Phylogenetic Position of the Genus Alulacris (Orthoptera: Acrididae: Melanoplinae: Podismini) Revealed by Complete Mitogenome Evidence
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Taxon Sampling
2.2. Sequencing, Assembly, and Annotation
2.3. Phylogenetic Analyses
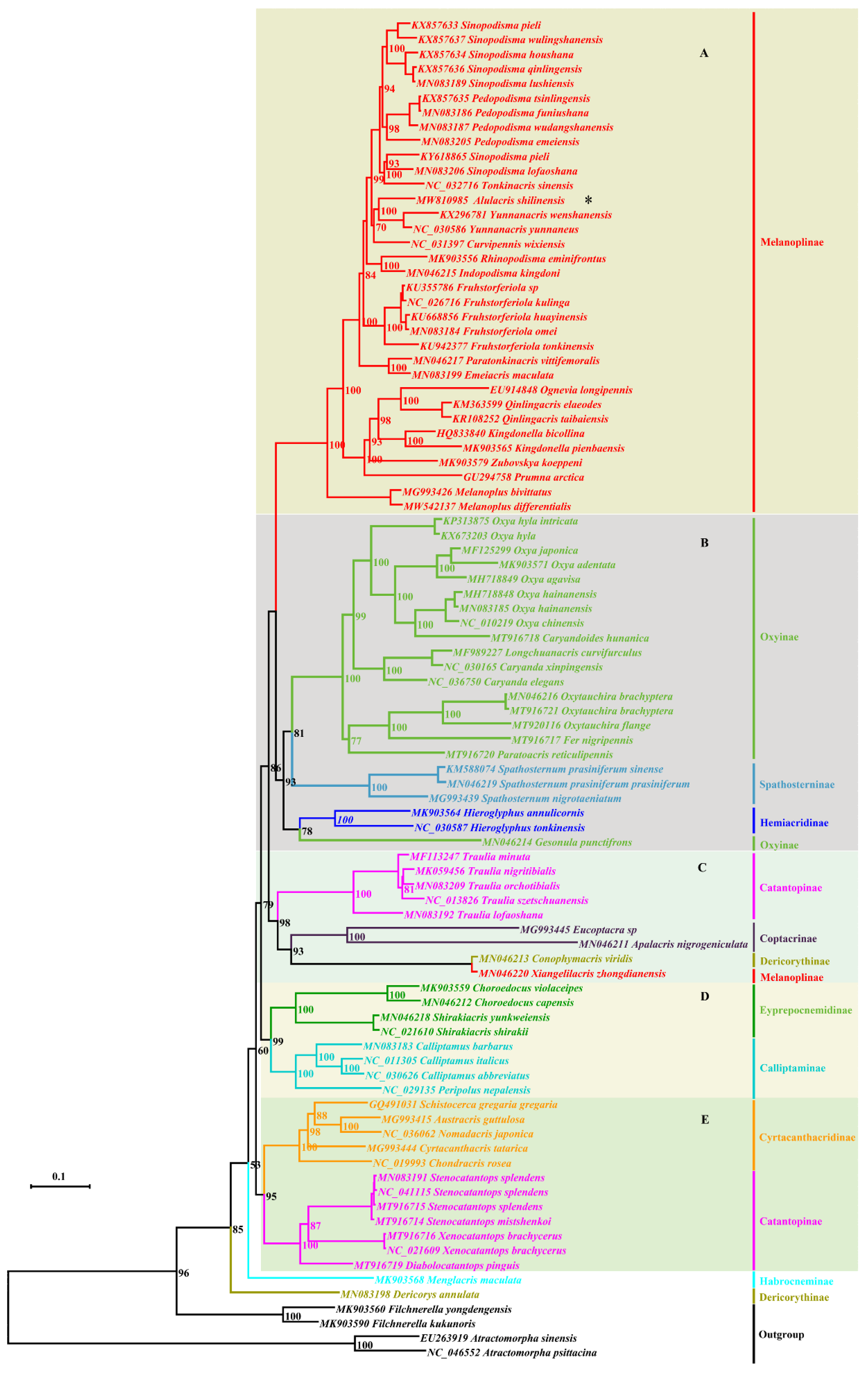
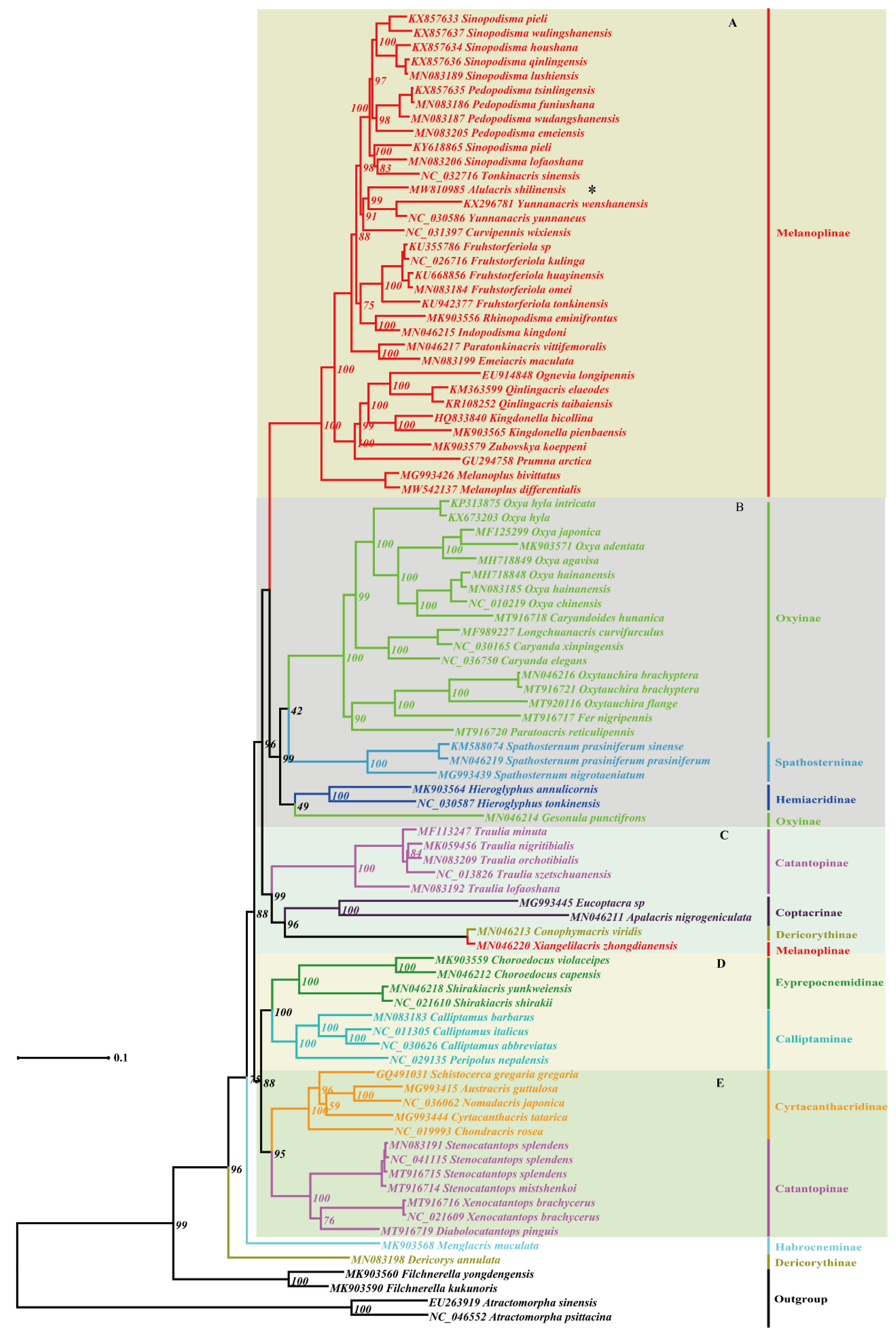
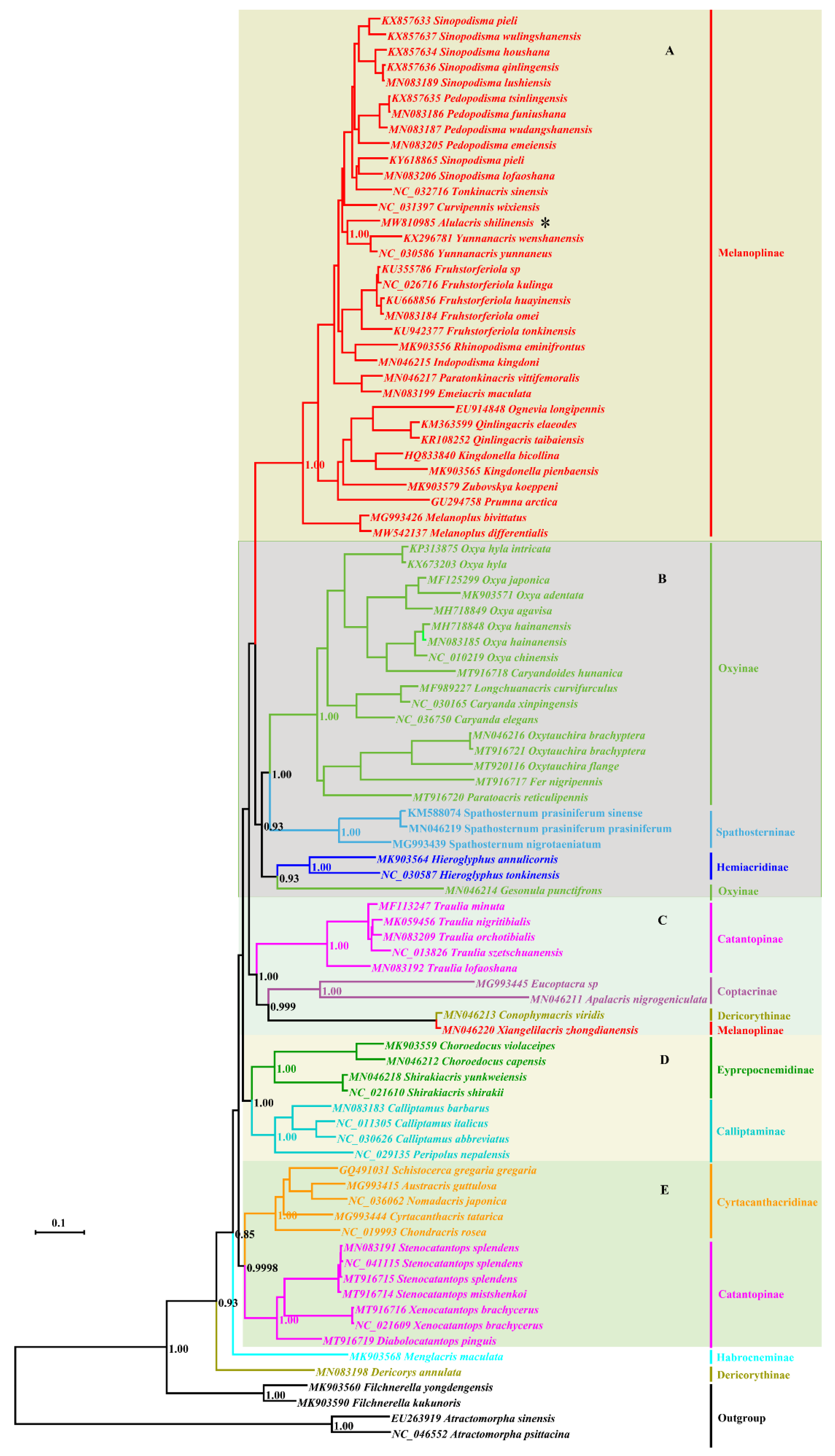
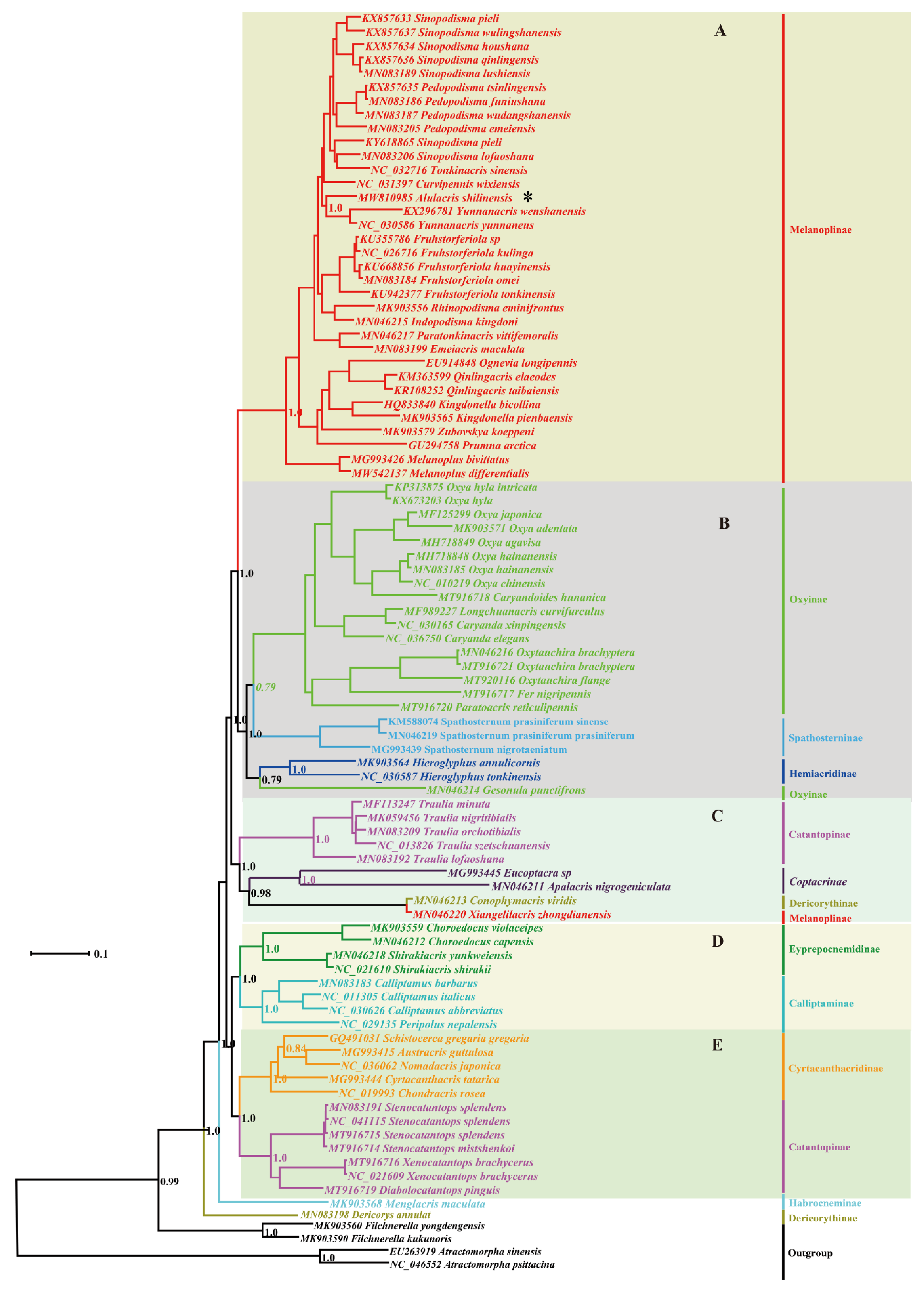
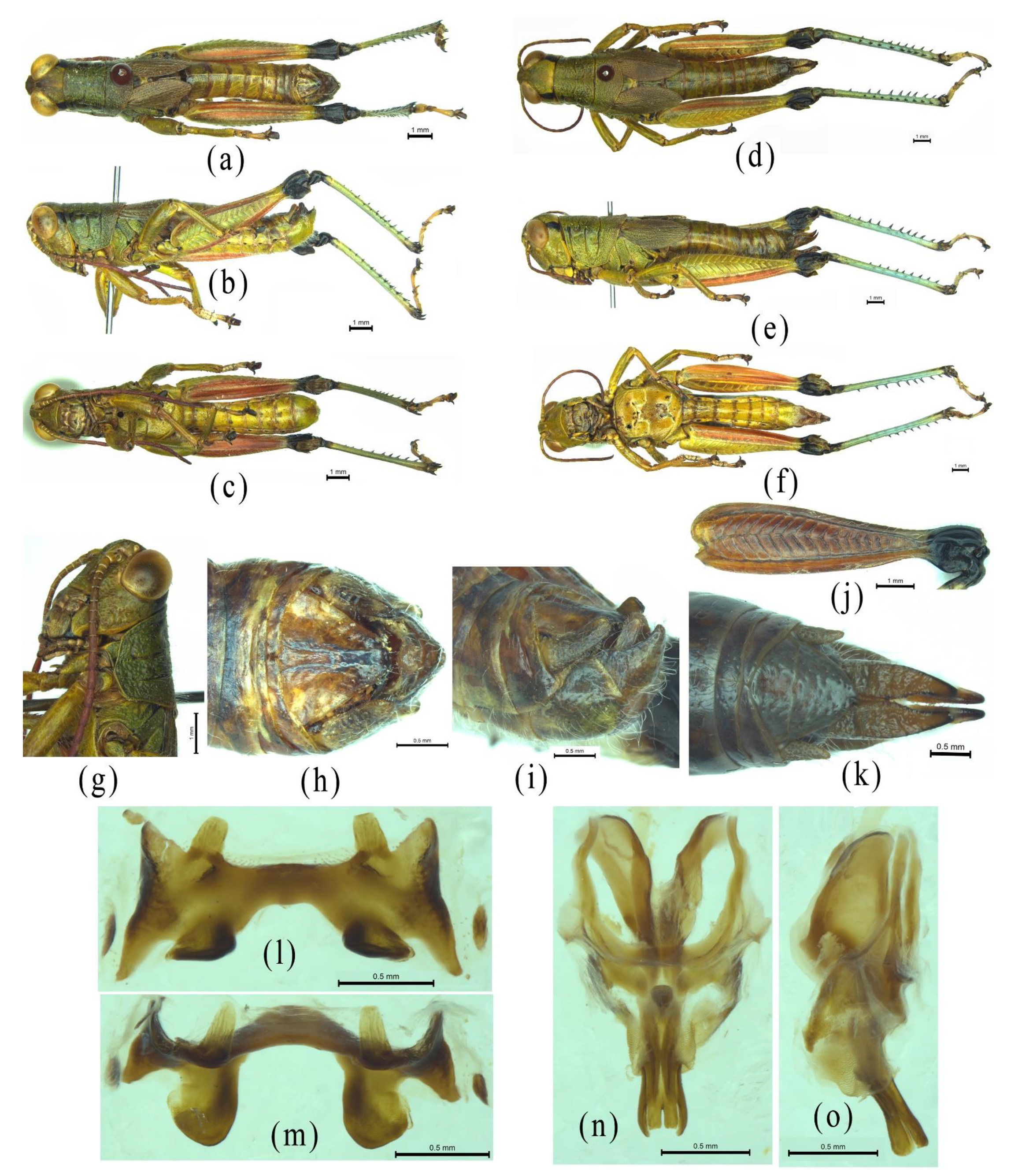
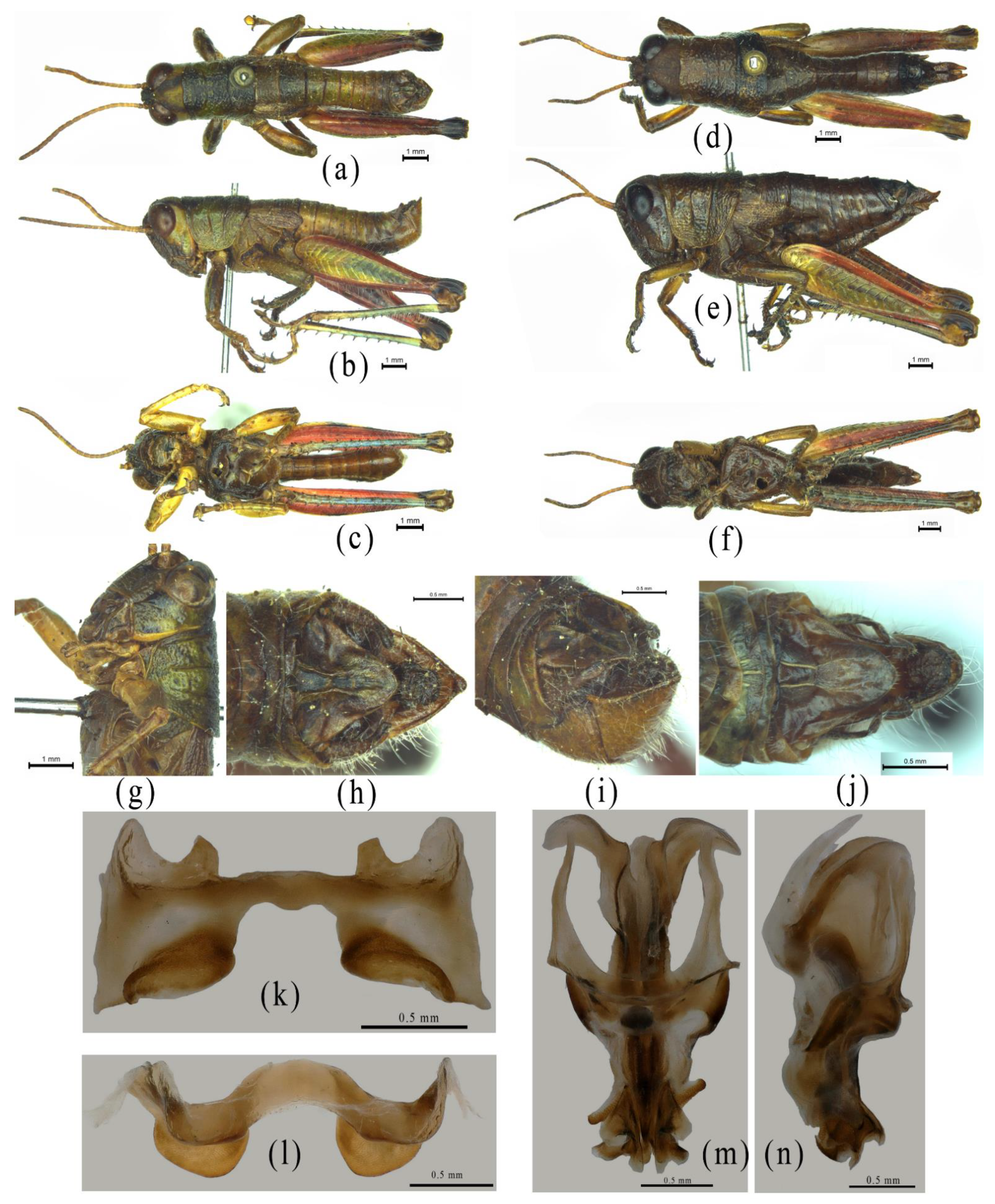
3. Results
3.1. Characteristics of the Mitogenome of Alulacris Shilinensis
3.2. Phylogeny
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [Green Version]
- Cameron, S.L. How to sequence and annotate insect mitochondrial genomes for systematic and comparative genomics research. Syst. Entomol. 2014, 39, 400–411. [Google Scholar] [CrossRef] [Green Version]
- Cameron, S.L.; Sullivan, J.; Song, H.; Miller, K.B.; Whiting, M.F. A mitochondrial genome phylogeny of the Neuropterida (lace-wings, alderflies and snakeflies) and their relationship to the other holometabolous insect orders. Zool. Scr. 2009, 38, 575–590. [Google Scholar] [CrossRef]
- Wan, X.; Kim, M.I.; Kim, M.J.; Kim, I. Complete mitochondrial genome of the free-living earwig, Challia fletcheri (Dermaptera: Pygidicranidae) and phylogeny of Polyneoptera. PLoS ONE 2012, 7, e42056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, X.; Winterton, S.L.; Yang, D. The first mitochondrial genome for the fishfly subfamily Chauliodinae and implications for the higher phylogeny of Megaloptera. PLoS ONE 2012, 7, e47302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, S.L.; Lambkin, C.L.; Barker, S.C.; Whiting, M.F. A mitochondrial genome phylogeny of Diptera: Whole genome sequence data accurately resolve relationships over broad timescales with high precision. Syst. Entomol. 2007, 32, 40–59. [Google Scholar] [CrossRef]
- Dowton, M.; Cameron, S.L.; Austin, A.D.; Whiting, M.F. Phylogenetic approaches for the analysis of mitochondrial genome sequence data in the Hymenoptera—A lineage with both rapidly and slowly evolving mitochondrial genomes. Mol. Phylogenet. Evol. 2009, 52, 512–519. [Google Scholar] [CrossRef]
- Timmermans, M.J.T.N.; Dodsworth, S.; Culverwell, C.L.; Bocak, L.; Ahrens, D.; Littlewood, D.T.J.; Pons, J.; Vogler, A.P. Why barcode? High-throughput multiplex sequencing of mitochondrial genomes for molecular systematics. Nucleic Acids Res. 2010, 38, e197. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Huang, D.-Y.; Sun, X.-Y.; Shi, Q.-H.; Hao, J.-S.; Zhang, L.-L.; Yang, Q. The first mitochondrial genome for the butterfly family Riodinidae (Abisara fylloides) and its systematic implications. Zool. Res. 2013, 34, E109–E119. [Google Scholar]
- Nelson, L.A.; Lambkin, C.L.; Batterham, P.; Wallman, J.F.; Dowton, M.; Whiting, M.F.; Yeates, D.K.; Cameron, S.L. Beyond barcoding: Genomic approaches to molecular diagnostics in blowflies (Diptera: Calliphoridae). Gene 2012, 511, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Winterton, S.L.; Liu, Z. Ancestral gene organization in the mitochondrial genome of Thyridosmylus langii (McLachlan, 1970) (Neuroptera: Osmylidae) and implications for lacewing evolution. PLoS ONE 2013, 8, e62943. [Google Scholar]
- Ma, C.; Yang, P.; Jiang, F.; Chapuis, M.-P.; Shali, Y.; Sword, G.A.; Kang, L. Mitochondrial genomes reveal the global phylogeography and dispersal routes of the migratory locust. Mol. Ecol. 2012, 21, 4344–4358. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, H.Y.; Gu, J.X.; Mao, B.Y.; Chen, Z.L.; Huang, Y.; Huang, J.H. Phylogenetic position of the genera Caryandoides, Paratoacris, Fer and Longchuanacris (Orthoptera: Acrididae) revealed by complete mitogenome sequences. Invert. Syst. 2021, 35, 725–741. [Google Scholar]
- Zheng, Z.M. New genera and new species of Acrididae from Yunnan-Kweichou Platau, China. Acta Entomol. Sinica 1977, 20, 303–313. [Google Scholar]
- Zheng, Z.M. New genera and new species of grasshoppers from Yunnan, Guizhou and Sichuan, China. Acta Zootax. Sinica 1981, 6, 60–68. [Google Scholar]
- Li, H.C.; Xia, K.L. Fauna Sinica, Insecta, vol. 43, Orthoptera, Acridoidea, Catantopidae; Science Press: Beijing, China, 2006. [Google Scholar]
- Mao, B.Y.; Ren, G.D.; Ou, X.H. Fauna, Distribution Pattern and Adaptability on Acridoidea from Yunnan; China Forestry Publishing House: Beijing, China, 2011. [Google Scholar]
- Zheng, Z.M.; Deng, W.A.; Wei, S.Z. One new species of the genus Alulacris Zheng (Orthoptera, Catantopidae) from Guizhou Province China. Acta Zootax. Sinica 2013, 38, 90–92. [Google Scholar]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. 2021. Available online: http://Orthoptera.SpeciesFile.org (accessed on 15 August 2021).
- Fenn, J.D.; Song, H.; Cameron, S.; Whiting, M.F. A preliminary mitochondrial genome phylogeny of Orthoptera (Insecta) and approaches to maximizing phylogenetic signal found within mitochondrial genome data. Mol. Phylogenet. Evol. 2008, 49, 59–68. [Google Scholar] [CrossRef]
- Sheffield, N.C.; Hiatt, K.D.; Valentine, M.C.; Song, H.; Whiting, M.F. Mitochondrial genomics in Orthoptera using MOSAS. Mitochondrial DNA 2010, 21, 87–104. [Google Scholar] [CrossRef]
- Zhou, Z.; Ye, H.; Huang, Y.; Shi, F. The phylogeny of Orthoptera inferred from mtDNA and description of Elimaea cheni (Tettigoniidae: Phaneropterinae) mitogenome. J. Genet. Genom. 2010, 37, 315–324. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, L.; Liu, N.; Guo, H.; Guan, B.; Di, J.; Shi, F. Towards a higher-level Ensifera phylogeny inferred from mitogenome sequences. Mol. Phylogenet. Evol. 2017, 108, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, J.R.; Hiatt, K.D.; Whiting, M.F.; Song, H. Searching for the optimal data partitioning strategy in mitochondrial phylogenomics: A phylogeny of Acridoidea (Insecta: Orthoptera: Caelifera) as a case study. Mol. Phylogenet. Evol. 2013, 67, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Huang, Y.; Lin, L.-L.; Wang, X.-Y.; Zheng, Z.-M. The phylogeny of the Orthoptera (Insecta) as deduced from mitogenomic gene sequences. Zool. Stud. 2013, 52, 37. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-L.; Zeng, H.-H.; Huang, Y.; Zheng, Z.-M. The complete mitochondrial genomes of three grasshoppers, Asiotmethis zacharjini, Filchnerella helanshanensis and Pseudotmethis rubimarginis (Orthoptera: Pamphagidae). Gene 2013, 517, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Amédégnato, C.; Cigliano, M.M.; Desutter-Grandcolas, L.; Heads, S.W.; Huang, Y.; Otte, D.; Whiting, M.F. 300 million years of diversification: Elucidating the patterns of orthopteran evolution based on comprehensive taxon and gene sampling. Cladistics 2015, 31, 621–651. [Google Scholar] [CrossRef]
- Song, H.; Mariño-Pérez, R.; A Woller, D.; Cigliano, M.M. Evolution, Diversification, and Biogeography of Grasshoppers (Orthoptera: Acrididae). Insect Syst. Divers. 2018, 2, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yan, L.; Jiang, G. The complete mitochondrial genome of the jumping grasshopper Sinopodisma pieli (Orthoptera: Acrididae) and the phylogenetic analysis of Melanoplinae. Zootaxa 2017, 4363, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Huang, Y. Characterization of the mitochondrial genomics and phylogeny of orthoptera (Insecta: Arthropoda). Chin. Bull. Life Sci. 2018, 30, 113–123. [Google Scholar]
- Li, R.; Shu, X.; Li, X.; Meng, L.; Li, B. Comparative mitogenome analysis of three species and monophyletic inference of Catantopinae (Orthoptera: Acridoidea). Genomics 2019, 111, 1728–1735. [Google Scholar] [CrossRef]
- Li, R.; Shu, X.; Deng, W.; Meng, L.; Li, B. Complete mitochondrial genome of Atractomorpha sagittaris (Orthoptera: Pyrgomorphidae) and its phylogenetic analysis for Acrididea. Biologia 2020, 75, 1571–1583. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Shu, X.; Meng, L.; Li, B. Complete mitochondrial genomes of three Oxya grasshoppers (Orthoptera) and their implications for phylogenetic reconstruction. Genomics 2020, 112, 289–296. [Google Scholar] [CrossRef]
- Qiu, Z.; Chang, H.; Yuan, H.; Huang, Y.; Lu, H.; Li, X.; Gou, X. Comparative mitochondrial genomes of four species of Sinopodisma and phylogenetic implications (Orthoptera, Melanoplinae). ZooKeys 2020, 969, 23–42. [Google Scholar]
- Li, X.-D.; Jiang, G.-F.; Yan, L.-Y.; Li, R.; Mu, Y.; Deng, W.-A. Positive selection drove the adaptation of mitochondrial genes to the demands of flight and high-altitude environments in grasshoppers. Front. Genet. 2018, 9, 605. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Nie, Y.; Zhang, N.; Zhang, X.; Sun, H.; Mao, Y.; Qiu, Z.Y.; Huang, Y. MtOrt: An empirical mitochondrial amino acid substitution model for evolutionary studies of Orthoptera insects. BMC Evol. Biol. 2020, 20, 57. [Google Scholar] [CrossRef]
- Chang, H.; Qiu, Z.; Yuan, H.; Wang, X.; Li, X.; Sun, H.; Guo, X.; Lu, Y.; Feng, X.; Majid, M.; et al. Evolutionary rates of and selective constraints on the mitochondrial genomes of Orthoptera insects with different wing types. Mol. Phylogenet. Evol. 2020, 145, 106734. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-P.; Zheng, F.-Y.; Bai, J.; Wang, J.-M.; Lv, C.-Y.; Li, X.; Zhi, Y.-C.; Li, X.-J. Comparative analysis of mitogenomes among six species of grasshoppers (Orthoptera: Acridoidea: Catantopidae) and their phylogenetic implications in wing-type evolution. Int. J. Biol. Macromol. 2020, 159, 1062–1072. [Google Scholar] [CrossRef]
- Hollis, D. A review of the subfamily Oxyinae (Orthoptera: Acridoidea). Bull. Brit. Mus. (Nat. Hist.), Entomol. Ser. 1975, 31, 189–234. [Google Scholar] [CrossRef]
- Uvarov, B.P. Grasshoppers and Locusts. A Handbook of General Acridology. Vol. 1. Anatomy, Physiology, Development, Phase Polymorphism, Introduction to Taxonomy; University of Cambridge Press: Cambridge, UK, 1966. [Google Scholar]
- Storozhenko, S.Y.; Kim, T.W.; Jeon, M.J. Monograph of Korean Orthoptera; National Institute of Biological Resources: Incheon, Korea, 2015.
- Dirsh, V.M. The phallic complex in Acridoidea (Orthoptera) in relation to taxonomy. T. Roy. Entomol. Soc. London 1956, 108, 223–356. [Google Scholar] [CrossRef]
- Hahn, C.; Bachmann, L.; Chevreux, B. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads—A baiting and iterative mapping approach. Nucleic Acids Res. 2013, 41, e129. [Google Scholar] [CrossRef] [Green Version]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Darty, K.; Denise, A.; Ponty, Y. VARNA: Interactive drawing and editing of the RNA secondary structure. Bioinformatics 2009, 25, 1974–1975. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Abascal, F.; Zardoya, R.; Telford, M.J. TranslatorX: Multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 2010, 38, W7–W13. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.-T.; Schmidt, H.A.; Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.-T.; Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [Green Version]
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hna, S.H.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Erixon, P.; Svennblad, B.; Britton, T.; Oxelman, B. Reliability of Bayesian posterior probabilities and bootstrap frequencies in phylogenetics. Syst. Biol. 2003, 52, 665–673. [Google Scholar] [CrossRef]
- Otte, D. Orthoptera Species File 4; The Orthopterists’ Society and The Academy of Natural Sciences of Philadelphia: Philadelphia, PA, USA, 1995. [Google Scholar]
- Key, K.H.L.; Colles, D.H. A higher classification of the Australian Acridoidea (Orthoptera). II. Subfamily Catantopinae. Invertebr. Tax. 1993, 7, 89–111. [Google Scholar] [CrossRef]
- Storozhenko, S.Y. A new species of the genus MesambriaStål, 1878 with notes on the tribe Mesambriini (Orthoptera: Acrididae, Catantopinae). Zootaxa 2018, 4418, 55–65. [Google Scholar] [CrossRef]
- Mistshenko, L.L. Locusts and Grasshoppers, Catantopinae. Fauna of USSR, Orthoptera. Tome 4. No. 2; Academy of Sciences of USSR Publ.: Moscow-Leningrad, Russia, 1952. [Google Scholar]
- Liu, D.F.; Dong, Z.M.; Zhang, D.Y.; Gu, Y.Z.; Guo, P.J.; Han, R.H.; Jiang, G.F. Molecular phylogeny of the higher category of Acrididae (Orthoptera: Acridoidea). Zool. Res. 2008, 29, 585–591. [Google Scholar] [CrossRef]
- Chapco, W. A note on the molecular phylogeny of small sample of catantopine grasshoppers. J. Orth. Res. 2013, 22, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Woller, D.A.; Fontana, P.; Mariño-Pérez, R.; Song, H. Studies in Mexican grasshoppers: Liladownsia fraile, a new genus and species of Dactylotini (Acrididae: Melanoplinae) and an updated molecular phylogeny of Melanoplinae. Zootaxa 2014, 3793, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Chapco, W.; Litzenberger, G.; Kuperus, W.R. A molecular biogeographic analysis of the relationship between North American melanoploid grasshoppers and their Eurasian and South American relatives. Mol. Phylogenet. Evol. 2001, 18, 460–466. [Google Scholar] [CrossRef]
- Litzenberger, G.; Chapco, W. Molecular phylogeny of selected Eurasian Podismine Grasshoppers (Orthoptera: Acrididae). Ann. Entomol. Soc. Am. 2001, 94, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Litzenberger, G.; Chapco, W. The North American Melanoplinae (Orthoptera: Acrididae): A molecular phylogenetic study of their origins and taxonomic relationships. Ann. Entomol. Soc. Am. 2003, 96, 491–497. [Google Scholar] [CrossRef]
- Amédégnato, C.; Chapco, W.; Litzenberger, G. Out of South America? Additional evidence for a southern origin of melanopline grasshoppers. Mol. Phylogenet. Evol. 2003, 29, 115–119. [Google Scholar] [CrossRef]
- Chintauan-Marquier, I.C.; Jordan, S.; Berthier, P.; Amédégnato, C.; Pompanon, F. Evolutionary history and taxonomy of a short-horned grasshopper subfamily: The melanoplinae (Orthoptera: Acrididae). Mol. Phylogenet. Evol. 2011, 58, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Chintauan-Marquier, I.C.; Amédégnato, C.; Nichols, R.A.; Pompanon, F.; Grandcolas, P.; Desutter-Grandcolas, L. Inside the Melanoplinae: New molecular evidence for the evolutionary history of the Eurasian Podismini (Orthoptera: Acrididae). Mol. Phylogenet. Evol. 2014, 71, 224–233. [Google Scholar] [CrossRef]
- Roberts, H.R. A comparative study of the subfamilies of the Acrididae primarily on the basis of their phallic structures. P. Acad. Nat. Sci. Phila. 1941, 93, 201–246. [Google Scholar]
- Rehn, J.A.G.; Randell, R.L. A preliminary analysis of the lines of the super-tribe Melanoplini (Orthoptera: Acrididae: Cyrtacanthacridinae). P. Acad. Nat. Sci. Phila. 1963, 115, 1–32. [Google Scholar]
- Willemse, C. Revision des Acridiodea, décrites par De Haan, avec descriptions de nouvelles espèces. Zool. Mededelingen 1928, 11, 1–27. [Google Scholar]
- Bhowmik, H.K.; Halder, P. A new species of Circocephalus Willemse, 1928 from the foothill of the eastern Himalaya. Geobios 1982, 1, 8–10. [Google Scholar]
- Zheng, Z.M. A new species of Circocephalus Willemse from Heilongjiang Province, China (Orthoptera: Catantopidae). Acta Zootax. Sin. 1985, 10, 403–405. [Google Scholar]
- Bhowmik, H.K. Grasshopper fauna of west Bengal, India (Orthoptera: Acrididae). Zool. Surv. India, Techn. Monogr. 1986, 14, 1–180. [Google Scholar]
- Bhowmik, H.K.; Saha, B.C.; Bhargava, R.N. Contribution to the Acridid fauna (Orthoptera) of north-eastern states of India. Rec. Zool. Surv. India 1990, 86, 217–227. [Google Scholar]
- Ito, G. A systematic study of the grasshopper tribe Podismini in Japan (Orthoptera: Acrididae). Insecta Mats. J. Fac. Agr. Hokkaido Univ. 2015, 71, 1–119. [Google Scholar]
| Model Selection | Best Model | Partition Names |
|---|---|---|
| AICC | GTR + I + G | ATP6_pos1, ATP6_pos2, ATP6_pos3 |
| ATP8_pos1, ATP8_pos2 | ||
| COX1_pos1, COX1_pos2, COX1_pos3 | ||
| COX2_pos1, COX2_pos2, COX2_pos3 | ||
| COX3_pos2, COX3_pos3 | ||
| CYTB_pos1, CYTB_pos2, CYTB_pos3 | ||
| ND1_pos2, ND1_pos3 | ||
| ND2_pos1, ND2_pos2, ND2_pos3 | ||
| ND3_pos1, ND3_pos2 | ||
| ND4_pos1, ND4_pos2, ND4_pos3 | ||
| ND4L_pos3 | ||
| ND5_pos1, ND5_pos2, ND5_pos3 | ||
| ND6_pos3 | ||
| GTR + G | COX3_pos1 | |
| ND1_pos1 | ||
| ND3_pos3 | ||
| ND4L_pos2 | ||
| ND6_pos1, ND6_pos2 | ||
| RrnL, rrnS | ||
| HKY+I+G | ATP8_pos3 | |
| COX3_pos2 | ||
| ND4L_pos1 |
| Feature | Length(bp) | A% | C% | G% | T% | A + T% | AT-skew | GC-skew |
|---|---|---|---|---|---|---|---|---|
| Whole genome | 16,950 | 41.2 | 14.6 | 11.3 | 32.9 | 74.1 | 0.1120 | −0.1274 |
| Protein-coding genes | 11,145 | 31.8 | 12.9 | 12.9 | 42.4 | 74.2 | −0.1429 | 0.0000 |
| First codon position | 3715 | 32.0 | 12.5 | 20.3 | 35.5 | 67.5 | −0.0519 | 0.2378 |
| Second codon position | 3715 | 19.4 | 20.5 | 14.3 | 45.8 | 65.2 | −0.4049 | −0.1782 |
| Third codon position | 3715 | 43.9 | 5.6 | 4.1 | 46.4 | 90.3 | −0.0277 | −0.1546 |
| Protein-coding genes-J | 6867 | 35.9 | 15.2 | 12.3 | 29.1 | 65.0 | 0.1046 | −0.1055 |
| First codon position | 2289 | 35.0 | 15.2 | 20.8 | 29.1 | 64.1 | 0.0920 | 0.1556 |
| Second codon position | 2289 | 19.8 | 22.2 | 13.7 | 44.3 | 64.1 | −0.3822 | −0.2368 |
| Third codon position | 2289 | 25.8 | 8.3 | 2.4 | 36.5 | 62.3 | −0.1717 | −0.5514 |
| Protein-coding genes-N | 4278 | 25.2 | 9.1 | 14.0 | 51.8 | 77.0 | −0.3455 | 0.2121 |
| First codon position | 1426 | 27.1 | 8.3 | 19.6 | 45.0 | 72.1 | −0.2483 | 0.4050 |
| Second codon position | 1426 | 18.7 | 17.7 | 15.4 | 48.2 | 66.9 | −0.4410 | −0.0695 |
| Third codon position | 1426 | 29.7 | 2.3 | 6.9 | 62.2 | 91.9 | −0.3536 | 0.5000 |
| ATP6 | 678 | 37.9 | 15.3 | 9.6 | 37.2 | 75.1 | 0.0093 | −0.2289 |
| ATP8 | 162 | 43.8 | 12.3 | 6.2 | 37.7 | 81.5 | 0.0748 | −0.3297 |
| COX1 | 1540 | 33.6 | 15.5 | 15.5 | 35.4 | 69.0 | −0.0261 | 0.0000 |
| COX2 | 684 | 36.5 | 16.1 | 13.6 | 33.8 | 70.3 | 0.0384 | −0.0842 |
| COX3 | 792 | 32.7 | 17.7 | 14.5 | 35.1 | 52.8 | −0.3295 | −0.0994 |
| CYTB | 1137 | 34.3 | 14.7 | 12.5 | 38.5 | 72.8 | −0.0577 | −0.0809 |
| ND1 | 942 | 50.1 | 13.3 | 9.8 | 26.9 | 77.0 | 0.3013 | −0.1515 |
| ND2 | 1018 | 37.8 | 15.6 | 36.5 | 25.6 | 63.4 | 0.1924 | 0.4012 |
| ND3 | 353 | 35.7 | 14.2 | 12.2 | 38.0 | 73.7 | −0.0312 | −0.0758 |
| ND4 | 1335 | 52.5 | 14.5 | 8.8 | 24.3 | 76.8 | 0.3672 | −0.2446 |
| ND4L | 294 | 57.8 | 14.3 | 7.5 | 20.4 | 78.2 | 0.4783 | −0.3119 |
| ND5 | 1719 | 51.0 | 13.8 | 9.2 | 25.9 | 76.9 | 0.3264 | −0.2000 |
| ND6 | 522 | 42.0 | 10.7 | 6.9 | 40.4 | 82.4 | 0.0194 | −0.2159 |
| rrnL | 1377 | 44.4 | 15.0 | 8.9 | 31.7 | 76.1 | 0.1669 | −0.2552 |
| rrnS | 793 | 39.8 | 16.6 | 10.5 | 33.0 | 72.8 | 0.0934 | −0.2251 |
| D-loop | 2121 | 35.6 | 13.5 | 14.1 | 36.9 | 72.5 | −0.0179 | 0.0217 |
| Name | Type | Minimum | Maximum | Length | Direction | START | END | Interval |
|---|---|---|---|---|---|---|---|---|
| trnI | tRNA | 1 | 66 | 66 | + | 4 | ||
| trnQ | tRNA | 71 | 139 | 69 | − | 1 | ||
| trnM | tRNA | 141 | 209 | 69 | + | 0 | ||
| ND2 | CDS | 210 | 1228 | 1019 | + | ATG | TA | 2 |
| trnW | tRNA | 1231 | 1298 | 68 | + | −8 | ||
| trnC | tRNA | 1291 | 1355 | 65 | − | 8 | ||
| trnY | tRNA | 1364 | 1429 | 66 | − | −8 | ||
| COX1 | CDS | 1422 | 2961 | 1540 | + | ATC | T | 0 |
| trnL2 | tRNA | 2962 | 3027 | 66 | + | 4 | ||
| COX2 | CDS | 3032 | 3715 | 684 | + | ATG | TAA | −2 |
| trnD | tRNA | 3714 | 3778 | 65 | + | 2 | ||
| trnK | tRNA | 3781 | 3851 | 71 | + | 14 | ||
| ATP8 | CDS | 3866 | 4027 | 162 | + | ATC | TAA | −7 |
| ATP6 | CDS | 4021 | 4698 | 678 | + | ATG | TAA | 4 |
| COX3 | CDS | 4703 | 5494 | 792 | + | ATG | TAA | 2 |
| trnG | tRNA | 5497 | 5563 | 67 | + | 0 | ||
| ND3 | CDS | 5564 | 5917 | 354 | + | ATT | TAA | −1 |
| trnA | tRNA | 5917 | 5981 | 65 | + | 2 | ||
| trnR | tRNA | 5984 | 6048 | 65 | + | 3 | ||
| trnN | tRNA | 6052 | 6120 | 69 | + | 0 | ||
| trnS1 | tRNA | 6121 | 6187 | 67 | + | 0 | ||
| trnE | tRNA | 6188 | 6253 | 66 | + | 0 | ||
| trnF | tRNA | 6254 | 6319 | 66 | − | 1 | ||
| ND5 | CDS | 6321 | 8039 | 1719 | − | ATT | TAA | 15 |
| trnH | tRNA | 8055 | 8119 | 65 | − | 4 | ||
| ND4 | CDS | 8124 | 9458 | 1335 | − | ATG | TAA | −7 |
| ND4L | CDS | 9452 | 9745 | 294 | − | ATG | TAA | 2 |
| trnT | tRNA | 9748 | 9816 | 69 | + | 0 | ||
| trnP | tRNA | 9817 | 9880 | 64 | − | 2 | ||
| ND6 | CDS | 9883 | 10,404 | 522 | + | ATG | TAA | 6 |
| CYTB | CDS | 10,411 | 11,550 | 1140 | + | ATG | TAA | 1 |
| trnS2 | tRNA | 11,552 | 11,621 | 70 | + | 16 | ||
| ND1 | CDS | 11,638 | 12,582 | 945 | − | ATA | TAG | 3 |
| trnL1 | tRNA | 12,586 | 12,651 | 66 | − | −51 | ||
| rrnL | rRNA | 12,601 | 13,977 | 1377 | − | −14 | ||
| trnV | tRNA | 13,964 | 14,034 | 71 | − | 2 | ||
| rrnS | rRNA | 14,037 | 14,829 | 793 | − | 0 | ||
| D-loop | unsure | 14,830 | 16,950 | 2121 | + |
| Amino Acid | Codon | No. | RSCU (%) | Amino Acid | Codon | No. | RSCU (%) |
|---|---|---|---|---|---|---|---|
| Phe | UUU | 297 | 1.73 | Tyr | UAU | 143 | 1.73 |
| UUC | 47 | 0.27 | UAC | 22 | 0.27 | ||
| Leu | UUA | 369 | 4.13 | End | UAA | 0 | 0 |
| UUG | 30 | 0.34 | UAG | 0 | 0 | ||
| Leu | CUU | 74 | 0.83 | His | CAU | 55 | 1.53 |
| CUC | 10 | 0.11 | CAC | 17 | 0.47 | ||
| CUA | 49 | 0.55 | Gln | CAA | 60 | 1.85 | |
| CUG | 4 | 0.04 | CAG | 5 | 0.15 | ||
| Ile | AUU | 334 | 1.8 | Asn | AAU | 144 | 1.71 |
| AUC | 37 | 0.2 | AAC | 24 | 0.29 | ||
| Met | AUA | 227 | 1.79 | Lys | AAA | 65 | 1.41 |
| AUG | 27 | 0.21 | AAG | 27 | 0.59 | ||
| Val | GUU | 104 | 2.12 | Asp | GAU | 68 | 1.74 |
| GUC | 3 | 0.06 | GAC | 10 | 0.26 | ||
| GUA | 84 | 1.71 | Glu | GAA | 68 | 1.68 | |
| GUG | 5 | 0.1 | GAG | 13 | 0.32 | ||
| Ser | UCU | 123 | 2.71 | Cys | UGU | 41 | 1.91 |
| UCC | 5 | 0.11 | UGC | 2 | 0.09 | ||
| UCA | 126 | 2.78 | Trp | UGA | 89 | 1.78 | |
| UCG | 1 | 0.02 | UGG | 11 | 0.22 | ||
| Pro | CCU | 67 | 1.99 | Arg | CGU | 17 | 1.19 |
| CCC | 5 | 0.15 | CGC | 0 | 0 | ||
| CCA | 60 | 1.78 | CGA | 38 | 2.67 | ||
| CCG | 3 | 0.09 | CGG | 2 | 0.14 | ||
| Thr | ACU | 63 | 1.3 | Ser | AGU | 30 | 0.66 |
| ACC | 11 | 0.23 | AGC | 3 | 0.07 | ||
| ACA | 117 | 2.41 | AGA | 75 | 1.65 | ||
| ACG | 3 | 0.06 | AGG | 0 | 0 | ||
| Ala | GCU | 76 | 1.72 | Gly | GGU | 86 | 1.54 |
| GCC | 8 | 0.18 | GGC | 4 | 0.07 | ||
| GCA | 89 | 2.01 | GGA | 115 | 2.05 | ||
| GCG | 4 | 0.09 | GGG | 19 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Mao, B.; Storozhenko, S.Y.; Huang, Y.; Chen, Z.; Huang, J. Phylogenetic Position of the Genus Alulacris (Orthoptera: Acrididae: Melanoplinae: Podismini) Revealed by Complete Mitogenome Evidence. Insects 2021, 12, 918. https://doi.org/10.3390/insects12100918
Xu H, Mao B, Storozhenko SY, Huang Y, Chen Z, Huang J. Phylogenetic Position of the Genus Alulacris (Orthoptera: Acrididae: Melanoplinae: Podismini) Revealed by Complete Mitogenome Evidence. Insects. 2021; 12(10):918. https://doi.org/10.3390/insects12100918
Chicago/Turabian StyleXu, Haiyang, Benyong Mao, Sergey Yu. Storozhenko, Yuan Huang, Zhilin Chen, and Jianhua Huang. 2021. "Phylogenetic Position of the Genus Alulacris (Orthoptera: Acrididae: Melanoplinae: Podismini) Revealed by Complete Mitogenome Evidence" Insects 12, no. 10: 918. https://doi.org/10.3390/insects12100918
APA StyleXu, H., Mao, B., Storozhenko, S. Y., Huang, Y., Chen, Z., & Huang, J. (2021). Phylogenetic Position of the Genus Alulacris (Orthoptera: Acrididae: Melanoplinae: Podismini) Revealed by Complete Mitogenome Evidence. Insects, 12(10), 918. https://doi.org/10.3390/insects12100918






