First Population Study on Winter Breeding Monarch Butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) in the Urban South Bay of San Francisco, California
Abstract
:Simple Summary
Abstract
1. Introduction
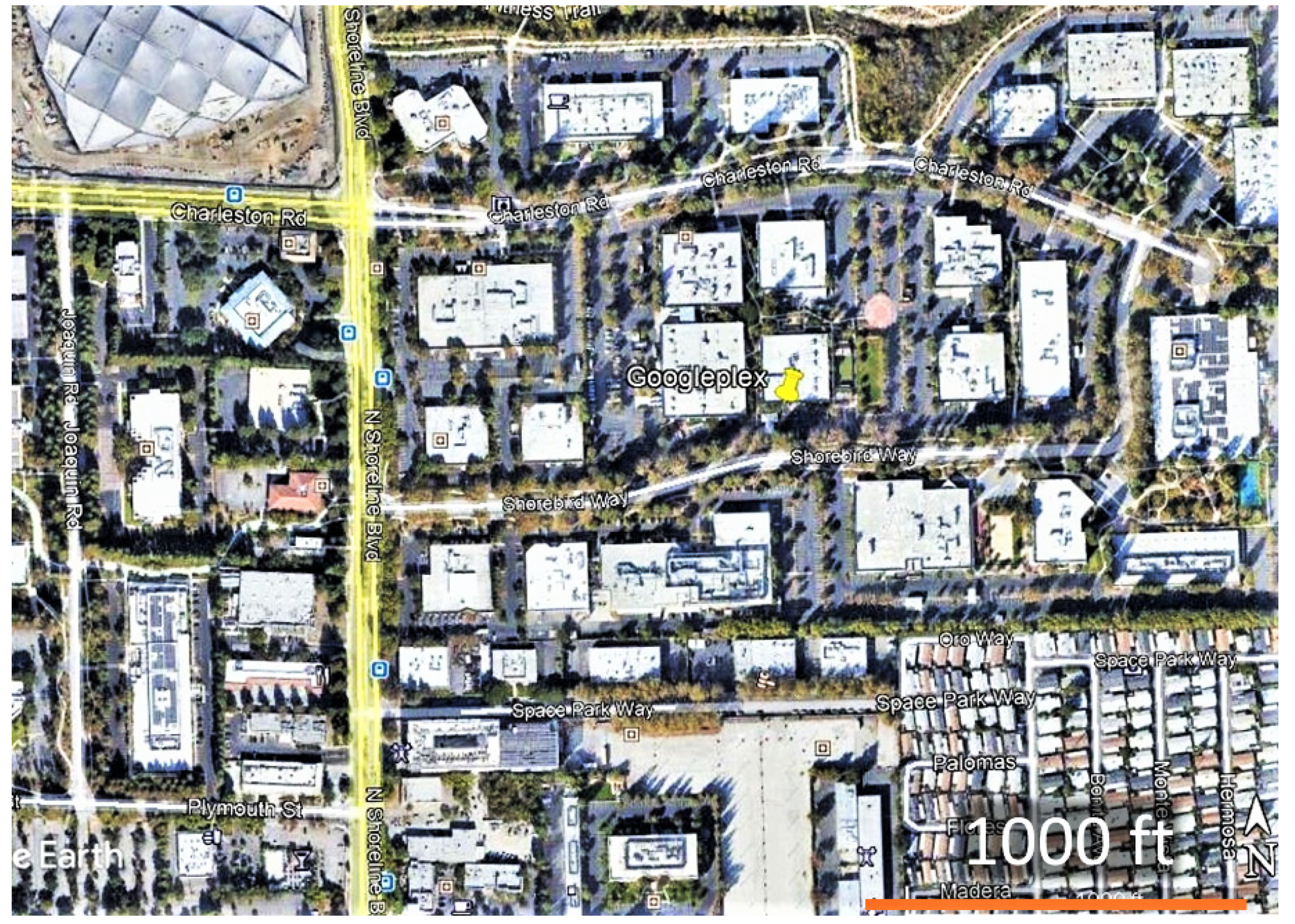
2. Materials and Methods
2.1. Adult Counts and Nectar Sources
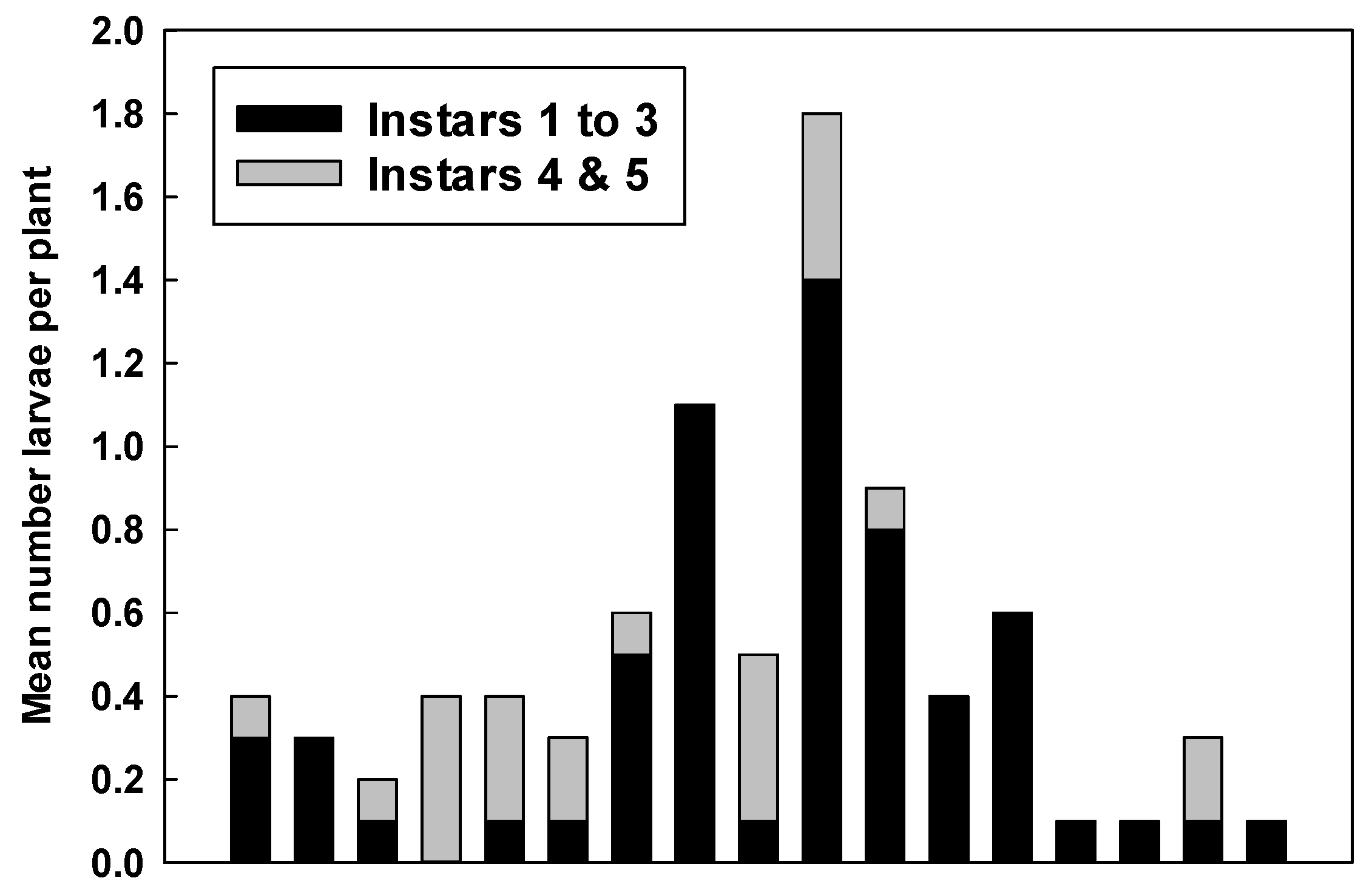
2.2. Counts of Eggs and Larvae
2.3. Infection of Monarch Adults by the Protozoan Parasite Ophryocystis Elektroscirrha (OE)
2.4. Dispersal and Movement of Adult Monarchs
3. Results
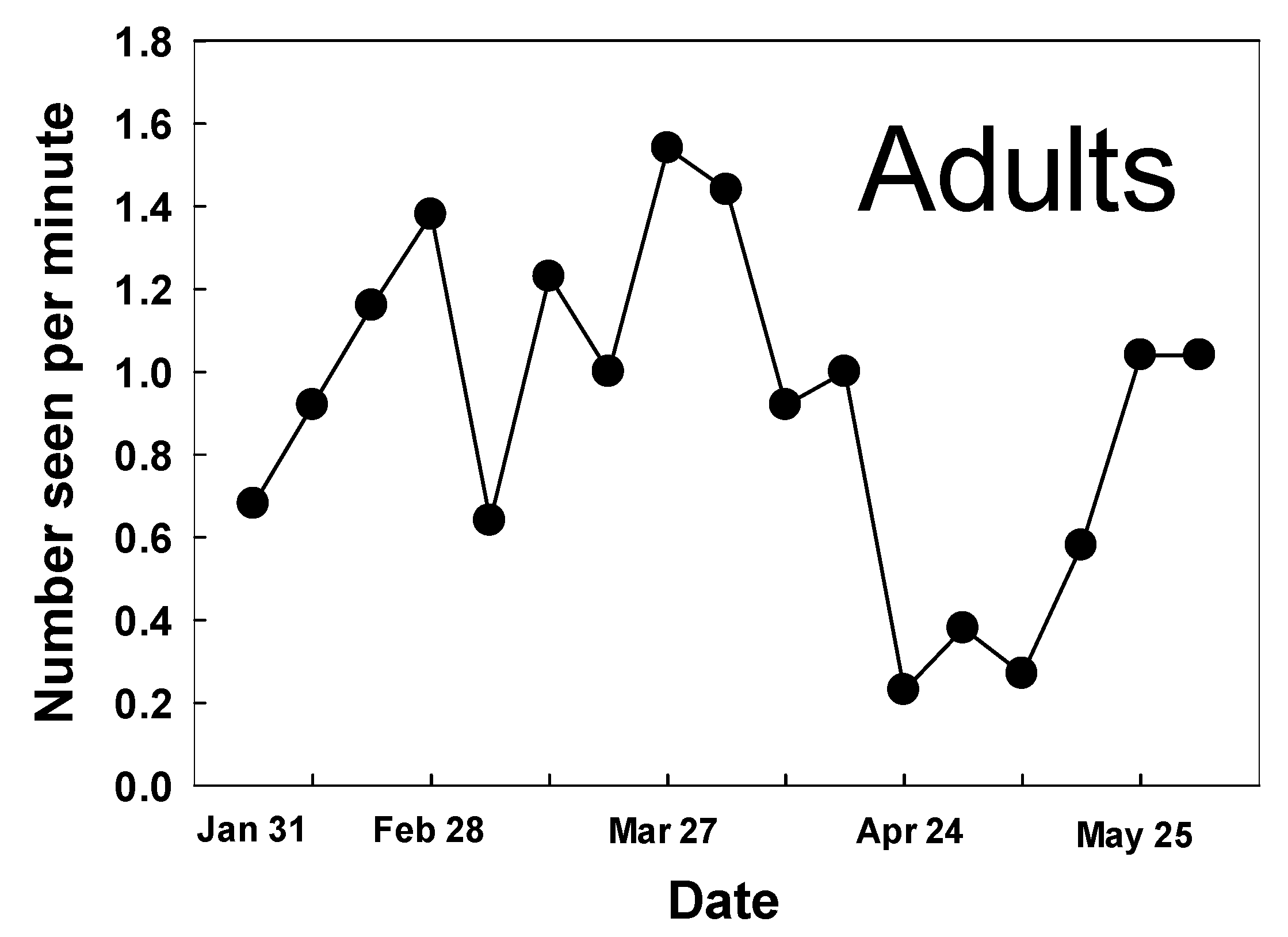
3.1. Adult Counts and Nectar Sources
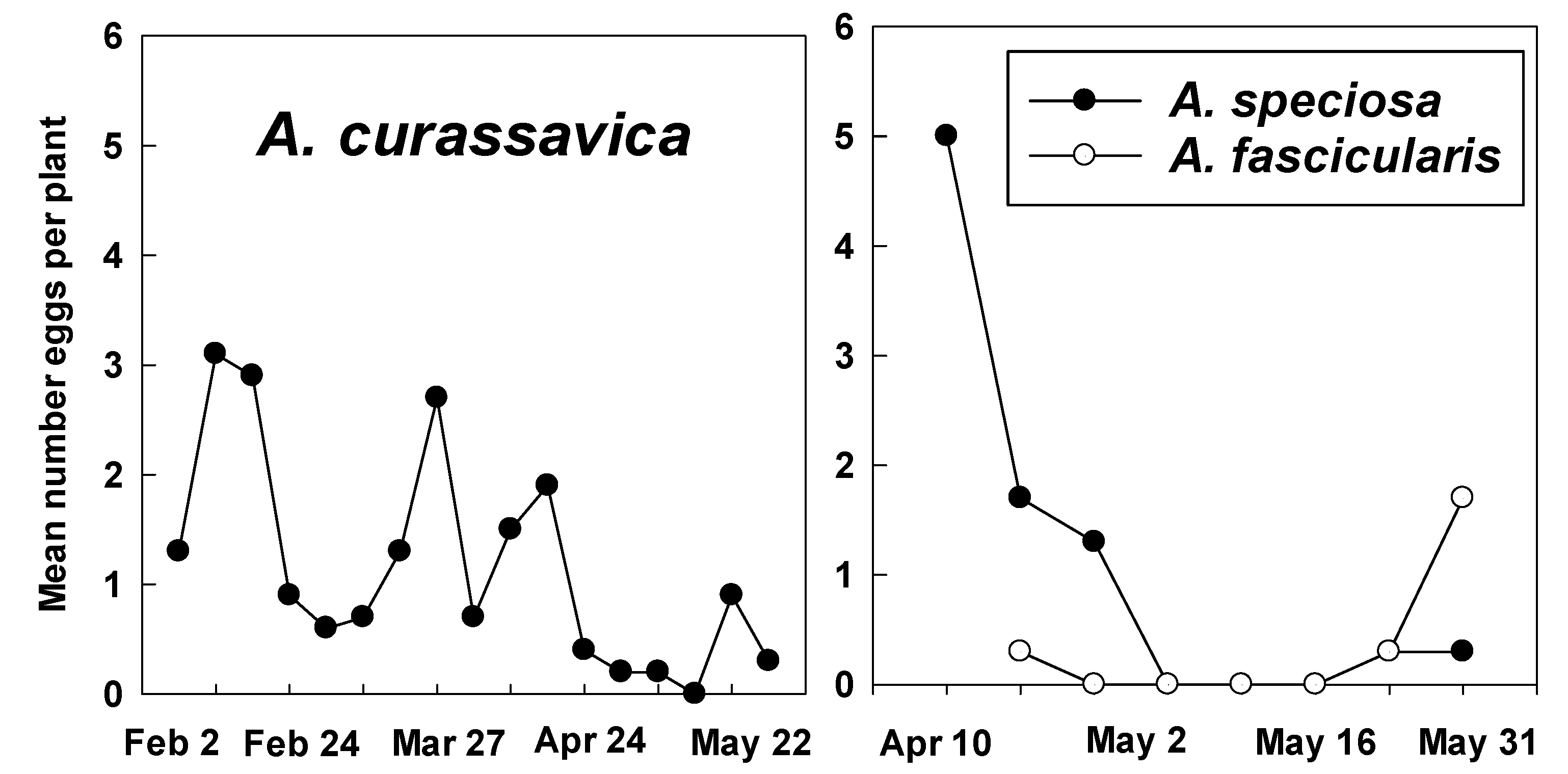
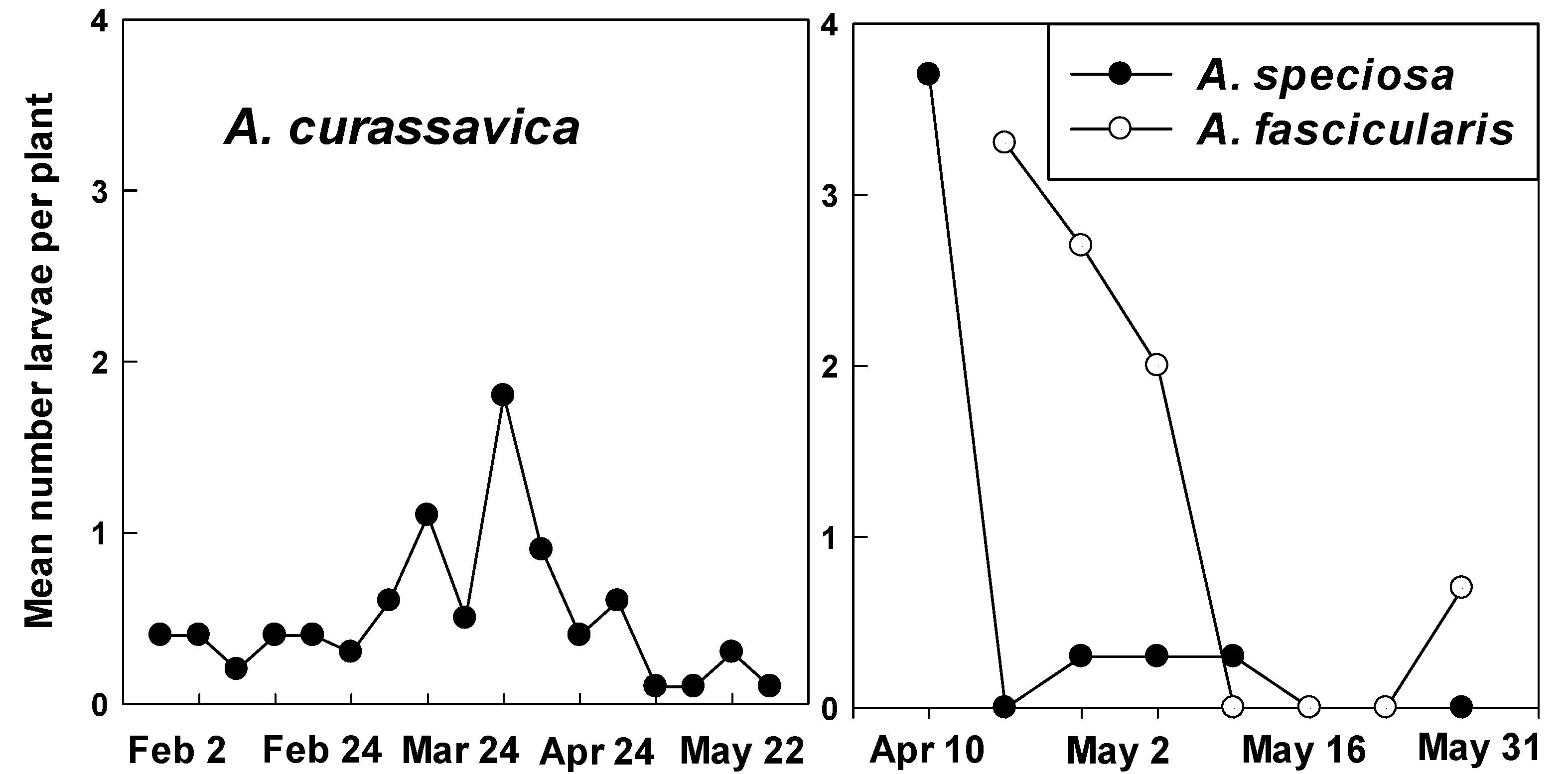
3.2. Counts of Eggs and Larvae
3.3. Infection of Monarch Adults by the Protozoan Parasite Ophryocystis elektroscirrha (OE)
3.4. Dispersal and Movement of Adult Monarchs
4. Discussion
4.1. Successful Winter Breeding
4.2. Spring Dispersal or Migration
4.3. Infection by O. elektroscirrha (OE)
4.4. Role of Non-Native Milkweeds in Winter Breeding
4.5. Extent of Winter-Breeding in California
4.6. Winter Breeding in the Bay Area May Contribute to the Western Population
4.7. Winter Breeding and Climate Warming
4.8. Dependence of Winter Breeding on Non-Native Milkweed
4.9. Monarchs Adapting to a Changing Environment
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Gustaffson, K.M.; Agrawal, A.A.; Lewenstein, B.V.; Wolf, S.A. The monarch butterfly through time and space: The social construction of an icon. Bioscience 2015, 65, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Freedman, M.G.; de Roode, J.C.; Forister, M.L.; Kronforst, M.R.; Pierce, A.A.; Schultz, C.B.; Taylor, O.R.; Crone, E.E. Are eastern and western monarch butterflies distinct populations? A review of evidence for ecological, phenotypic, and genetic differentiation and implications for conservation. Conserv. Sci. Pract. 2021, 3, e432. [Google Scholar]
- Morris, G.M.; Kline, C.; Morris, S.M. Status of Danaus plexippus populations in Arizona. J. Lepid. Soc. 2015, 69, 91–107. [Google Scholar]
- James, D.G.; James, T.S.; Seymour, L.; Kappen, L.; Russell, T.; Harryman, B.; Bly, C. Citizen scientist tagging reveals destinations of migrating monarch butterflies, Danaus plexippus (L.) from the Pacific Northwest. J. Lepid. Soc. 2018, 72, 127–144. [Google Scholar] [CrossRef]
- James, D.G.; Kappen, L. Further insights on the migration biology of monarch butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) from the Pacific Northwest. Insects 2021, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- James, D.G. Western North American monarchs: Spiraling into oblivion or adapting to a changing environment? Anim. Migr. 2021, 8, 19–26. [Google Scholar] [CrossRef]
- James, D.G. Do some fall migrants from the Pacific Northwest augment winter breeding populations of monarch butterflies in southern California? J. Lepid. Soc. 2018, 72, 244–246. [Google Scholar] [CrossRef]
- Crone, E.E.; Schultz, C.B. Resilience or catastrophe? A possible state change for monarch butterflies in western North America. Ecol. Lett. 2021, 24, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, D.A.; Villablanca, F.X.; Maerz, J.C.; Altizer, S. Migratory monarchs wintering in California experience low infection risk compared to monarchs breeding year-round on non-native milkweed. Integr. Comp. Biol. 2016, 56, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altizer, S.M.; Oberhauser, K.S.; Brower, L.P. Associations between host migration and the prevalence of a protozoan parasite in natural populations of adult monarch butterflies. Ecol. Entomol. 2000, 25, 125–139. [Google Scholar] [CrossRef]
- James, D.G. Population biology of monarch butterflies, Danaus plexippus L. (Lepidoptera: Nymphalidae) at a milkweed-rich summer breeding site in central Washington. J. Lep. Soc. 2016, 70, 182–193. [Google Scholar] [CrossRef]
- Nagano, C.D.; Sakai, W.H.; Malcolm, S.B.; Cockrell, B.J.; Donahue, J.P.; Brower, L.P. Spring migration of monarch butterflies in California. In Biology and Conservation of the Monarch Butterfly; Malcolm, S., Zalucki, M.P., Eds.; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1993; pp. 219–232. [Google Scholar]
- Leong, K.L.H.; Kaya, H.K.; Yoshimura, M.A.; Frey, D.F. The occurrence and effect of a protozoan parasite Ophryocystis elektroscirra (Neogregarinida: Ophryocystidae) on overwintering monarch butterflies, Danaus plexippus (Lepidoptera: Danaidae) from two California overwintering sites. Ecol. Entomol. 1991, 17, 338–342. [Google Scholar] [CrossRef] [Green Version]
- De Roode, J.; Chi, C.J.; Rarick, R.M.; Altizer, S. Strength in numbers; high parasite burdens increase transmission of a protozoan parasite of monarch butterflies Danaus plexippus. Oecologia 2009, 161, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.; Altizer, S.M. Parasites hinder monarch butterfly flight ability: Implications for disease spread in migratory hosts. Ecol. Lett. 2005, 8, 290–300. [Google Scholar] [CrossRef]
- Majewska, A.A.; Altizer, S. Exposure to non-native tropical milkweed promotes reproductive development in migratory monarch butterflies. Insects 2019, 10, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodson, R.E. The North American species of Asclepias L. Ann. Mo. Bot. Gdn. 1954, 41, 1–211. [Google Scholar] [CrossRef]
- Urquhart, F.A.; Urquhart, N.R.; Munger, F. A study of a continuously breeding population of Danaus plexippus in southern California compared to a migratory population and its significance in the study of insect movement. J. Res. Lep. 1970, 7, 169–181. [Google Scholar]
- James, D.G. Migration biology of the monarch butterfly in Australia. In Biology and Conservation of the Monarch Butterfly; Malcolm, S., Zalucki, M.P., Eds.; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1993; pp. 189–200. [Google Scholar]
- James, D.G. Studies on a winter breeding population of Danaus plexippus (L.) (Lepidoptera: Nymphalidae) at Spencer, News South Wales. Gen. Appl. Ent. 1981, 13, 47–53. [Google Scholar]
- James, D.G. Population and general biology of non-reproductive colonies of the monarch butterfly, Danaus plexippus, in Australia. Aust. J. Zool. 1984, 32, 663–670. [Google Scholar] [CrossRef]
- James, D.G.; James, T.A. Migration and overwintering in Australian monarch butterflies, Danaus plexippus (L.) (Lepidoptera: Nymphalidae): A review with new observations and research needs. J. Lep. Soc. 2019, 73, 177–190. [Google Scholar] [CrossRef]




| Plant Species | Blooming at Googleplex 2021 | Blooming Period: Santa Clara County | Native or Ornamental |
|---|---|---|---|
| Limonium perezii (Stapf) | 31 Jan–31 May | Year-round | Ornamental |
| Verbena lilicina Greene | 31 Jan–31 May | Year-round | Ornamental |
| Lantana camara L. | 31 Jan–31 May | Year-round | Ornamental |
| Gaillardia aristata Pursh | 31 Jan–31 May | Year-round | Native |
| Achillea millefolium L. | 31 Jan–31 May | Year-round | Native |
| Erigeron glaucus Ker Gawl | 31 Jan–31 May | Year-round | Native |
| Lobularia maritima (L.) Desv. | 31 Jan–31 May | Year-round | Ornamental |
| Arctostaphylos sp. | 31 Jan–27 Mar | Winter | Native |
| Ribes malvaceum Sm. | 31 Jan–11 April | Winter | Native |
| Lupinus spp. | 31 Jan–18 April | Spring | Ornamental |
| Rhus ovatra S. Watson | 31 Jan–31 Mar | Winter–spring | Native |
| Solidago californica Nutt. | 31 Jan–2 April | Summer–fall | Native |
| Symphotrichum chilense (Nees) G.L Nesom | 31 Jan–21 Feb | Summer–fall | Native |
| Frangula californica(Eschsch) A. Gray | 21 Feb–21 Mar 16–31 May | Spring–summer | Native |
| Ceanothus spp. | 21 Feb–24 April | Spring | Native |
| Salvia sonomensis Greene | 6 March–7 May | Spring–summer | Native |
| Echium candicans L.f. | 27 March–30 April | Spring–summer | Ornamental |
| Lupinus albifrons Benth | 27 March–24 April | Spring–Summer | Native |
| Nepeta faasenii Bergmans ex Stearn | 11 Apr–31 May | Spring–summer | Ornamental |
| Asclepias fascicularis Dcne | 16–31 May | Spring–fall | Ornamental |
| Monardella villosa Benth | 31 May | Spring–summer | Native |
| Months | Jan–Feb | March | April | May–June |
|---|---|---|---|---|
| % infected (>100 spores) | 73.0 | 69.3 | 70.1 | 77.5 |
| Mean (±SE) OE Rating | 3.05 (0.097) | 2.90 (0.098) | 2.92 (0.111) | 3.13 (0.110) |
| No. examined | 137 | 137 | 107 | 111 |
| Months | Jan–Feb | March | April | May–June |
|---|---|---|---|---|
| No. Tagged | 130 | 134 | 108 | 127 |
| No. Recovered | 25 | 15 | 14 | 30 |
| % Recovered | 19.2 | 11.9 | 13.0 | 23.6 |
| Mean (±SE) period (days) from release to recovery | 27.8 (4.4) | 19.3 (3.3) | 21.1 (4.3) | 20.6 (2.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, D.G.; Schaefer, M.C.; Krimmer Easton, K.; Carl, A. First Population Study on Winter Breeding Monarch Butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) in the Urban South Bay of San Francisco, California. Insects 2021, 12, 946. https://doi.org/10.3390/insects12100946
James DG, Schaefer MC, Krimmer Easton K, Carl A. First Population Study on Winter Breeding Monarch Butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) in the Urban South Bay of San Francisco, California. Insects. 2021; 12(10):946. https://doi.org/10.3390/insects12100946
Chicago/Turabian StyleJames, David G., Maria C. Schaefer, Karen Krimmer Easton, and Annie Carl. 2021. "First Population Study on Winter Breeding Monarch Butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) in the Urban South Bay of San Francisco, California" Insects 12, no. 10: 946. https://doi.org/10.3390/insects12100946
APA StyleJames, D. G., Schaefer, M. C., Krimmer Easton, K., & Carl, A. (2021). First Population Study on Winter Breeding Monarch Butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) in the Urban South Bay of San Francisco, California. Insects, 12(10), 946. https://doi.org/10.3390/insects12100946







