Preferential Attraction of Oviposition-Ready Oriental Fruit Flies to Host Fruit Odor over Protein Food Odor
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Host Fruits and Torula Yeast
2.3. Attraction and Oviposition Response Bioassay
2.4. Comparison of Mated B. dorsalis Attraction and Oviposition Response to Host Fruit versus Torula Yeast
2.5. Selection of a Preferred Host Fruit Using Multiple-Choice Test
2.6. Headspace Volatile Collection
2.7. Comparison of Mated B. dorsalis Attraction and Oviposition Response to Headspace Extracts of Host Fruit versus Torula Yeast
2.8. Oviposition Response of Mated B. dorsalis Attracted to Guava Juice versus Torula Yeast
2.9. Statistical Analyses
3. Results
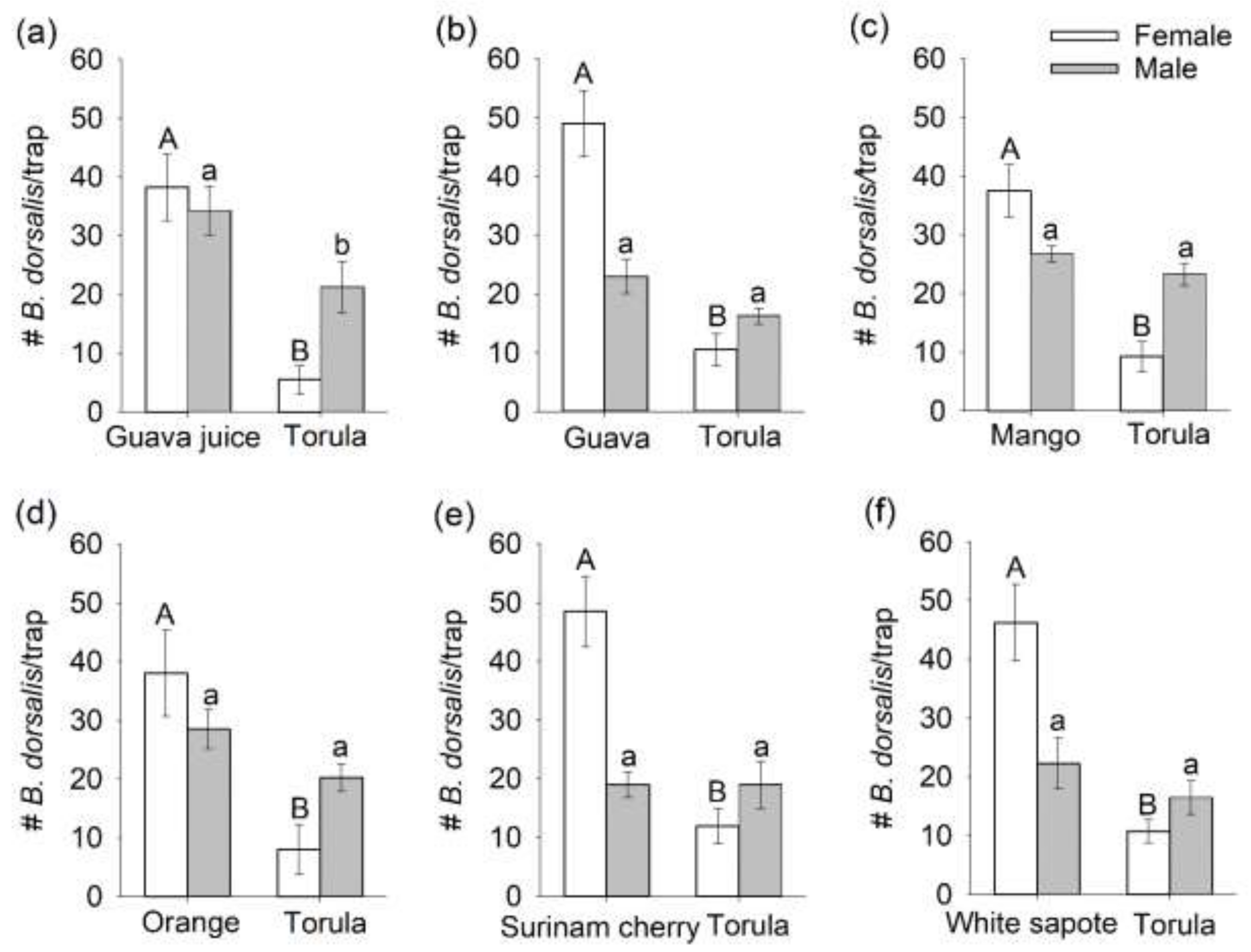
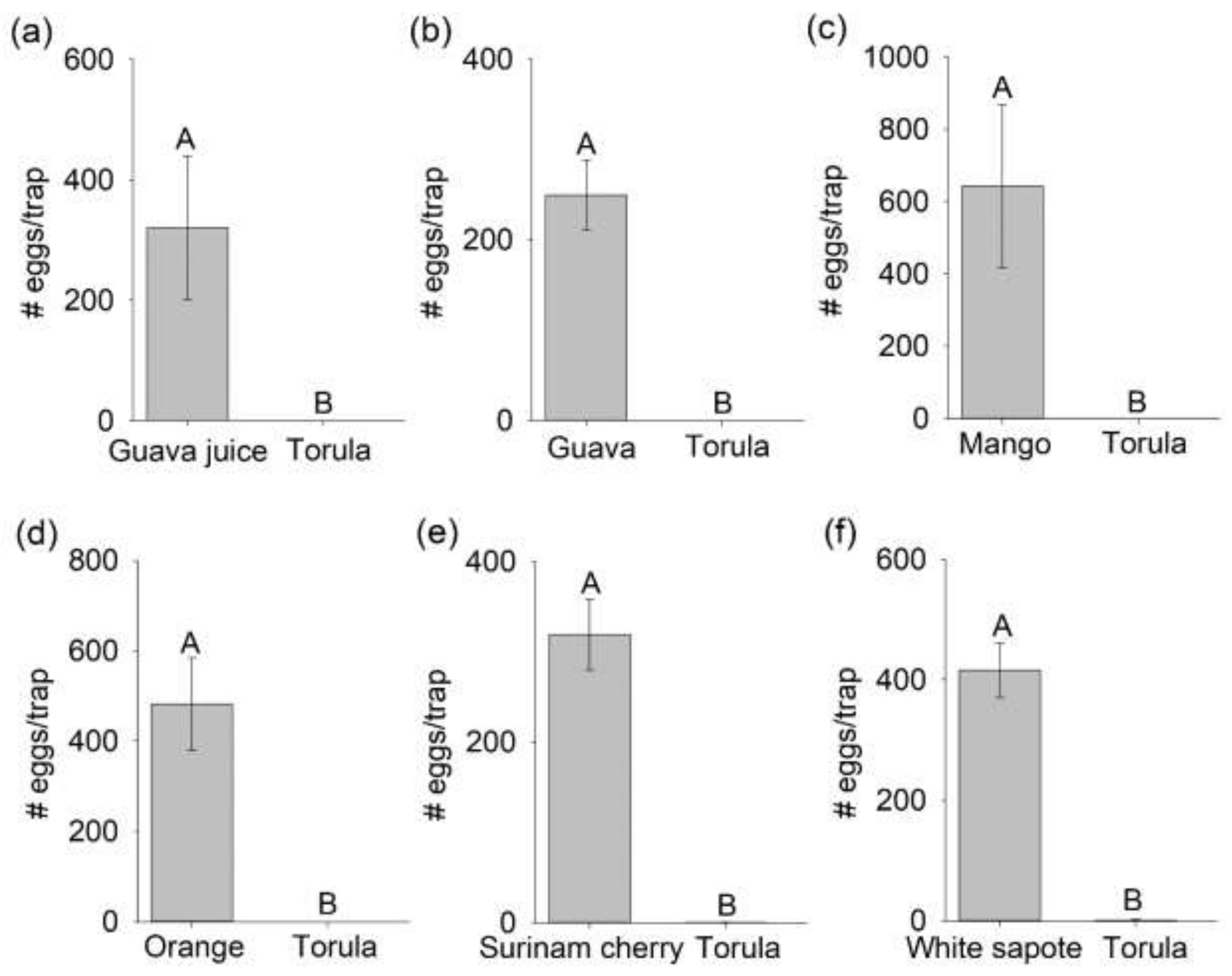
3.1. Comparison of Mated B. dorsalis Attraction and Oviposition Response to Host Fruit versus Torula Yeast
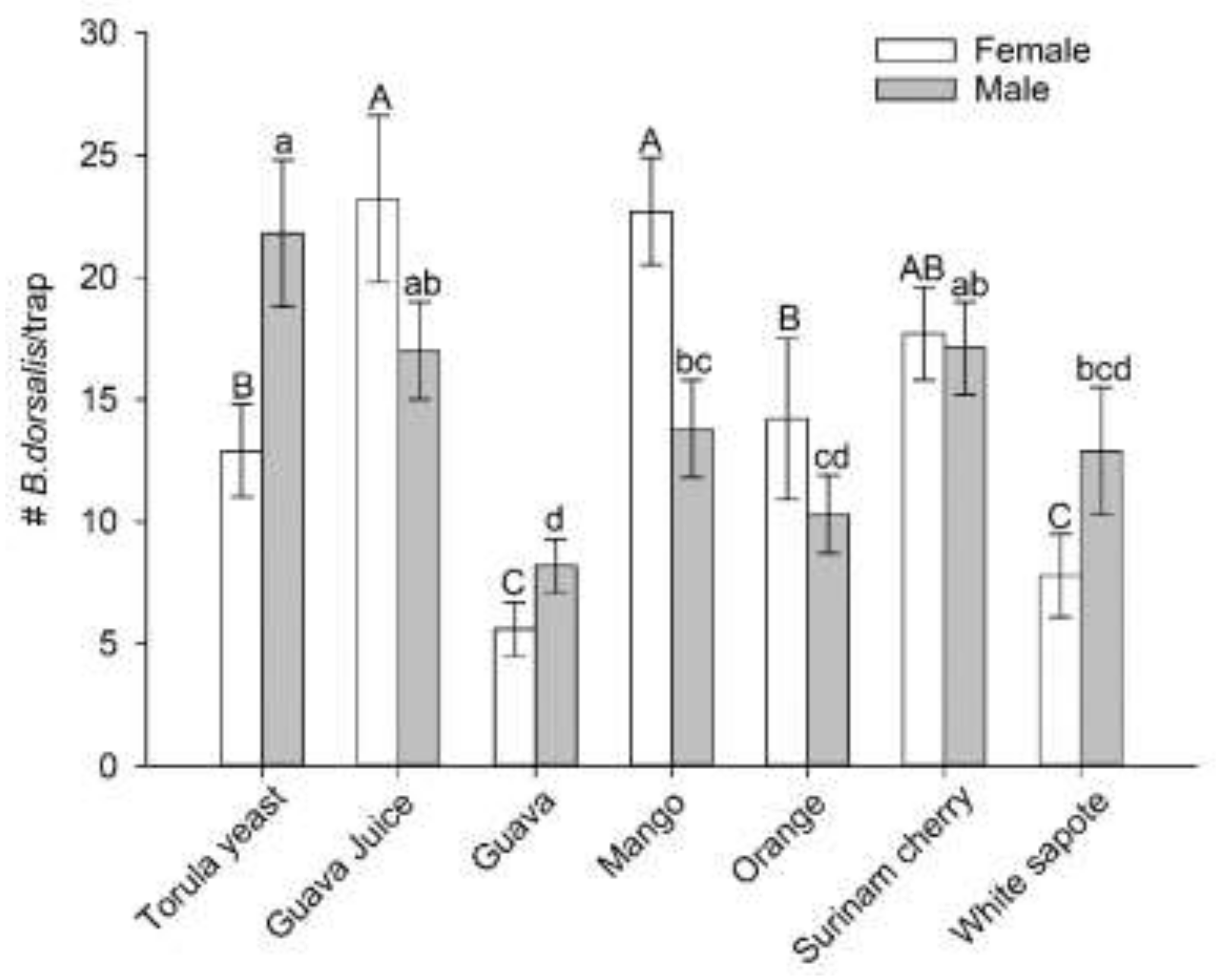
3.2. Selection of a Preferred Host Using Multiple-Choice Test
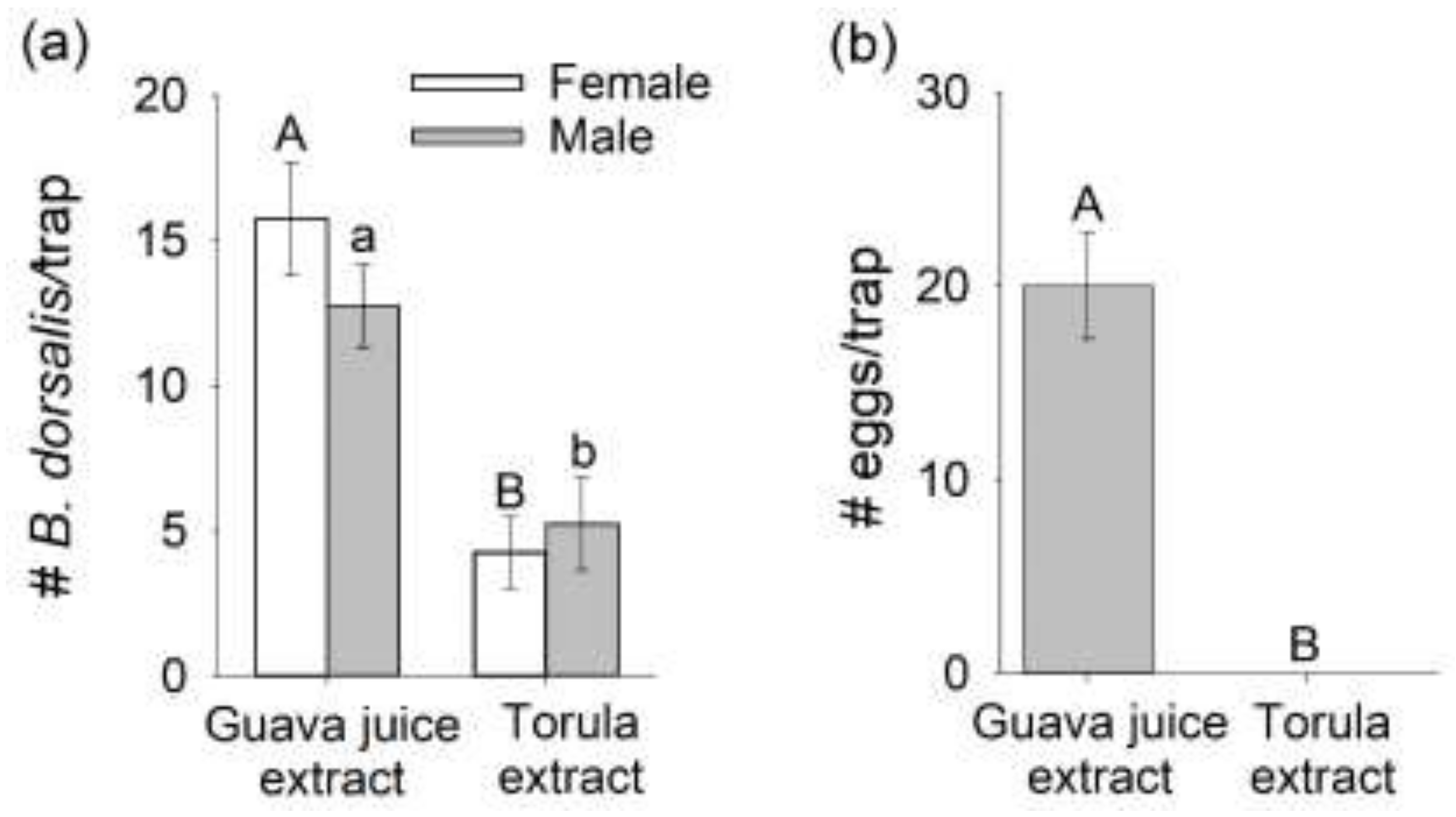
3.3. Comparison of Mated B. dorsalis Attraction and Oviposition Response to Headspace Extracts of Host Fruit versus Torula Yeast
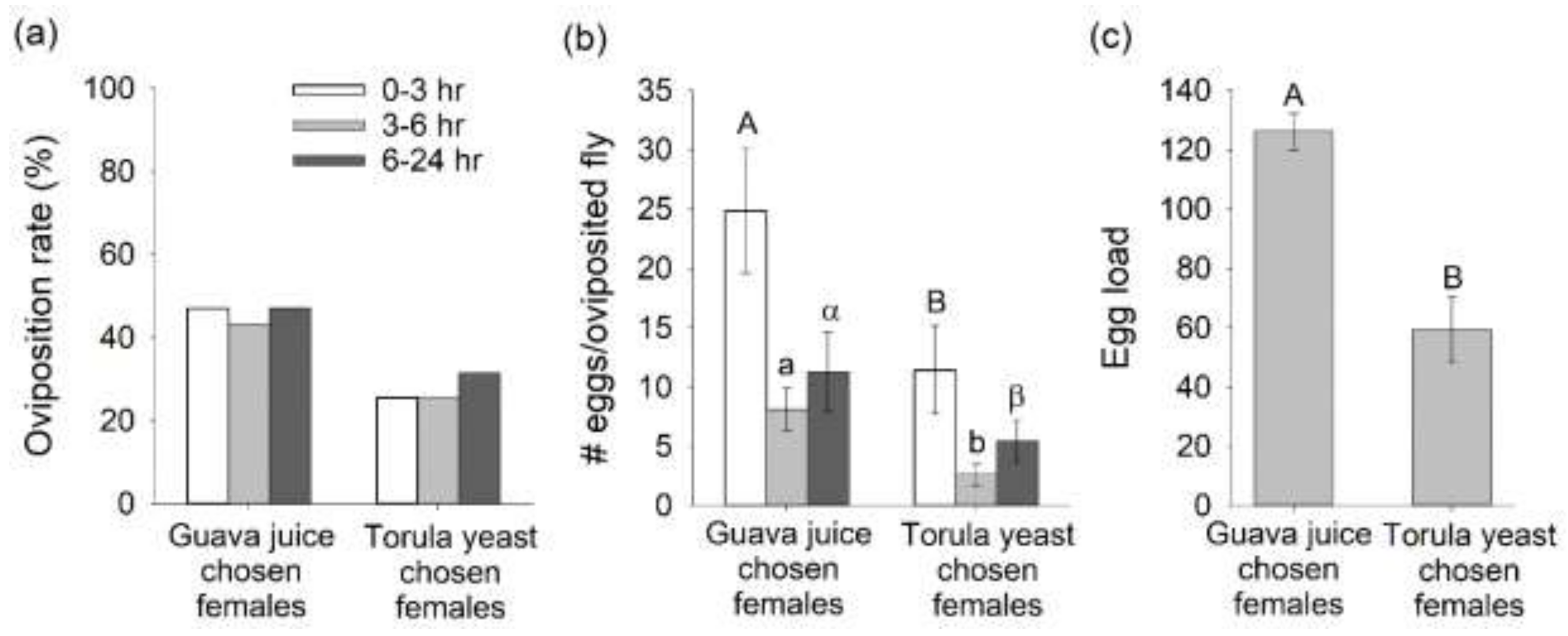
3.4. Oviposition Response from Mated B. dorsalis Preferentially Attracted to Guava Juice versus Torula Yeast
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Weems, H.V.; Heppner, J.B.; Nation, J.L.; Steck, G.J. Oriental Fruit Fly, Bactrocera dorsalis (Hedndel) (Insecta: Diptera: Tephritidae); EENY-083, IFAS Extension; University of Florida: Gainesville, FL, USA, 2016; Available online: https://edis.ifas.ufl.edu/publication/IN240 (accessed on 7 June 2021).
- Dohino, T.; Hallman, G.J.; Grout, T.G.; Clarke, A.R.; Follett, P.A.; Cugala, D.R.; Tu, D.M.; Murdita, W.; Hernandez, E.; Pereira, R.; et al. Phytosanitary treatments against Bactorocera dorsalis (Diptera: Tephritidae): Current situation and future prospects. J. Econ. Entol. 2017, 110, 67–79. [Google Scholar]
- Steiner, L.F.; Mitchell, W.C.; Harris, E.J.; Kozuma, T.T.; Fujimoto, M.S. Oriental fruit fly eradication by male annihilation. J. Econ. Entomol. 1965, 58, 961–964. [Google Scholar] [CrossRef]
- Steck, G.J.; Fox, A.J.; Carrillo, D.; Dean, D.; Roda, A.; Epsky, N.D.; Smith, T.R. Oriental fruit fly eradication in Florida 2015–2016: Program implementation, unique aspects, and lessons learned. Am. Entomol. 2019, 65, 108–121. [Google Scholar] [CrossRef]
- Cunningham, R.T.; Suda, D.Y. Male annihilation through mass-trapping of male flies with methyl eugenol to reduce infestation of oriental fruit fly (Diptera: Tephritidae) larvae in papaya. J. Econ. Entomol. 1986, 79, 1580–1582. [Google Scholar] [CrossRef]
- Vargas, R.I.; Mau, R.F.L.; Jang, E.B.; Faust, R.M.; Wong, L. The Hawaii fruit fly area-wide pest management program. In Areawide Pest Management: Theory and Implementation; Koul, O., Cuperus, G.W., Elliott, N.C., Eds.; CABI Publishing: Wallingford, UK, 2008; pp. 300–325. [Google Scholar]
- Shelly, T.; Epsky, N.D.; Jang, E.; Reyes-Flores, J.; Vargas, R. Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Fletcher, B.; Prokopy, R.J. Host location and oviposition in tephritid fruit flies. In Reproductive Behavior of Insects; Bailey, W.J., Ridsdill-Smith, J., Eds.; Chapman and Hall: New York, NY, USA, 1991; pp. 139–171. [Google Scholar]
- Robacker, D.C.; Heath, R.R. Attraction of Mexican fruit flies (Diptera: Tephritidae) to lures emitting host-fruit volatiles in a citrus orchard. Fla. Entomol. 1996, 79, 600–602. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Hu, X.P.; Jand, E.B.; Vargas, R.I.; Warthen, J.D. Attraction of mature Ceratitis capitate females to 2-heptanone a component of coffee fruit odour. J. Chem. Ecol. 1998, 24, 1293–1304. [Google Scholar] [CrossRef]
- Cornelius, M.L.; Nergel, L.; Duan, J.J.; Messing, R.H. Responses of female oriental fruit flies (Diptera: Tephritidae) to protein and host fruit odours in field cage and open field tests. Environ. Entomol. 2000, 29, 14–19. [Google Scholar] [CrossRef][Green Version]
- Barry, J.D.; Miller, N.W.; Pinero, J.C.; Tuttle, A.; Mau, R.F.L.; Vargas, R.I. Effectiveness of protein baits on melon fly and oriental fruit fly (Diptera: Tephritidae): Attraction and feeding. J. Econ. Entomol. 2006, 99, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Siderhurst, M.; Jang, E. Female-biased attraction of oriental fruit fly, Bactrocera dorsalis (Hendel), to a blend of host fruit volatiles from Terminalia catappa L. J. Chem. Ecol. 2006, 32, 2513–2524. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Drew, R.A.; Sabine, B.N.; Lloyd, A.C.; Hamacek, E. Effect of physiological and experiential state of Bactrocera tryoni flies on intra-tree foraging behavior for food (bacteria) and host fruit. Oecologia 1991, 87, 394–400. [Google Scholar] [CrossRef]
- Díaz-Fleischer, F.; Piñero, J.C.; Shelly, T.E. Interactions between tephritid fruit fly physiological state and stimuli from baits and traps: Looking for the Pied Piper of Hamelin to lure pestiferous fruit flies. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 145–172. [Google Scholar]
- Miller, N.W.; Vargas, R.I.; Prokopy, R.J.; Mackey, B.E. State dependent attractiveness of protein bait and host fruit odor to Bactrocera cucurbitae (Diptera: Tephritidae) females. Ann. Entomol. Soc. Am. 2004, 97, 1063–1068. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Mateo, D.M.; Puche, H.; Epsky, N.D.; Heath, R.R. Effect of age on EAG response and attraction of female Anastrepha suspensa (Diptera: Tephritidae) to ammonia and carbon dioxide. Environ. Entomol. 2005, 34, 584–590. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Epsky, N.D.; Heath, R.R. Electroantennogram and behavioral responses of Anastrepha suspensa (Diptera: Tephritidae) to putrescine and ammonium bicarbonate lures. Environ. Entomol. 2009, 38, 1259–1266. [Google Scholar] [CrossRef]
- Epsky, N.D.; Kendra, P.E.; Schnell, E.Q. History and development of food-based attractants. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 75–118. [Google Scholar]
- Tan, K.H.; Nishida, R.; Jang, E.B.; Shelly, T.E. Pheromones, male lures, and trapping of tephritid fruit flies. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T.E., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R.I., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 15–74. [Google Scholar]
- Piñero, J.C.; Mau, R.F.L.; Vargas, R.I. Managing oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae), through spinosad-based protein bait sprays and sanitation in papaya orchards in Hawaii. J. Econ. Entomol. 2009, 102, 1123–1132. [Google Scholar] [CrossRef]
- Shelly, T.; Kurashima, R.; Fezza, T. Field evaluation of three-component solid food-based dispenser versus torula yeast for capturing Mediterranean and oriental fruit flies (Diptera: Tephritidae). J. Asia Pac. Entomol. 2020, 23, 825–831. [Google Scholar] [CrossRef]
- Robacker, D.C. Specific hunger in Anastrepha ludens (Diptera: Tephritidae): Effects on attractiveness of proteinaceous and fruit-derived lures. Environ. Entomol. 1991, 20, 1680–1686. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Vargas, R.I. Attraction of Ceratitis capitata (Diptera: Tephritidae) flies to odor of coffee fruit. J. Chem. Ecol. 1996, 22, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, M.L.; Duan, J.J.; Messing, R.H. Volatile fruit odours as attractants for the oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2000, 93, 93–100. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Epsky, N.D.; Heath, R.R. Assessment of female reproductive status in Anastrepha suspensa (Diptera: Tephritidae). Fla. Entomol. 2006, 89, 144–151. [Google Scholar] [CrossRef]
- Wang, F.; Chambi, C.; Li, Z.; Huang, C.; Ma, Y.; Li, C.; Tian, X.; Sangija, F.; Ntambo, M.S.; Kankonda, O.M.; et al. Influence of supplemental protein on the life expectancy and reproduction of the Chinese citrus fruit fly, Bactrocera minax (Enderlein) (Tetradacus minax) (Diptera: Tephritidae). J. Insect Sci. 2018, 18, 25. [Google Scholar] [CrossRef]
- Papaj, D.R. Ovarian dynamics and host use. Annu. Rev. Entomol. 2000, 45, 423–448. [Google Scholar] [CrossRef]
- Aluja, M.; Birke, A.; Guillen, L.; Díaz-Fleischer, F.; Nestel, D. Coping with an unpredictable and stressful environment: The life history and metabolic response to variable food and host availability in a polyphagous tephritid fly. J. Insect Physiol. 2011, 57, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Migani, V.; Ekesi, S.; Hoffmeister, T.S. Physiology vs. Environment: What drives oviposition decisions in mango fruit flies (Bactrocera invadens and Ceratitis cosyra)? J. Appl. Entomol. 2014, 138, 395–402. [Google Scholar] [CrossRef]
- Tanaka, N.; Steiner, L.F.; Ohinata, K.; Okamoto, R. Low-cost larval rearing medium for mass production of oriental and Mediterranean fruit flies. J. Econ. Entomol. 1969, 62, 967–968. [Google Scholar] [CrossRef]
- Jang, E.B.; Carvalho, L.A.; Stark, J.D. Attraction of female oriental fruit fly, Bactrocera dorsalis, to volatile semiochemicals from leaves and extracts of a non host plant, Panax (Polyscias guilfoylei) in laboratory and olfactometer assays. J. Chem. Ecol. 1997, 23, 1389–1401. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Rogg, H.; Landolt, P.J. Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing drosophila. Drosophila Suzukii. J. Chem. Ecol. 2012, 38, 1419–1431. [Google Scholar] [CrossRef]
- Cha, D.H.; Roh, G.H.; Hesler, S.P.; Wallingford, A.; Stockton, D.G.; Park, S.K.; Loeb, G.M. 2-Pentylfuran: A Novel Repellent of Drosophila Suzukii. Pest Manag. Sci. 2021, 77, 1757–1764. [Google Scholar] [CrossRef]
- Chou, M.Y.; Mau, R.F.L.; Jang, E.B.; Vargas, R.I.; Pinero, J.C. Morphological features of the ovaries during oogenesis of the oriental fruit fly, Bactrocera dorsalis, in relation to the physiological state. J. Insect Sci. 2012, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. SAS/STAT 9.2 User’s Guide, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Robacker, D.C.; Moreno, D.S.; Demilo, A.B. Attractiveness to Mexican fruit flies of combinations of acetic acid with ammonium/amino attractants with emphasis on effects of hunger. J. Chem. Ecol. 1996, 22, 499–511. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Resilva, S.S.; Vargas, R.I. Post-alighting behavior of Ceratitis capitata flies on odor baited traps. Fla. Entomol. 1996, 79, 422–428. [Google Scholar] [CrossRef]
- Manrakhan, A.; Lux, S.A. Effect of food deprivation on attractiveness of food sources, containing natural and artificial sugar and protein, to three African fruit flies: Ceratitis cosyra, Ceratitis fasciventris, and Ceratitis capitata. Ent. Exp. Appl. 2008, 127, 133–143. [Google Scholar] [CrossRef]
- Cohen, H.; Voet, H. Effect of physiological state of young Ceratitis capitata females, on resource foraging behaviour. Entomol. Exp. Appl. 2002, 104, 345–351. [Google Scholar] [CrossRef]
- Tsitsipis, J.A. Nutrition: Requirements. In World Crop Pests, Fruit Flies: Their Biology, Natural Enemies and Control; Robinson, A.S., Hooper, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 3, pp. 103–119. [Google Scholar]
- Aluja, M.; Díaz-Fleischer, F.; Papaj, D.R.; Lagunes, G.; Sivinski, J. Effects of age, diet, female density, and the host resource on egg load in Anastrepha ludens and Anastrepha obliqua (Diptera: Tephritidae). J. Insect Physiol. 2001, 47, 975–988. [Google Scholar] [CrossRef]
- Jayanthi, K.P.D.; Woodcock, C.M.; Caulfield, J.; Birkett, M.A.; Bruce, T.J.A. Isolation and identification of host cues from mango, Mangifera indica, that attract gravid female oriental fruit fly, Bactrocera dorsalis. J. Chem. Ecol. 2012, 38, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, K.P.D.; Kempraj, V.; Aurade, R.M.; Venkataramanappa, R.K.; Namdagopal, B.; Verghese, A.; Bruce, T.J.A. Specific volatile compounds from mango elicit oviposition in gravid Bactrocera dorsalis females. J. Chem. Ecol. 2014, 40, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Meats, A.; Leighton, S.M. Protein consumption by mated, unmated, sterile and fertile adults of the Queensland fruit fly, Bactrocera tryoni and its relation to egg production. Physiol. Entomol. 2004, 29, 176–182. [Google Scholar] [CrossRef]
- Jayanthi, P.D.K.; Kumar, P.S.; Vyas, M. Odour cue from fruit arils of Artocarpus heterophyllus attract both sexes of oriental fruit flies. J. Chem. Ecol. 2021, 47, 552–563. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Yuval, B. The evolution of fruit fly feeding behavior. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 731–749. [Google Scholar]
- Zhou, X.W.; Niu, C.Y.; Han, P.; Desneux, N. Field evaluation of attractive lures for the fruit fly Bactrocera minax (Diptera: Tephritidae) and their potential use in spot sprays in Hubei Province (China). J. Econ. Entomol. 2012, 105, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.A.; Maoz, Y.; Cohen, B.; Golani, M.; Fefer, D.; Levi-Zada, A. Protecting avocado trees from ambrosia beetles by repellents and mass trapping (push-pull): Experiments and simulations. J. Pest Sci. 2021, 94, 991–1002. [Google Scholar] [CrossRef]
- Omoy, T.R.; Sudarwohadi, S.; Soelakson, S. The attractive ability of methyl eugenol and protein hydrolisate against fruit fly on hot pepper. J. Hort. 1999, 6, 469–476. [Google Scholar]
- Revis, H.C.; Miller, N.W.; Vargas, R.I. Effects of aging and dilution and toxicity of GF120 fruit fly bait spray for melon fly control in Hawaii. J. Econ. Entomol. 2004, 97, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, L.; Vargas, R.I.; Rubinoff, D. Captures of pest fruit flies (Diptera: Tephritidae) and nontarget insects in BioLure and torula yeast traps in Hawaii. Environ. Entomol. 2010, 39, 1626–1630. [Google Scholar] [CrossRef]
- Leblanc, L.; Vargas, R.I.; Rubinoff, D. Attraction of Ceratitis capitata (Diptera: Tephritidae) and endemic and introduced nontarget insects to BioLure bait and its individual components in Hawaii. Environ. Entomol. 2010, 39, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Llopis, V.; Vacas, S. Mass trapping for fruit fly control. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 513–555. [Google Scholar]
- Alyokhin, A.V.; Messing, R.H.; Duan, J.J. Visual and olfactory stimuli and fruit maturity affect trap captures of oriental fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 2000, 93, 644–649. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, G.-H.; Kendra, P.E.; Cha, D.H. Preferential Attraction of Oviposition-Ready Oriental Fruit Flies to Host Fruit Odor over Protein Food Odor. Insects 2021, 12, 909. https://doi.org/10.3390/insects12100909
Roh G-H, Kendra PE, Cha DH. Preferential Attraction of Oviposition-Ready Oriental Fruit Flies to Host Fruit Odor over Protein Food Odor. Insects. 2021; 12(10):909. https://doi.org/10.3390/insects12100909
Chicago/Turabian StyleRoh, Gwang-Hyun, Paul E. Kendra, and Dong H. Cha. 2021. "Preferential Attraction of Oviposition-Ready Oriental Fruit Flies to Host Fruit Odor over Protein Food Odor" Insects 12, no. 10: 909. https://doi.org/10.3390/insects12100909
APA StyleRoh, G.-H., Kendra, P. E., & Cha, D. H. (2021). Preferential Attraction of Oviposition-Ready Oriental Fruit Flies to Host Fruit Odor over Protein Food Odor. Insects, 12(10), 909. https://doi.org/10.3390/insects12100909





