Oilseed Rape Shares Abundant and Generalized Pollinators with Its Co-Flowering Plant Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
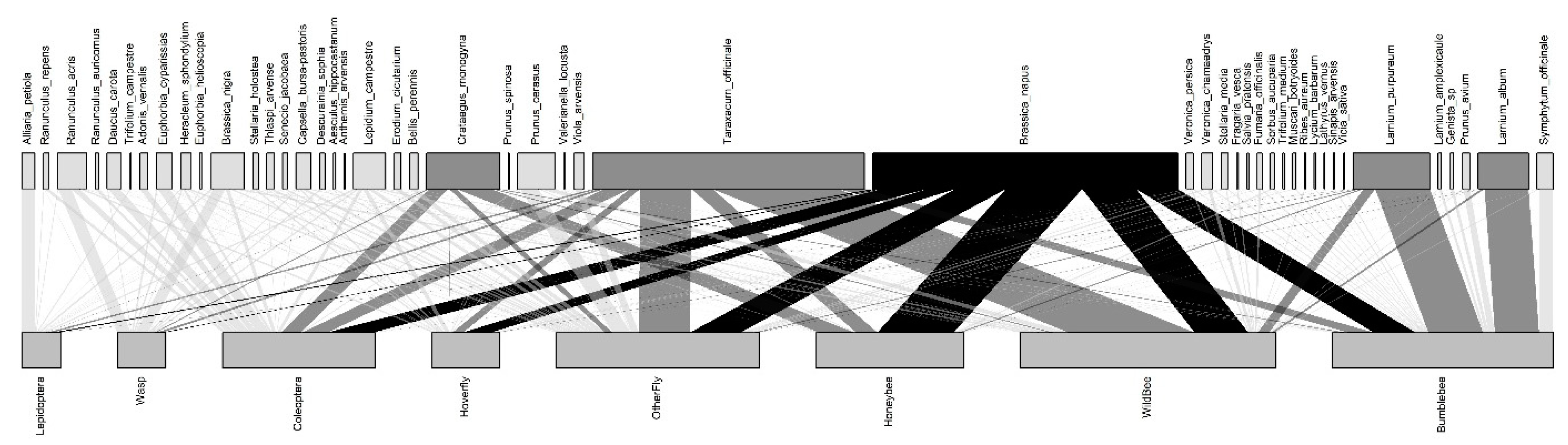
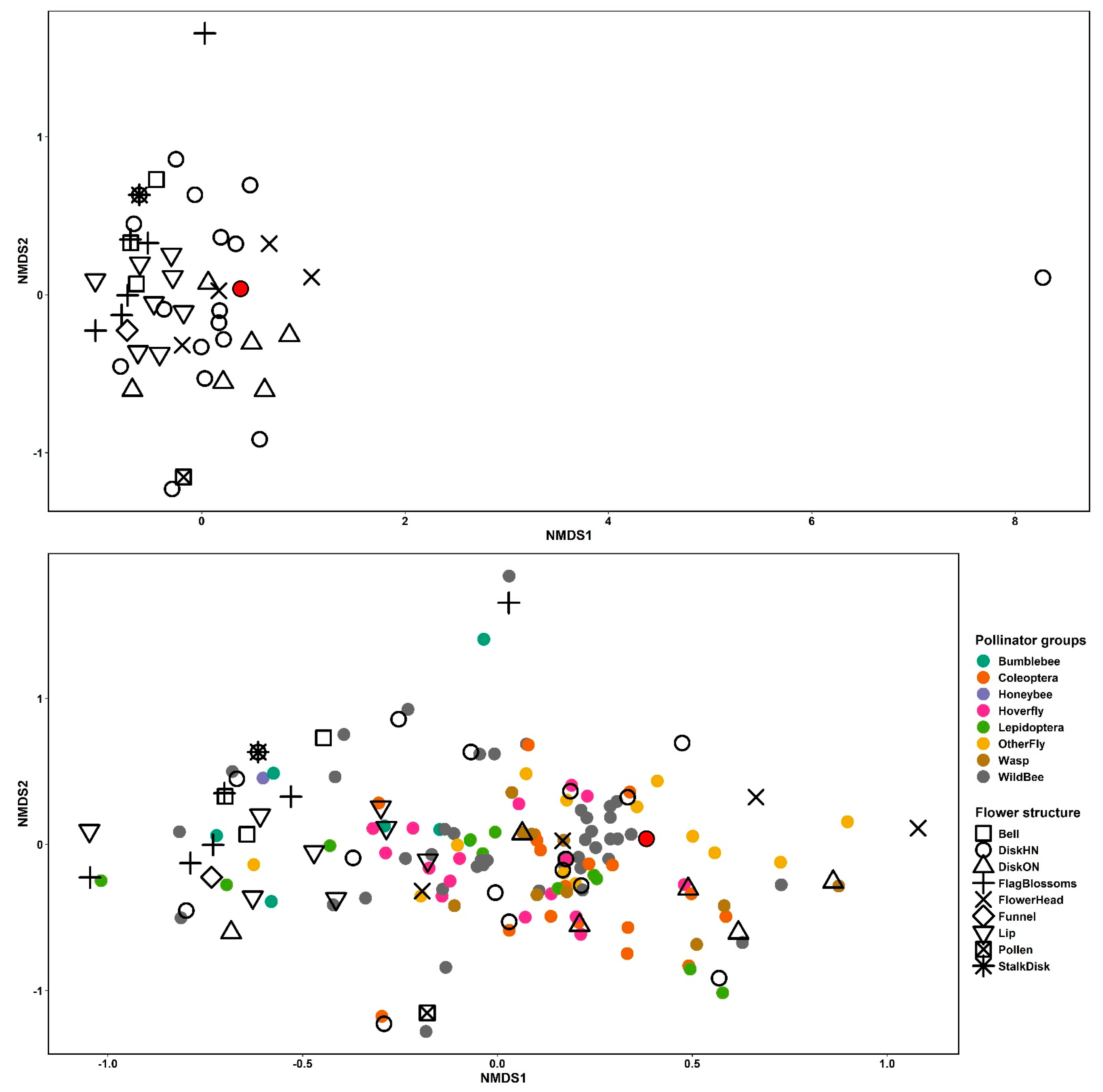
3.1. Plant-Pollinator Interactions and Composition
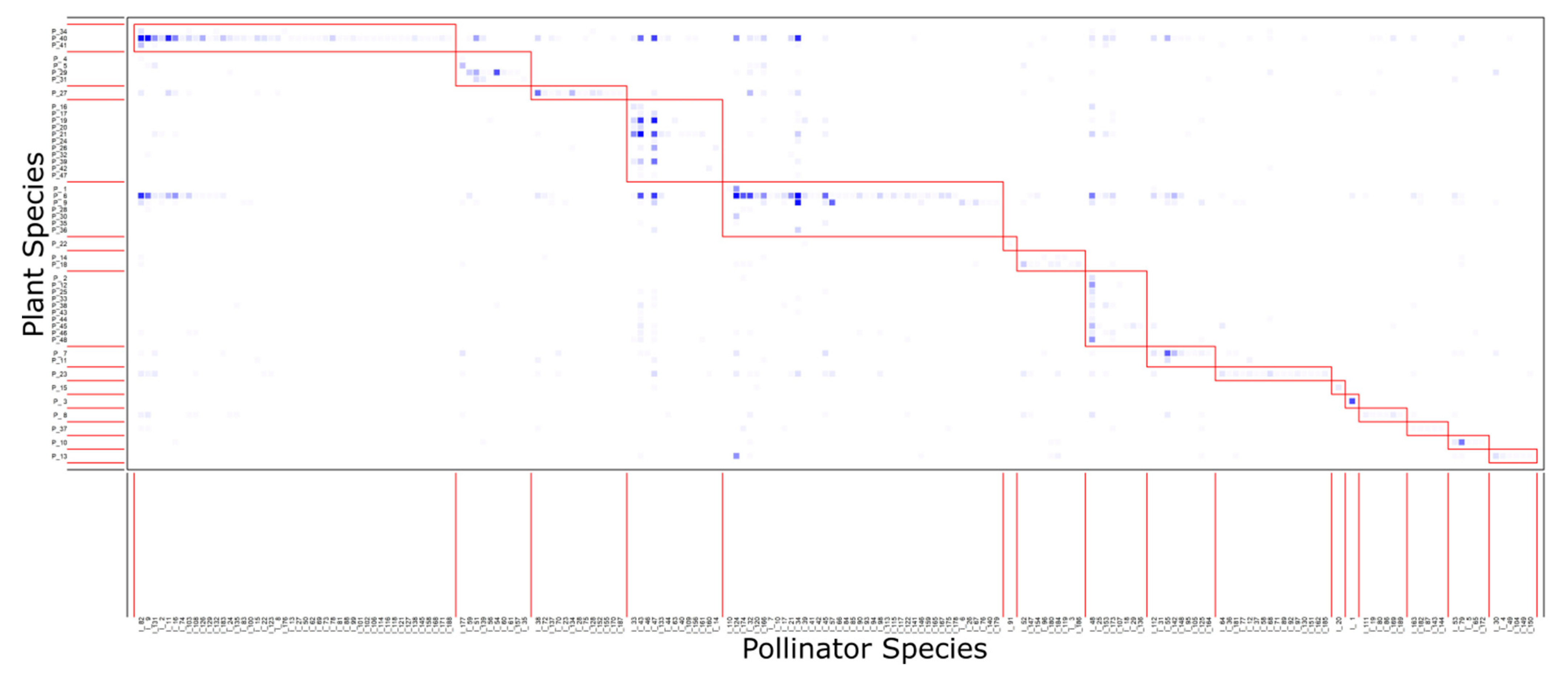
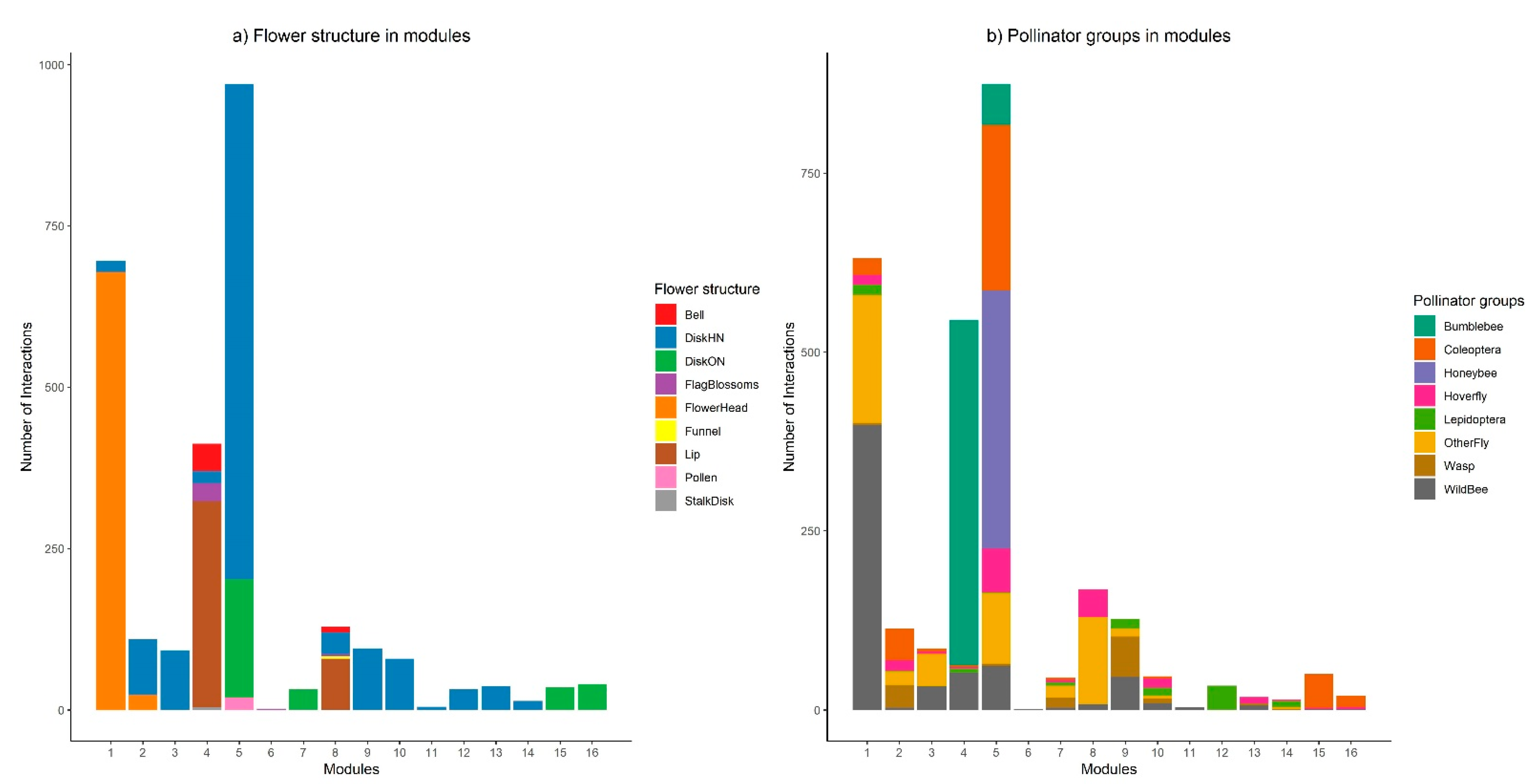
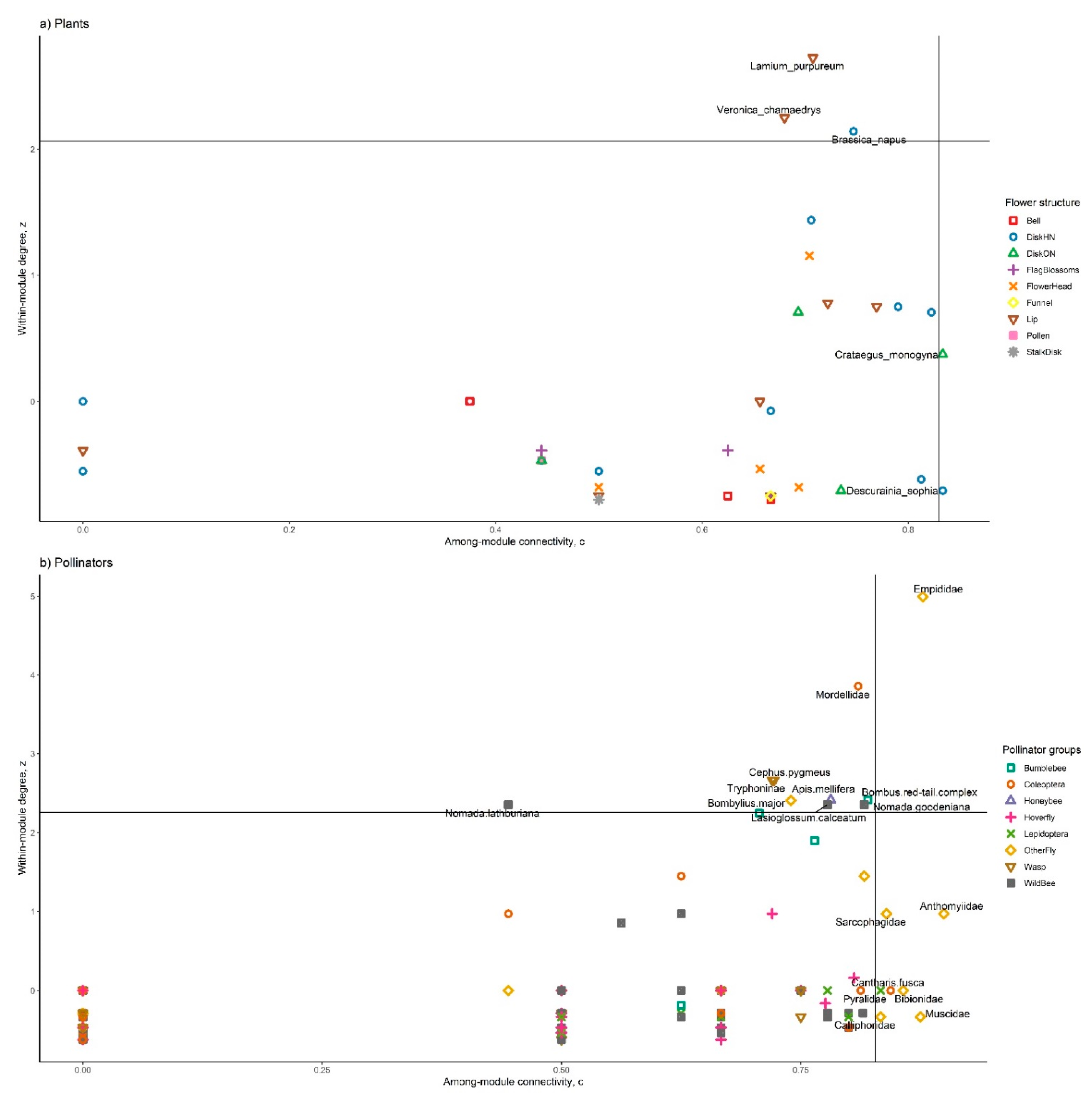
3.2. Network Modularity
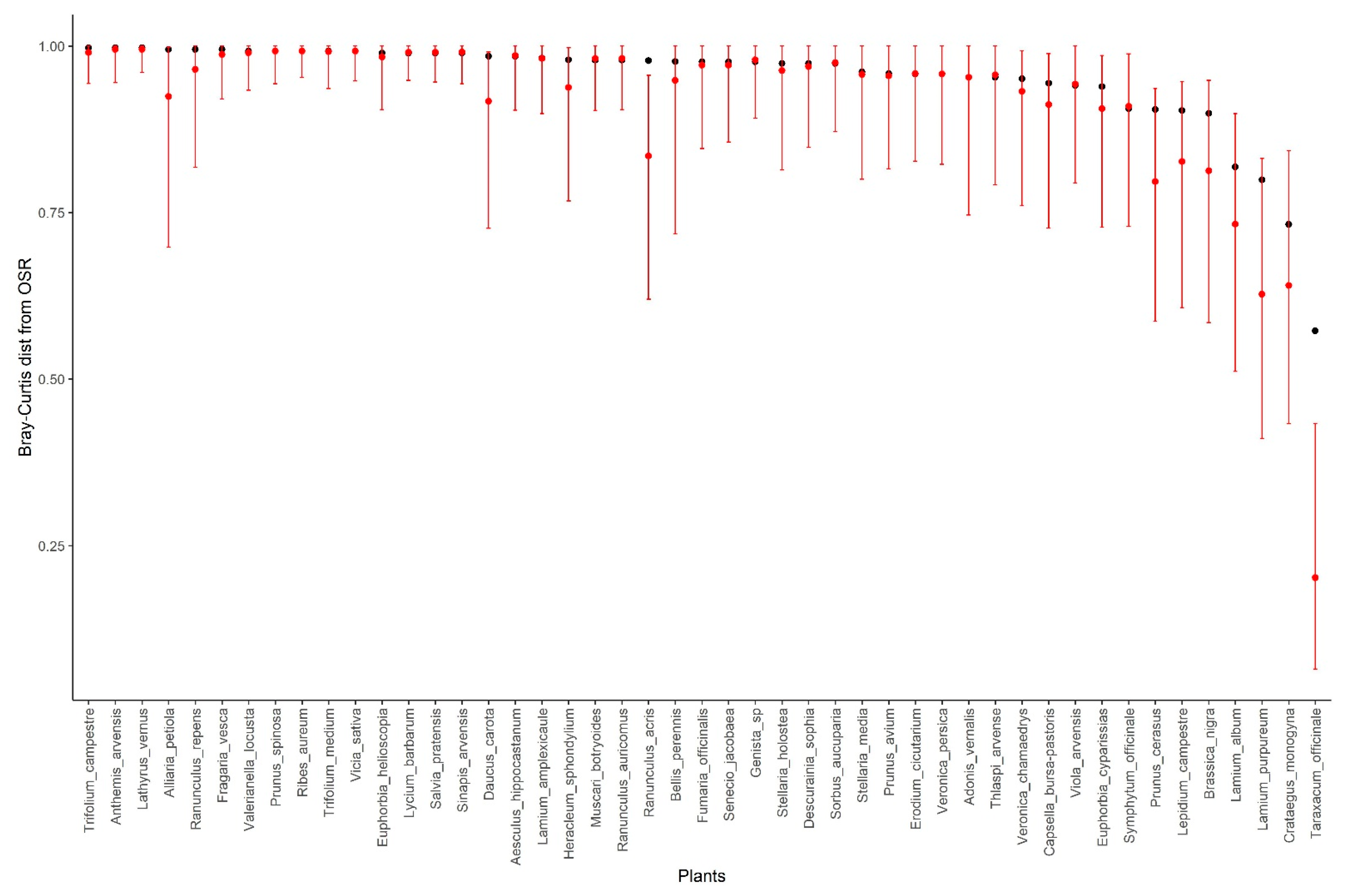
3.3. Müller Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; LeBuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef]
- Winfree, R.; Williams, N.M.; Dushoff, J.; Kremen, C. Native bees provide insurance against ongoing honey bee losses. Ecol. Lett. 2007, 10, 1105–1113. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Kremen, C.; Morales, J.M.; Bommarco, R.; Cunningham, S.A.; Carvalheiro, L.G.; Chacoff, N.P.; Dudenhöffer, J.H.; Greenleaf, S.S.; et al. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol. Lett. 2011, 14, 1062–1072. [Google Scholar] [CrossRef]
- Klein, A.M.; Müller, C.; Hoehn, P.; Kremen, C. Understanding the role of species richness for crop pollination services. In Biodiversity, Ecosystem Functioning, and Human Wellbeing: An Ecological and Economic Perspective; Oxford University Press: Oxford, UK, 2009; ISBN 9780191720345. [Google Scholar]
- Rader, R.; Bartomeus, I.; Garibaldi, L.A.; Garratt, M.P.D.; Howlett, B.G.; Winfree, R.; Cunningham, S.A.; Mayfield, M.M.; Arthur, A.D.; Andersson, G.K.S.; et al. Non-bee insects are important contributors to global crop pollination. Proc. Natl. Acad. Sci. USA 2016, 113, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.M.; Kremen, C.; M’Gonigle, L.K.; Rader, R.; et al. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 2015, 6, 10841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Taylor, H.; Winfree, R.; Brittain, C.; Alana, L.; Cariveau, D.; Ricketts, T.H.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Simba, L.D.; Foord, S.H.; Thébault, E.; van Veen, F.J.F.; Joseph, G.S.; Seymour, C.L. Indirect interactions between crops and natural vegetation through flower visitors: The importance of temporal as well as spatial spillover. Agric. Ecosyst. Environ. 2018, 253, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Holzschuh, A.; Dormann, C.F.; Tscharntke, T.; Steffan-Dewenter, I. Mass-flowering crops enhance wild bee abundance. Oecologia 2013, 172, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Burkle, L.A.; Delphia, C.M.; O’Neill, K.M. A dual role for farmlands: Food security and pollinator conservation. J. Ecol. 2017, 105, 890–899. [Google Scholar] [CrossRef] [Green Version]
- Hass, A.L.; Kormann, U.G.; Tscharntke, T.; Clough, Y.; Baillod, A.B.; Sirami, C.; Fahrig, L.; Martin, J.-L.L.; Baudry, J.; Bertrand, C.; et al. Landscape configurational heterogeneity by small-scale agriculture, not crop diversity, maintains pollinators and plant reproduction in western Europe. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172242. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.J.; Brocklehurst, S.; Robertson, D.; Harrison, W.; McCracken, D.I. Exploring the interactions between resource availability and the utilisation of semi-natural habitats by insect pollinators in an intensive agricultural landscape. Agric. Ecosyst. Environ. 2017, 246, 157–167. [Google Scholar] [CrossRef]
- Zou, Y.; Bianchi, F.J.J.J.A.; Jauker, F.; Xiao, H.; Chen, J.; Cresswell, J.; Luo, S.; Huang, J.; Deng, X.; Hou, L.; et al. Landscape effects on pollinator communities and pollination services in small-holder agroecosystems. Agric. Ecosyst. Environ. 2017, 246, 109–116. [Google Scholar] [CrossRef]
- Ferreira, P.A.; Boscolo, D.; Viana, B.F. What do we know about the effects of landscape changes on plant-pollinator interaction networks? Ecol. Indic. 2013, 31, 35–40. [Google Scholar] [CrossRef]
- Mandelik, Y.; Winfree, R.; Neeson, T.; Kremen, C. Complementary habitat use by wild bees in agro-natural landscapes. Ecol. Appl. 2012, 22, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Rader, R.; Cunningham, S.A.; Howlett, B.G.; Inouye, D.W. Non-bee insects as visitors and pollinators of crops: Biology, ecology, and management. Annu. Rev. Entomol. 2020, 65, 391–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dormann, C.F.; Fründ, J.; Schaefer, H.M. Identifying Causes of Patterns in Ecological Networks: Opportunities and Limitations. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 559–584. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, I.P.; Gotelli, N.J.; Memmott, J.; Pearson, C.E.; Woodward, G.; Symondson, W.O.C. econullnetr: An R package using null models to analyse the structure of ecological networks and identify resource selection. Methods Ecol. Evol. 2018, 9, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Dormann, C.F.; Frund, J.; Bluthgen, N.; Gruber, B. Indices, Graphs and Null Models: Analyzing Bipartite Ecological Networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Müller, C.; Adriaanse, I.; Belshaw, R.; Godfray, H. The structure of an aphid-parasitoid community. J. Anim. Ecol. 1999, 68, 346–370. [Google Scholar] [CrossRef]
- Newman, M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef] [Green Version]
- Dupont, Y.L.; Padrón, B.; Olesen, J.M.; Petanidou, T. Spatio-temporal variation in the structure of pollination networks. Oikos 2009, 118, 1261–1269. [Google Scholar] [CrossRef]
- May, R.M. Will a Large Complex System be Stable? Nature 1972, 238, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.E.; Frank, K.A.; Mason, D.M.; Ulanowicz, R.E.; Taylor, W.W. Compartments revealed in food-web structure. Nature 2003, 426, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.H.; Martin, A.P.; White, R.P. The pollination requirements of oil-seed rape (Brassica napus L.). J. Agric. Sci. 1986, 106, 27–30. [Google Scholar] [CrossRef]
- Langridge, D.F.; Goodman, R.D. Honeybee pollination of oilseed rape, cultivar Midas. Aust. J. Exp. Agric. Anim. Husb. 1982, 22, 124–126. [Google Scholar] [CrossRef]
- Manning, R.; Wallis, I.R. Seed yields in canola (Brassica napus cv. Karoo) depend on the distance of plants from honeybee apiaries. Aust. J. Exp. Agric. 2005, 45, 1307–1313. [Google Scholar] [CrossRef]
- Sabbahi, R.; De Oliveira, D.; Marceau, J. Influence of honey bee (Hymenoptera: Apidae) density on the production of canola (Crucifera: Brassicacae). J. Econ. Entomol. 2005, 98, 367–372. [Google Scholar] [CrossRef]
- Bommarco, R.; Marini, L.; Vaissière, B.E. Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia 2012, 169, 1025–1032. [Google Scholar] [CrossRef]
- Stanley, D.A.; Gunning, D.; Stout, J.C. Pollinators and pollination of oilseed rape crops (Brassica napus L.) in Ireland: Ecological and economic incentives for pollinator conservation. J. Insect Conserv. 2013, 17, 1181–1189. [Google Scholar] [CrossRef]
- Bartomeus, I.; Potts, S.G.; Ste, I.; Vaissi, B.E.; Woyciechowski, M.; Krewenka, K.M.; Steffan-Dewenter, I.; Vaissière, B.E.; Woyciechowski, M.; Krewenka, K.M.; et al. Contribution of insect pollinators to crop yield and quality varies with agricultural intensification. PeerJ 2014, 2, e328. [Google Scholar] [CrossRef] [Green Version]
- Jauker, F.; Wolters, V. Hover flies are efficient pollinators of oilseed rape. Oecologia 2008, 156, 819–823. [Google Scholar] [CrossRef]
- Jauker, F.; Bondarenko, B.; Becker, H.C.; Steffan-Dewenter, I. Pollination efficiency of wild bees and hoverflies provided to oilseed rape. Agric. For. Entomol. 2012, 14, 81–87. [Google Scholar] [CrossRef]
- Lindström, S.A.M.; Herbertsson, L.; Rundlöf, M.; Bommarco, R.; Smith, H.G. Experimental evidence that honeybees depress wild insect densities in a flowering crop. Proc. R. Soc. B Biol. Sci. 2016, 283. [Google Scholar] [CrossRef] [Green Version]
- Beduschi, T.; Kormann, U.G.; Tscharntke, T.; Scherber, C. Spatial community turnover of pollinators is relaxed by semi-natural habitats, but not by mass-flowering crops in agricultural landscapes. Biol. Conserv. 2018, 221, 59–66. [Google Scholar] [CrossRef]
- Magrach, A.; Holzschuh, A.; Bartomeus, I.; Riedinger, V.; Roberts, S.P.M.M.; Rundlöf, M.; Vujić, A.; Wickens, J.B.; Wickens, V.J.; Bommarco, R.; et al. Plant–pollinator networks in semi-natural grasslands are resistant to the loss of pollinators during blooming of mass-flowering crops. Ecography 2018, 41, 62–74. [Google Scholar] [CrossRef] [Green Version]
- Beyer, N.; Gabriel, D.; Westphal, C. Contrasting effects of past and present mass-flowering crop cultivation on bee pollinators shaping yield components in oilseed rape. Agric. Ecosyst. Environ. 2021, 319, 107537. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dainese, M.; González-Varo, J.P.; Mudri-Stojnić, S.; Riedinger, V.; Rundlöf, M.; Scheper, J.; Wickens, J.B.; Wickens, V.J.; Bommarco, R.; et al. Mass-flowering crops dilute pollinator abundance in agricultural landscapes across Europe. Ecol. Lett. 2016, 19, 1228–1236. [Google Scholar] [CrossRef]
- Rollin, O.; Benelli, G.; Benvenuti, S.; Decourtye, A.; Wratten, S.D.; Canale, A.; Desneux, N. Weed-insect pollinator networks as bio-indicators of ecological sustainability in agriculture. A review. Agron. Sustain. Dev. 2016, 36, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Stanley, D.A.; Stout, J.C. Pollinator sharing between mass-flowering oilseed rape and co-flowering wild plants: Implications for wild plant pollination. Plant Ecol. 2014, 215, 315–325. [Google Scholar] [CrossRef]
- Zacharias, S.; Bogena, H.; Samaniego, L.; Mauder, M.; Fuß, R.; Pütz, T.; Frenzel, M.; Schwank, M.; Baessler, C.; Butterbach-Bahl, K.; et al. A Network of Terrestrial Environmental Observatories in Germany. Vadose Zo. J. 2011, 10, 955–973. [Google Scholar] [CrossRef] [Green Version]
- Amiet, F. Fauna Helvetica. Apidae 1: Apis, Bombus, Psithyrus, 12th ed.; Schweizerische Entomologische Gesellschaft: Neuchâtel, Switzerland, 1996. [Google Scholar]
- Van Veen, M.P. Hoverflies of Northwest Europe: Identification Keys to the Syrphidae, 2nd ed.; KNNV Publishing: Zeist, The Netherlands, 2009. [Google Scholar]
- Oosterbroek, P. The European Families of the Diptera; KNNV Publishing: Zeist, The Netherlands, 2015. [Google Scholar]
- Bartsch, H. Tvåvingar: Blomflugor Vol. 1 & 2; ArtDatabanken: Uppsala, Sweden, 2009; Volume 1 & 2. [Google Scholar]
- Amiet, F.; Müller, A.; Neumeyer, R. Fauna Helvetica. Apidae 2: Colletes, Dufourea, Hylaeus, Nomia, Nomioides, Rhophitoides, Rophites, Sphecodes, Systropha, 4th ed.; Centre Suisse de Cartographie de la Faune (CSCF): Neuchâtel, Switzerland, 1999. [Google Scholar]
- Amiet, F.; Herrmann, M.; Müller, A.; Neumeyer, R. Fauna Helvetica. Apidae 3: Halictus, Lasioglossum, 6th ed.; Centre Suisse de Cartographie de la Faune (CSCF): Neuchâtel, Switzerland, 2001. [Google Scholar]
- Amiet, F.; Herrmann, M.; Müller, A.; Neumeyer, R. Fauna Helvetica. Apidae 4: Anthidium, Chelostoma, Coelioxys, Dioxys, Heriades, Lithurgus, Megachile, Osmia, Stelis, 9th ed.; Centre Suisse de Cartographie de la Faune (CSCF): Neuchâtel, Switzerland, 2004. [Google Scholar]
- Amiet, F.; Herrmann, M.; Müller, A.; Neumeyer, R. Fauna Helvetica. Apidae 5: Ammobates, Ammobatoides, Anthophora, Biastes, Ceratina, Dasypoda, Epeoloides, Epeolus, Eucera, Macropis, Melecta, Melitta, Nomada, Pasites, Tetralonia, Thyreus, Xylocopa, 20th ed.; Centre Suisse de Cartographie de la Faune (CSCF): Neuchâtel, Switzerland, 2007. [Google Scholar]
- Amiet, F.; Herrmann, M.; Müller, A.; Neumeyer, R. Fauna Helvetica. Apidae 6: Andrena, Melitturga, Panurginus, Panurgus, 26th ed.; Centre Suisse de Cartographie de la Faune (CSCF): Neuchâtel, Switzerland, 2010. [Google Scholar]
- Klotz, S.; Durka, W. BIOLFLOR—Eine Datenbank zu Biologisch-Ökologischen Merkmalen der Gefäßpflanzen in Deutschland; Bundesamt für Naturschutz: Bonn, Germany, 2002; Volume 38. [Google Scholar]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R package version 2.5-7; 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 2 December 2021).
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dormann, C.F.; Strauss, R. A method for detecting modules in quantitative bipartite networks. Methods Ecol. Evol. 2014, 5, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Carvalheiro, L.G.; Biesmeijer, J.C.; Benadi, G.; Fründ, J.; Stang, M.; Bartomeus, I.; Kaiser-Bunbury, C.N.; Baude, M.; Gomes, S.I.F.; Merckx, V.; et al. The potential for indirect effects between co-flowering plants via shared pollinators depends on resource abundance, accessibility and relatedness. Ecol. Lett. 2014, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, S.; Dormann, C.F.; Martín González, A.M.; Ollerton, J. The influence of floral traits on specialization and modularity of plant-pollinator networks in a biodiversity hotspot in the Peruvian Andes. Ann. Bot. 2016, 118, 415–429. [Google Scholar] [CrossRef]
- Dupont, Y.L.; Olesen, J.M. Ecological modules and roles of species in heathland plant-insect flower visitor networks. J. Anim. Ecol. 2009, 78, 346–353. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dormann, C.F.; Tscharntke, T.; Steffan-Dewenter, I. Expansion of mass-flowering crops leads to transient pollinator dilution and reduced wild plant pollination. Proc. R. Soc. B Biol. Sci. 2011, 278, 3444–3451. [Google Scholar] [CrossRef] [Green Version]
- Morvan, N.; Delettre, Y.R.; Trehen, P. The distribution of Empididae ( Diptera ) in hedgerow network landscapes. In Proceedings of the BCPC Monograph. Field Margins: Integrating Agriculture and Conservation, Coventry, UK, 18–20 April 1994. [Google Scholar]
- Pornon, A.; Baksay, S.; Escaravage, N.; Burrus, M.; Andalo, C. Pollinator specialization increases with a decrease in a mass-flowering plant in networks inferred from DNA metabarcoding. Ecol. Evol. 2019, 9, 13650–13662. [Google Scholar] [CrossRef]
- Diekötter, T.; Kadoya, T.; Peter, F.; Wolters, V.; Jauker, F. Oilseed rape crops distort plant-pollinator interactions. J. Appl. Ecol. 2010, 47, 209–214. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, A.; Ștefan, V.; Knight, T.M. Oilseed Rape Shares Abundant and Generalized Pollinators with Its Co-Flowering Plant Species. Insects 2021, 12, 1096. https://doi.org/10.3390/insects12121096
Thompson A, Ștefan V, Knight TM. Oilseed Rape Shares Abundant and Generalized Pollinators with Its Co-Flowering Plant Species. Insects. 2021; 12(12):1096. https://doi.org/10.3390/insects12121096
Chicago/Turabian StyleThompson, Amibeth, Valentin Ștefan, and Tiffany M. Knight. 2021. "Oilseed Rape Shares Abundant and Generalized Pollinators with Its Co-Flowering Plant Species" Insects 12, no. 12: 1096. https://doi.org/10.3390/insects12121096
APA StyleThompson, A., Ștefan, V., & Knight, T. M. (2021). Oilseed Rape Shares Abundant and Generalized Pollinators with Its Co-Flowering Plant Species. Insects, 12(12), 1096. https://doi.org/10.3390/insects12121096





