The Pupal Pigmentation Pattern and Pupal Development in the Species of Aphytis Howard (Hymenoptera: Aphelinidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Aphytis Pupae
2.2. Identification of Aphytis Species
2.3. Photography of Specimens
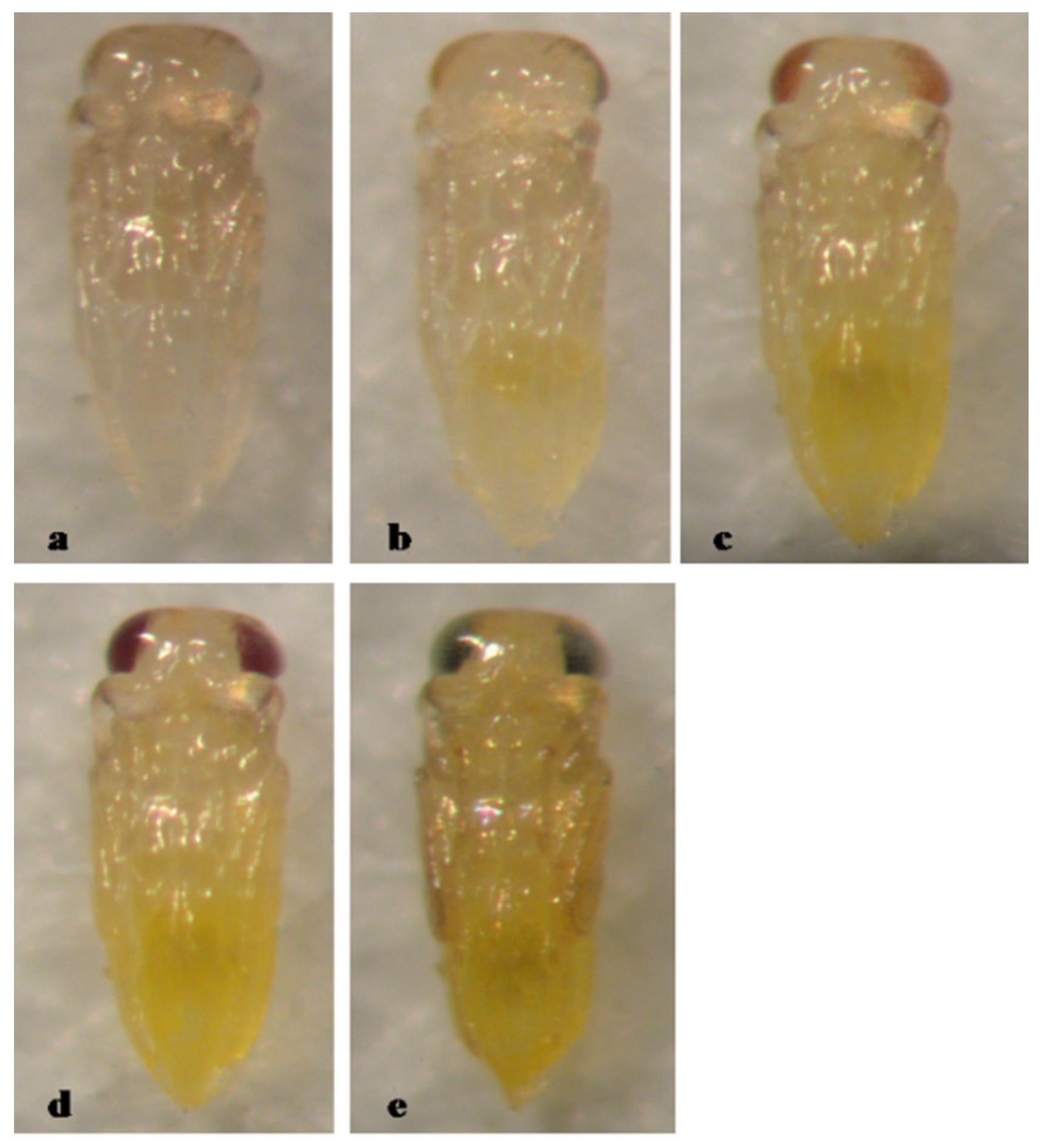
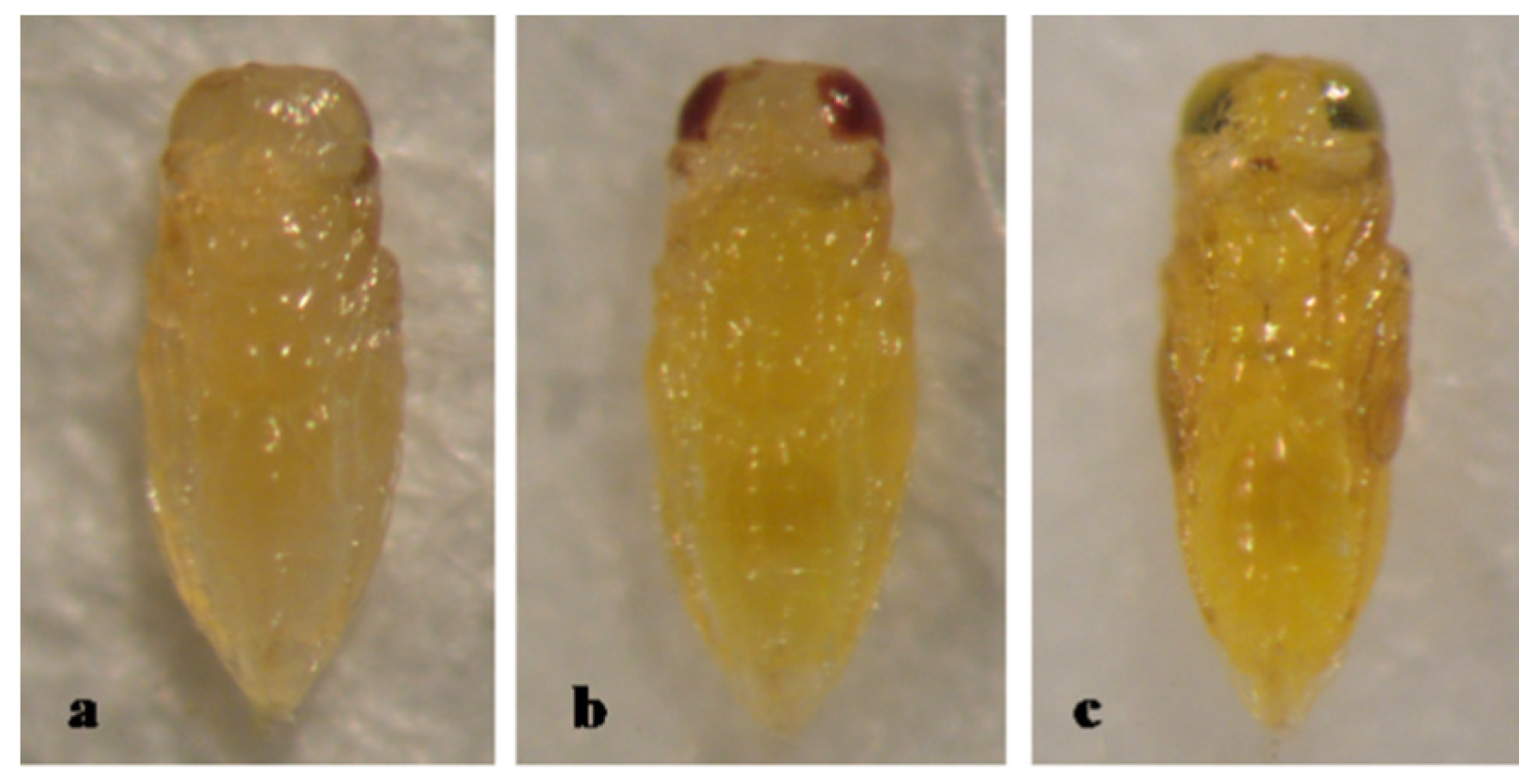
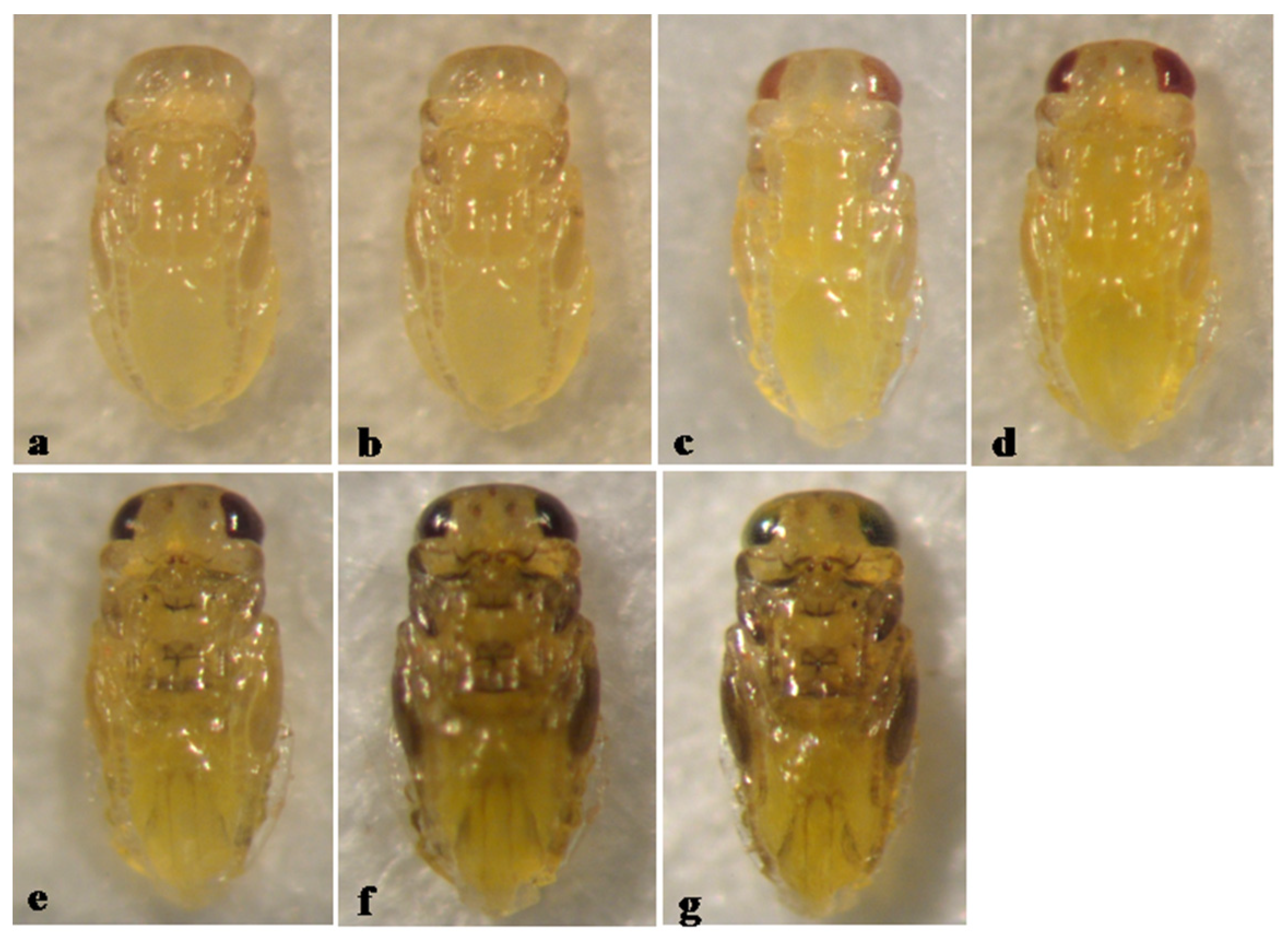
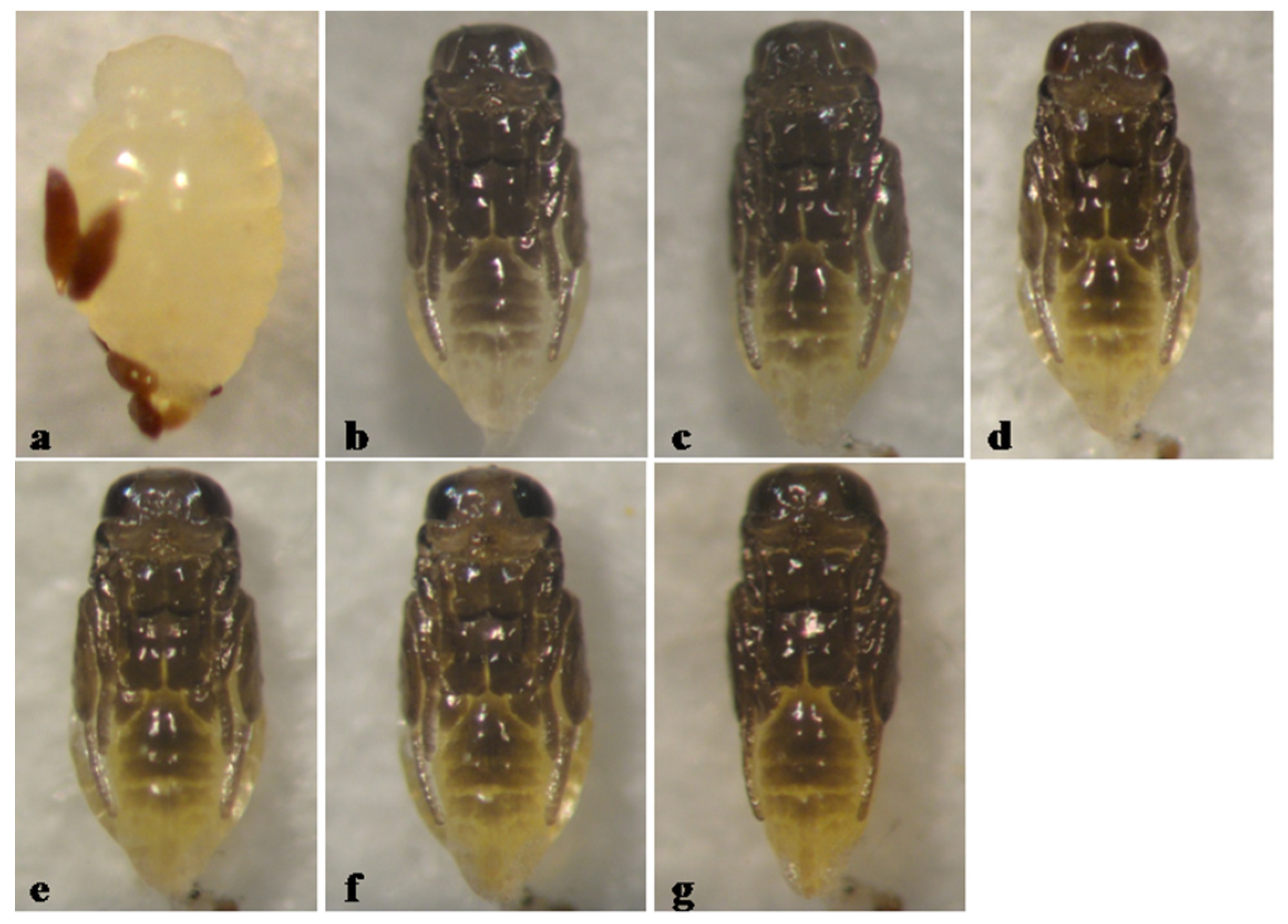
3. Results
3.1. Type 1, Entirely Yellow Pupa
3.1.1. Aphytis gordoni DeBach & Rosen (funicularis Group)
3.1.2. Aphytis lepidosaphes Compere (chrysomphali Group)
3.1.3. Aphytis fisheri DeBach (lingnanensis Group)
3.1.4. Aphytis hispanicus (Mercet) (proclia Group)
3.2. Type 2, Partly Dark Brown Pupa
3.2.1. Aphytis lingnanensis Compere (lingnanensis Group)
3.2.2. Aphytis holoxanthus DeBach (lingnanensis Group)
3.2.3. Aphytis melinus DeBach (lingnanensis Group)
3.3. Type 3, Entirely or Predominantly Black Pupa
3.3.1. Aphytis longicaudus Rosen & DeBach (Unassigned Species)
3.3.2. Aphytis sankarani Rosen & DeBach (Species Group Placement Intermediate between lingnanensis and mytilaspidis Groups)
3.4. Type 4, Partly Black Pupa
Aphytis sp. (Unassigned Species)
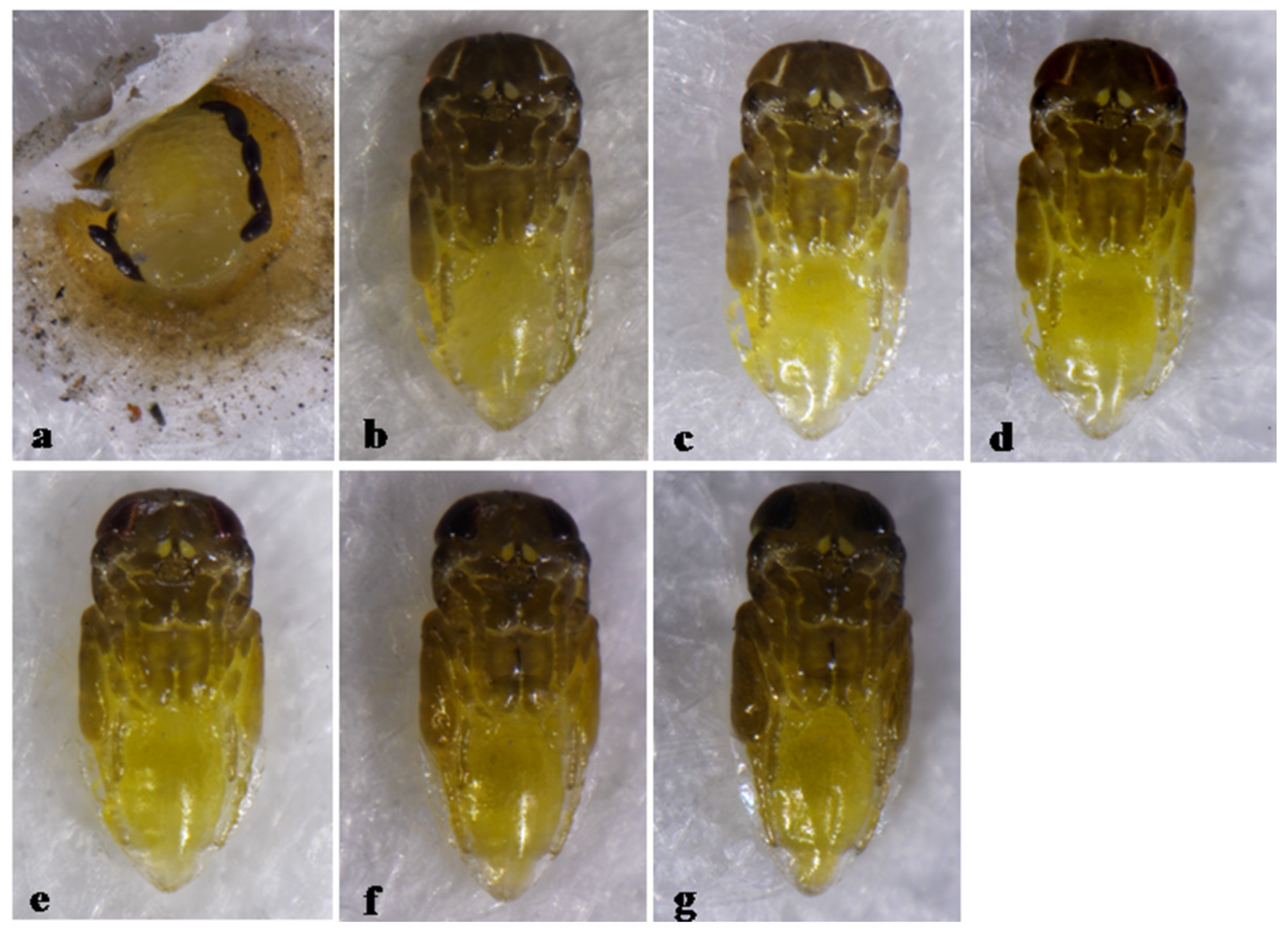
3.5. Pupal Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, D.; DeBach, P. Species of Aphytis of the World; Springer: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Rosen, D. Parasitic Hymenoptera in biological control: The genus Aphytis. In Advances in Parasitic Hymenoptera Research; Gupta, V., Ed.; EJBrill: Leiden, The Netherlands; New York, NY, USA, 1988; pp. 411–416. [Google Scholar]
- Huang, J. Systematic Studies on Aphelinidae of China (Hymenoptera: Chalcidoidea); Chongqing Publishing House: Chongqing, China, 1994. [Google Scholar]
- Noyes, J. Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). Ann. Mag. Nat. Hist. 1982, 16, 315–334. [Google Scholar] [CrossRef]
- Taylor, T.H.C. The Campaign against Aspidiotus destructor, Sign., in Fiji. Bull. Entomol. Res. 1935, 26, 1. [Google Scholar] [CrossRef]
- Flanders, S.E. Aphelinid biologies with implications for taxonomy. Ann. Entomol. Soc. Am. 1953, 46, 84–94. [Google Scholar] [CrossRef]
- Compere, H. A Systematic Study of the Genus Aphytis Howard (Hymenoptera, Aphelinidae) with Descriptions of New Species; University of California Press: Berkeley, CA, USA, 1955; pp. 277–321. [Google Scholar]
- DeBach, P. New species and strains of Aphytis (Hymenoptera, Eulophidae) parasitic on the California red scale, Aonidiella Aurantii (Mask.), in the Orient. Ann. Entomol. Soc. Am. 1959, 52, 354–362. [Google Scholar] [CrossRef]
- DeBach, P. Aphytis riyadhi n. sp. (Hymenoptera: Aphelinidae), a parasite of Aonidiella spp. (Homoptera: Diaspididae). Entomophaga 1979, 131–138. [Google Scholar]
- Quednau, F.W. A contribution on the genus Aphytis Howard in South Africa. (Hymenoptera: Aphelinidae). J. Entomol. Soc. S. Afr. 1964, 27, 86–116. [Google Scholar]
- Rosen, D.; DeBach, P. Systematics, morphology and biological control. Entomophaga 1973, 18, 215–222. [Google Scholar] [CrossRef]
- Bedford, E.C.G. Red scale, Aonidiella aurantii (Mask.). In Citrus Pests in the Republic of South Africa; Bedford, E.C.G., Ed.; Department of Agricultural Technical Services, Republic of South Africa, Science Bulletin; The Government Printer: Pretoria, South Africa, 1978; Volume 391, pp. 109–118. [Google Scholar]
- Yasnosh, V.A. A contribution to biosystematic description of species of the genus Aphytis Howard (Chalcidoidea, Aphelinidae) which are parasites of scale insects in the USSR. Entomol. Rev. 1972, 51, 146–152. [Google Scholar]
- Prinsloo, G.L. An illustrated guide to the parasitic wasps associated with citrus pests in the Republic of South Africa. Repub. S. Afr. Dep. Agric. Sci. Bull. 1984, 402, 1–119. [Google Scholar]
- Huang, J. Notes on Aphytis holoxanthus DeBach (Hymenoptera: Aphelinidae), a parasite of the Florida red scale, Chrysomphalus aonidum (L.) (Homoptera: Diaspididae). J. Fujian Agric. Coll. 1989, 18, 403–408. [Google Scholar]
- Prinsloo, G.L.; Neser, O.C. The Aphytis fauna of the Afrotropical region. In Advances in the Study of Aphytis (Hymenoptera: Aphelinidae); Rosen, D., Ed.; Intercept Ltd.: Andover, UK, 1994; pp. 295–298. [Google Scholar]
- Ren, H. Two new species of Aphytis from China (Hymenoptera, Aphelinidae). Entomotaxonomia 1988, 10, 220–221. [Google Scholar]
- Rosen, D.; Rose, M. Aphytis fioriniae, sp. nov. (Hymenoptera: Aphelinidae), a parasite of tea scale, Fiorinia theae Green, from India. Orient. Insects 1989, 23, 269–273. [Google Scholar] [CrossRef]
- Abd-Rabou, S. Revision of the genus Aphytis (hymenoptera: Aphelinidae) with descriptions of two new species from Egypt. Insect Sci. 2008, 11, 149–164. [Google Scholar] [CrossRef]
- Rose, M.; Rosen, D. A new species of Aphytis (Hymenoptera: Aphelinidae) parasitic upon the arrow-head scale, Unaspis yanonensis (kuwana), from the people’s republic of China. Entomotaxonomia 1991, 2, 120–123. [Google Scholar]
- DeBach, P. Aphytis simmondsiae n. sp. (Hymenoptera: Aphelinidae), a parasite of jojoba scale, Diaspis simmondsiae (Homoptera: Diaspididae). Folia Entomol. Mex. 1984, 60, 104–114. [Google Scholar]
- DeBach, P.; Rosen, D. Aphytis yanonensis n. sp. (Hymenoptera, Aphelinidae), a parasite of Unaspis yanonensis (Kuwana) (Homoptera, Diaspididae). Jpn. J. Entomol. 1982, 50, 626–634. [Google Scholar]
- Abdrabou, S. The holotype deposition of Aphytis sinaii Abd-Rabou (Hymenoptera: Aphelinidae), an external parasitoid of the California red scale, Aonidiella aurantii (Maskell). Egypt. J. Biol. Pest Control 2005, 15, 159. [Google Scholar]
- Li, C.; Yang, Q. A systematic study on the genus Aphytis Howard (Hymenoptera:Aphelinidae) from South Korea. Entomotaxonomia 2004, 26, 307–312. [Google Scholar]
- Hanan, R.M.; Hedaya, K.H. Aphytis alexandrina (Hymenoptera: Chalcidoidea: Aphelinidae) a new species from Alexandria, Egypt. Egypt. J. Entomol. 2013, 10, 49–52. [Google Scholar] [CrossRef][Green Version]
- Li, C. Two new species of Aphelinidae (Hymenoptera: Chalcidoidea) from northeastern region of China. J. North-East For. Univ. 1996, 24, 98–101. [Google Scholar]
- Rosen, D.; DeBach, P. Three new species of Aphytis (Hymenoptera: Aphelinidae), parasites of Pseudaulacaspis spp. (Homoptera: Diaspididae) in India and Australia. Entomophaga 1986, 31, 139–151. [Google Scholar] [CrossRef]
- Hayat, M. Aphelinidae of India (Hymenoptera: Chalcidoidea): A Taxonomic Revision; Associated Publishers: Gainesville, FL, USA, 1998. [Google Scholar]
- Hayat, M.; Veenakumari, K.A. Aphelinidae (Hymenoptera: Chalcidoidea) from Andaman & Nicobar Islands. J. Insect Syst. 2015, 1, 92–106. [Google Scholar]
- Myartseva, S.N.; Ruíz-Cancino, E.; Coronado-Blanco, J.M. El género Aphytis Howard (Hymenoptera: Chalcidoidea: Aphelinidae) en México, clave de especies y descripción de una especie nueva. Dugesiana 2010, 17, 81–94. [Google Scholar]
- Yasnosh, V.A. Aphytis species occurring in the former USSR and their role in biological control. In Advances in the Study of Aphytis (Hymenoptera: Aphelinidae); Rosen, D., Ed.; Intercept Ltd.: Andover, UK, 1994; pp. 317–333. [Google Scholar]
- Noyes, J. Universal Chalcidoidea Database. Available online: http://www.nhm.ac.uk/chalcidoids (accessed on 16 October 2020).
- Pina, T.; Verdú, M.; Urbaneja, A.; Sabater-Muñoz, B. The use of integrative taxonomy in determining species limits in the convergent pupa coloration pattern of Aphytis species. Biol. Control 2012, 61, 64–70. [Google Scholar] [CrossRef]





| Sp. Group | No. of sp. + Related sp. | No. of sp. Recorded with Pupal Pigmentation | Type of Pupal Pigmentation | References |
|---|---|---|---|---|
| chilensis | 5 + 2 | 2 | entirely or partly black | [1,16] |
| chrysomphali | 17 | 5 | entirely yellow, or some with brownish appendages | [1,3,16,17,18,19] |
| funicularis | 5 | 2 | entirely yellow | [1,20] |
| lingnanensis | 19 | 11 | partly dark brown; entirely yellow | [1,3,9,17,19,21,22,23,24,25] |
| mytilaspidis | 8 + 5 | 5 | entirely or predominantly black | [1,26] |
| proclia | 13 + 6 | generally entirely yellow (2) | [1,27,28,29] | |
| unassigned sp. | 15 | 3 | entirely or predominantly black | [1,27,28,30,31] |
| Total sp. (3) | 95 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Si, Y.; Zhang, H.; Zhang, Z.; Polaszek, A.; Huang, J. The Pupal Pigmentation Pattern and Pupal Development in the Species of Aphytis Howard (Hymenoptera: Aphelinidae). Insects 2021, 12, 399. https://doi.org/10.3390/insects12050399
Wang Z, Si Y, Zhang H, Zhang Z, Polaszek A, Huang J. The Pupal Pigmentation Pattern and Pupal Development in the Species of Aphytis Howard (Hymenoptera: Aphelinidae). Insects. 2021; 12(5):399. https://doi.org/10.3390/insects12050399
Chicago/Turabian StyleWang, Zhuhong, Yu Si, Hui Zhang, Zhengli Zhang, Andrew Polaszek, and Jian Huang. 2021. "The Pupal Pigmentation Pattern and Pupal Development in the Species of Aphytis Howard (Hymenoptera: Aphelinidae)" Insects 12, no. 5: 399. https://doi.org/10.3390/insects12050399
APA StyleWang, Z., Si, Y., Zhang, H., Zhang, Z., Polaszek, A., & Huang, J. (2021). The Pupal Pigmentation Pattern and Pupal Development in the Species of Aphytis Howard (Hymenoptera: Aphelinidae). Insects, 12(5), 399. https://doi.org/10.3390/insects12050399







