attenuation of adhesion-dependent signaling and cell spreading in transformed fibroblasts lacking protein tyrosine phosphatase-1B. J. Biol. Chem. 2001, 276, 25848–25855. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Matsumoto, H.; Furihata, S.; Ryuda, M.; Tanaka, H.; Sung, E.J.; Bird, G.S.; Zhou, Y.; Shears, S.B.; Hayakawa, Y. Switching between humoral and cellular immune responses in Drosophila is guided by the cytokine GBP. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Faivre-Sarrailh, C.; Banerjee, S.; Li, J.; Hortsch, M.; Laval, M.; Bhat, M.A. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development 2004, 131, 4931–4942. [Google Scholar] [CrossRef]
- Clark, K.D.; Strand, M.R. Hemolymph melanization in the silkmoth Bombyx mori involves formation of a high molecular mass complex that metabolizes tyrosine. J. Biol. Chem. 2013, 288, 14476–14487. [Google Scholar] [CrossRef]
- Tang, H. Regulation and function of the melanization reaction in Drosophila. Fly 2009, 3, 105–111. [Google Scholar] [CrossRef]
- Siva-Jothy, M.T.; Thompson, J.J.W. Short-term nutrient deprivation affects immune function. Physiol. Entomol. 2002, 27, 206–212. [Google Scholar] [CrossRef]
- Shu, M.; Mang, D.; Fu, G.S.; Tanaka, S.; Endo, H.; Kikuta, S.; Sato, R. Mechanisms of nodule-specific melanization in the hemocoel of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2016, 70, 10–23. [Google Scholar] [CrossRef]
- Imler, J.-L.; Bulet, P. Antimicrobial peptides in Drosophila: Structures, activities and gene regulation. Mech. Epithel. Def. 2005, 86, 1–21. [Google Scholar]
- Hanson, M.A.; Lemaitre, B. New insights on Drosophila antimicrobial peptide function in host defense and beyond. Curr. Opin. Immunol. 2020, 62, 22–30. [Google Scholar] [CrossRef]
- Parvy, J.-P.; Yu, Y.; Dostalova, A.; Kondo, S.; Kurjan, A.; Bulet, P.; Lemaitre, B.; Vidal, M.; Cordero, J.B. The antimicrobial peptide defensin cooperates with tumour necrosis factor to drive tumour cell death in Drosophila. eLife 2019, 8, e45061. [Google Scholar] [CrossRef]
- Yakovlev, A.Y.; Nesin, A.P.; Simonenko, N.P.; Gordya, N.A.; Tulin, D.V.; Kruglikova, A.A.; Chernysh, S.I. Fat body and hemocyte contribution to the antimicrobial peptide synthesis in Calliphora vicina R.-D. (Diptera: Calliphoridae) larvae. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kallio, J.; Leinonen, A.; Ulvila, J.; Valanne, S.; Ezekowitz, R.A.; Rämet, M. Functional analysis of immune response genes in Drosophila identifies JNK pathway as a regulator of antimicrobial peptide gene expression in S2 cells. Microbes Infect. 2005, 7, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Tanji, T.; Shiraishi, H.; Natori, S.; Ohashi-Kobayashi, A. Differential activation of the lectin and antimicrobial peptide genes in Sarcophaga peregrina (the flesh fly). Arch. Insect Biochem. Physiol. 2008, 69, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Xiao, X.; Liu, Y.; Zhang, R.; Liu, J.; Liu, Q.; Wang, P.; Cheng, G. Mosquito C-type lectins maintain gut microbiome homeostasis. Nat. Microbiol. 2016, 1, 1–11. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, Y.; Wang, P.; Xiao, X. Mosquito defense strategies against viral infection. Trends Parasitol. 2016, 32, 177–186. [Google Scholar] [CrossRef]
- Pereira-da-Silva, G.; Moreno, A.N.; Marques, F.; Oliver, C.; Jamur, M.C.; Panunto-Castelo, A.; Roque-Barreira, M.C. Neutrophil activation induced by the lectin KM+ involves binding to CXCR2. Biochim. Biophys. Acta General Subj. 2006, 1760, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Toledo, K.A.; Scwartz, C.; Oliveira, A.F.; Conrado, M.C.A.V.; Bernardes, E.S.; Fernandes, L.C.; Roque-Barreira, M.C.; Pereira-da-Silva, G.; Moreno, A.N. Neutrophil activation induced by ArtinM: Release of inflammatory mediators and enhancement of effector functions. Immunol. Lett. 2009, 123, 14–20. [Google Scholar] [CrossRef]
- Ganiko, L.; Martins, A.R.; Freymüller, E.; Mortara, R.A.; Roque-Barreira, M.-C. Lectin KM+-induced neutrophil haptotaxis involves binding to laminin. Biochim. Biophys. Acta General Subj. 2005, 1721, 152–163. [Google Scholar] [CrossRef]
- Moreno, A.N.; Jamur, M.C.; Oliver, C.; Roque-Barreira, M.C. Mast cell degranulation induced by lectins: Effect on neutrophil recruitment. Int. Arch. Allergy Immunol. 2003, 132, 221–230. [Google Scholar] [CrossRef]
- Coltri, K.C.; Oliveira, L.L.; Pinzan, C.F.; Vendruscolo, P.E.; Martinez, R.; Goldman, M.H.; Panunto-Castelo, A.; Roque-Barreira, M.-C. Therapeutic administration of KM+ lectin protects mice against Paracoccidioides brasiliensis infection via interleukin-12 production in a toll-like receptor 2-dependent mechanism. Am. J. Pathol. 2008, 173, 423–432. [Google Scholar] [CrossRef]
- Roudaire, T.; Héloir, M.-C.; Wendehenne, D.; Zadoroznyj, A.; Dubrez, L.; Poinssot, B. Cross kingdom immunity: The role of immune receptors and downstream signaling in animal and plant cell death. Front. Immunol. 2021, 11, 3894. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.C.N.; Correia, M.T.D.S. Plant lectins and Toll-like receptors: Implications for therapy of microbial infections. Front. Microbiol. 2014, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Azevedo, R.; Roque-Barreira, M.-C.; Gay, N.J. Targeting and recognition of toll-like receptors by plant and pathogen lectins. Front. Immunol. 2017, 8, 1820. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Jin, H.; Ke, X.; Man, Z.; Wang, Y.; Tan, Y.; Lu, Z.; Liu, H. The role of serotonin in Concanavalin A-induced liver injury in mice. Oxid. Med. Cell. Longev. 2020, 2020, 7504521. [Google Scholar] [CrossRef]
- Sodhi, A.; Tarang, S.; Kesherwani, V. Concanavalin A induced expression of Toll-like receptors in murine peritoneal macrophages in vitro. Int. Immunopharmacol. 2007, 7, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.A.; Carvalho, F.C.; Ruas, L.P.; Ricci-Azevedo, R.; Roque-Barreira, M.C. The immunomodulatory effect of plant lectins: A review with emphasis on ArtinM properties. Glycoconj. J. 2013, 30, 641–657. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.C.N.; Alves, N.M.P.; de Castro, M.C.A.B.; Pereira, V.R.A.; da Paz, N.V.N.; Coelho, L.C.B.B.; de Figueiredo, R.C.B.Q.; dos Santos Correia, M.T. Immunomodulatory effects of pCramoll and rCramoll on peritoneal exudate cells (PECs) infected and non-infected with Staphylococcus aureus. Int. J. Biol. Macromol. 2015, 72, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Jandú, J.J.; Costa, M.C.; Santos, J.R.A.; Andrade, F.M.; Magalhães, T.F.; Silva, M.V.; Castro, M.C.A.B.; Coelho, L.C.B.B.; Gomes, A.G.; Paixão, T.A. Treatment with pCramoll alone and in combination with fluconazole provides therapeutic benefits in C. gattii infected mice. Front. Cell. Infect. Microbiol. 2017, 7, 211. [Google Scholar] [CrossRef] [PubMed]
- Clement, F.; Venkatesh, Y.P. Dietary garlic (Allium sativum) lectins, ASA I and ASA II, are highly stable and immunogenic. Int. Immunopharmacol. 2010, 10, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Pereira-da-Silva, G.; Caroline Carvalho, F.; Cristina Roque-Barreira, M. Neutrophil activation induced by plant lectins: Modulation of inflammatory processes. Inflamm. Allergy Drug Targets Former. Curr. Drug Targets Inflamm. Allergy 2012, 11, 433–441. [Google Scholar] [CrossRef]
- Oliveira Brito, P.K.M.; Gonçalves, T.E.; Fernandes, F.F.; Miguel, C.B.; Rodrigues, W.F.; Lazo Chica, J.E.; Roque-Barreira, M.C.; da Silva, T.A. Systemic effects in naïve mice injected with immunomodulatory lectin ArtinM. PLoS ONE 2017, 12, e0187151. [Google Scholar] [CrossRef]
- Coriolano, M.C.; de Santana Brito, J.; de Siqueira Patriota, L.L.; de Araujo Soares, A.K.; de Lorena, V.; Paiva, P.M.G.; Napoleao, T.H.; Coelho, L.C.B.B.; de Melo, C.M.L. Immunomodulatory effects of the water-soluble lectin from Moringa oleifera seeds (WSMoL) on human peripheral blood mononuclear cells (PBMC). Protein Pept. Lett. 2018, 25, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.J.C.A.; da Silva Barros, B.R.; de Souza Aguiar, L.M.; de Siqueira Patriota, L.L.; de Albuquerque Lima, T.; Zingali, R.B.; Paiva, P.M.G.; Napoleão, T.H.; de Melo, C.M.L.; Pontual, E.V. Schinus terebinthifolia leaf lectin (SteLL) is an immunomodulatory agent by altering cytokine release by mice splenocytes. Biotech 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Jandú, J.J.B.; Moraes Neto, R.N.; Zagmignan, A.; de Sousa, E.M.; Brelaz-de-Castro, M.C.A.; dos Santos Correia, M.T.; da Silva, L.C.N. Targeting the immune system with plant lectins to combat microbial infections. Front. Pharmacol. 2017, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Ekowati, H.; Arai, J.; Putri, A.S.D.; Nainu, F.; Shiratsuchi, A.; Nakanishi, Y. Protective effects of Phaseolus vulgaris lectin against viral infection in Drosophila. Drug Discov. Ther. 2017, 11, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-M.; Sun, L.; Mittapalli, O.; Muir, W.M.; Xie, J.; Wu, J.; Schemerhorn, B.J.; Sun, W.; Pittendrigh, B.R.; Murdock, L.L. Transcriptional signatures in response to wheat germ agglutinin and starvation in Drosophila melanogaster larval midgut. Insect Mol. Biol. 2009, 18, 21–31. [Google Scholar] [CrossRef]
- Tsaneva, M.; Van Damme, E.J.M. 130 years of plant lectin research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Van Holle, S.; Van Damme, E.J.M. Signaling through plant lectins: Modulation of plant immunity and beyond. Biochem. Soc. Trans. 2018, 46, 217–233. [Google Scholar] [CrossRef]
- Kutschera, A.; Dawid, C.; Gisch, N.; Schmid, C.; Raasch, L.; Gerster, T.; Schäffer, M.; Smakowska-Luzan, E.; Belkhadir, Y.; Corina Vlot, A.; et al. Bacterial medium-chain 3-hydroxy fatty acid metabolites trigger immunity in Arabidopsis plants. Science 2019, 364, 178–181. [Google Scholar] [PubMed]
- Choi, J.; Tanaka, K.; Cao, Y.; Qi, Y.; Qiu, J.; Liang, Y.; Lee, S.Y.; Stacey, G. Identification of a plant receptor for extracellular ATP. Science 2014, 343, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, M.; Zhang, X.; Yao, J.; Zhang, Y.; Mou, Z. A lectin receptor kinase as a potential sensor for extracellular nicotinamide adenine dinucleotide in Arabidopsis thaliana. eLife 2017, 6, e25474. [Google Scholar] [CrossRef]
- Gust, A.A.; Pruitt, R.; Nürnberger, T. Sensing danger: Key to activating plant immunity. Trends Plant Sci. 2017, 22, 779–791. [Google Scholar] [CrossRef]
- Trautmann, A. Extracellular ATP in the immune system: More than just a “danger signal”. Sci. Signal. 2009, 2, pe6. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; De Schutter, K.; Pauwels, J.; Gevaert, K.; Van Damme, E.J.M.; Smagghe, G. The lectin Orysata induces phosphatase-mediated and carbohydrate-independent aggregation of insect cells. J. Insect Physiol. 2021, 131, 104241. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; De Schutter, K.; Van Damme, E.J.M.; Smagghe, G. Can Plant Lectins Help to Elucidate Insect Lectin-Mediated Immune Response? Insects 2021, 12, 497. https://doi.org/10.3390/insects12060497
Chen P, De Schutter K, Van Damme EJM, Smagghe G. Can Plant Lectins Help to Elucidate Insect Lectin-Mediated Immune Response? Insects. 2021; 12(6):497. https://doi.org/10.3390/insects12060497
Chicago/Turabian StyleChen, Pengyu, Kristof De Schutter, Els J. M. Van Damme, and Guy Smagghe. 2021. "Can Plant Lectins Help to Elucidate Insect Lectin-Mediated Immune Response?" Insects 12, no. 6: 497. https://doi.org/10.3390/insects12060497
APA StyleChen, P., De Schutter, K., Van Damme, E. J. M., & Smagghe, G. (2021). Can Plant Lectins Help to Elucidate Insect Lectin-Mediated Immune Response? Insects, 12(6), 497. https://doi.org/10.3390/insects12060497








