Synaptic Interactions in Scorpion Peg Sensilla Appear to Maintain Chemosensory Neurons within Dynamic Firing Range
Abstract
Simple Summary
Abstract
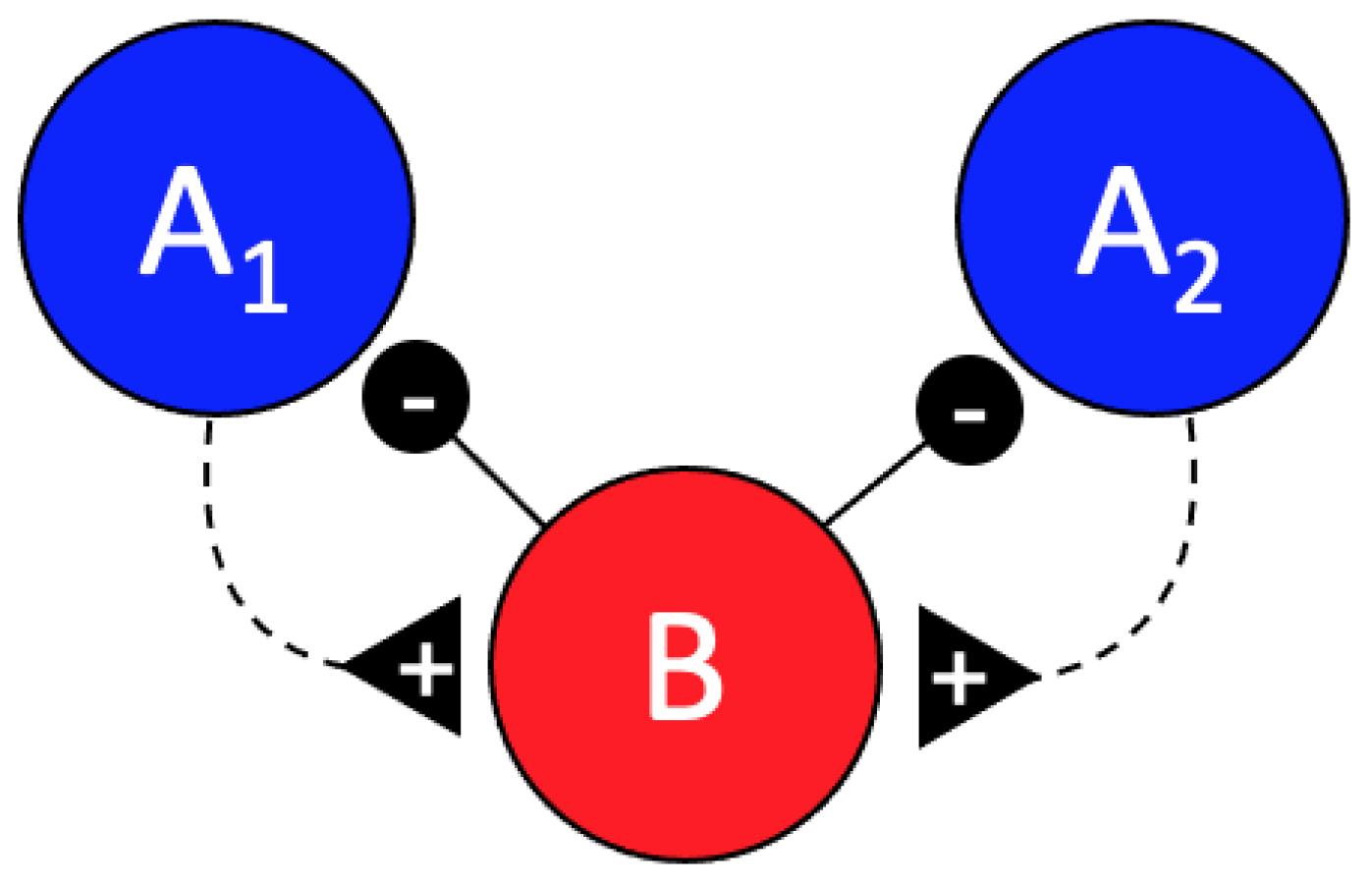
1. Introduction
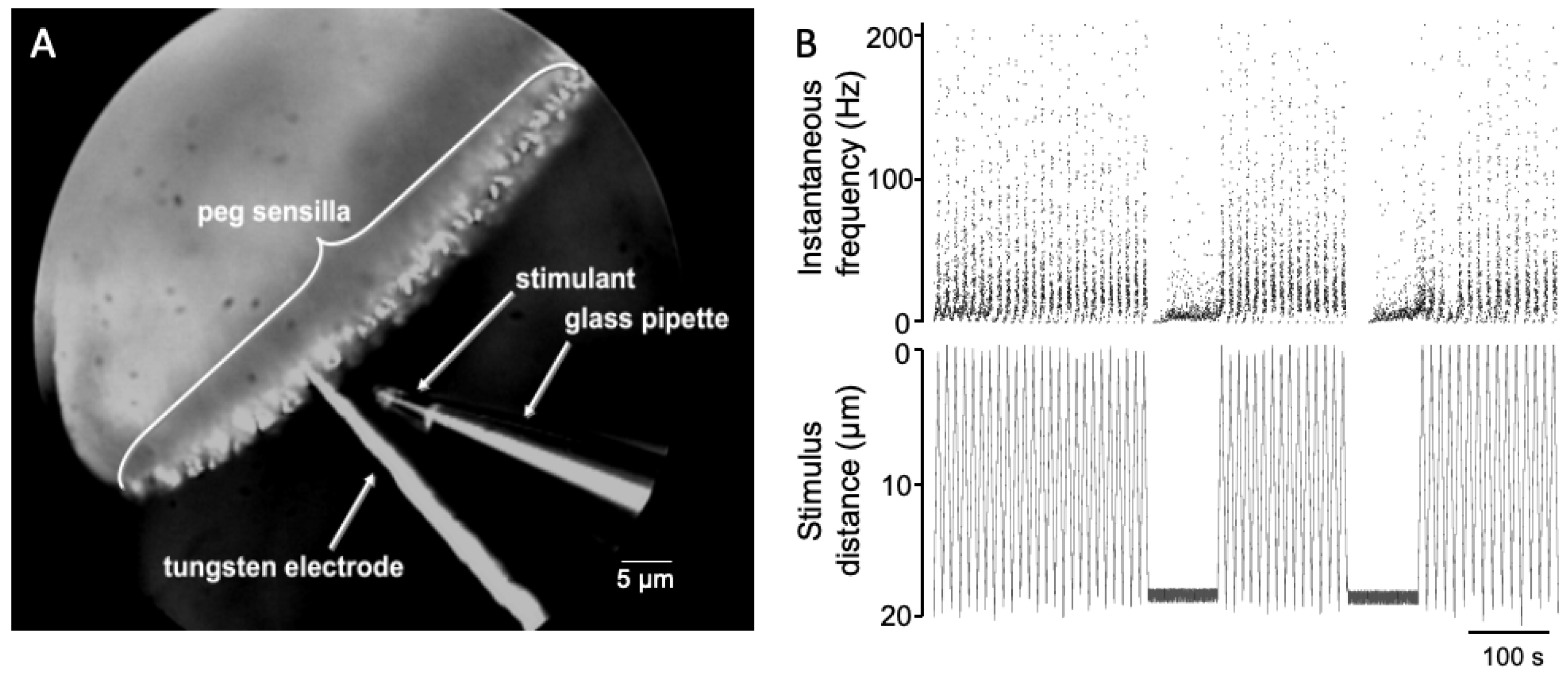
2. Materials and Methods
2.1. Collection of Scorpions
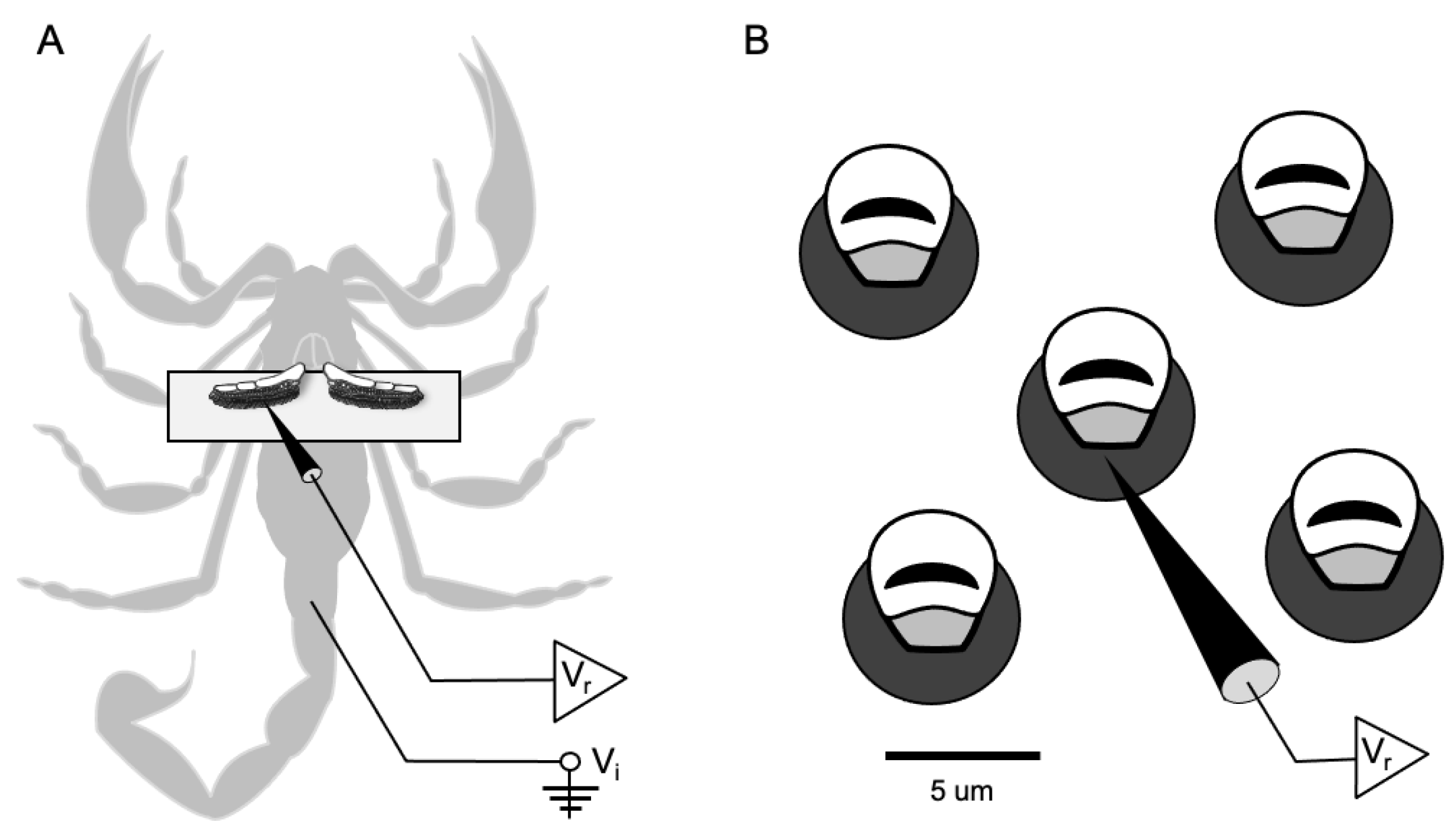
2.2. Preparation for Recording
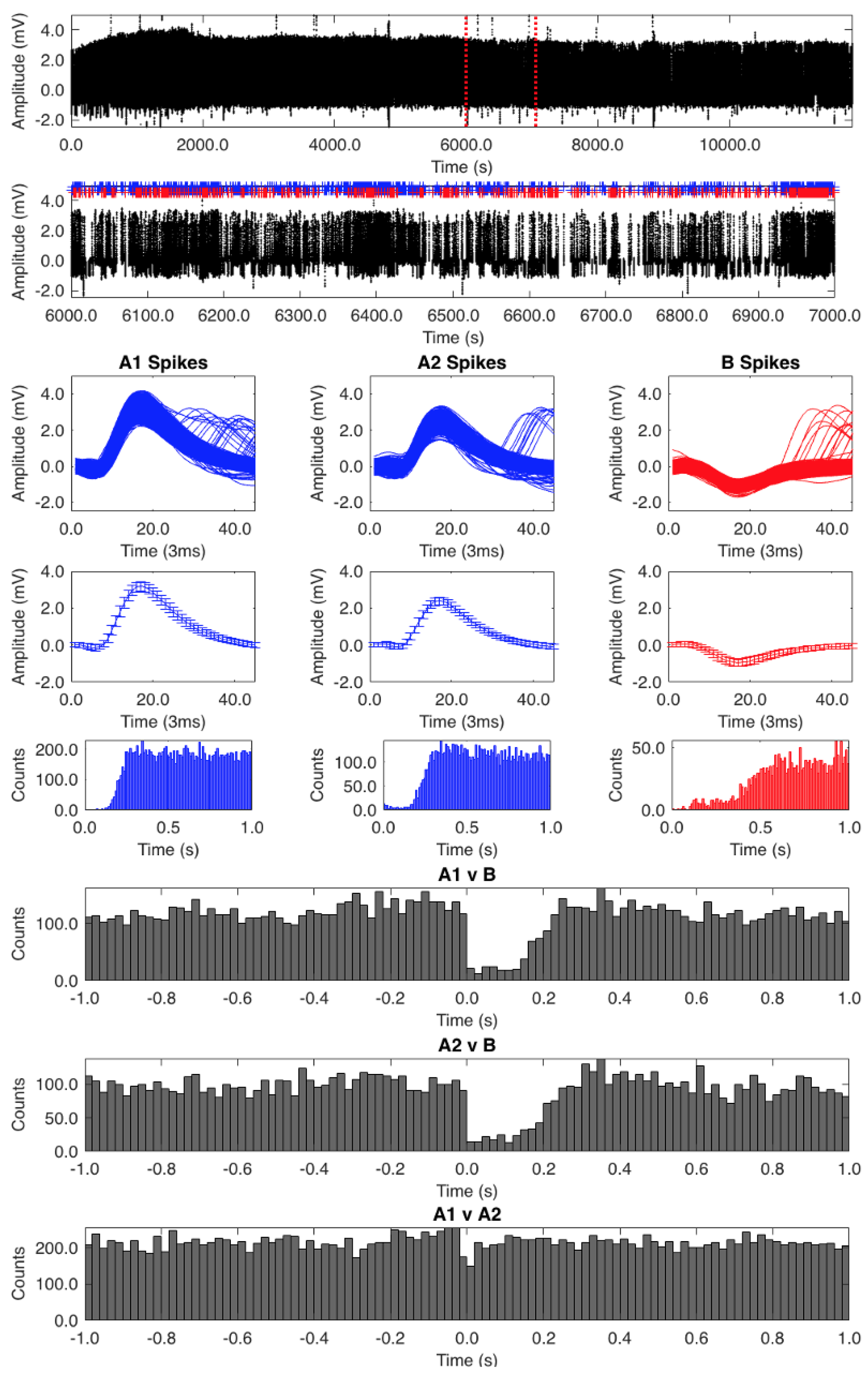
2.3. Processing of Action Potentials
2.4. Auto- and Cross-Correlation Analysis
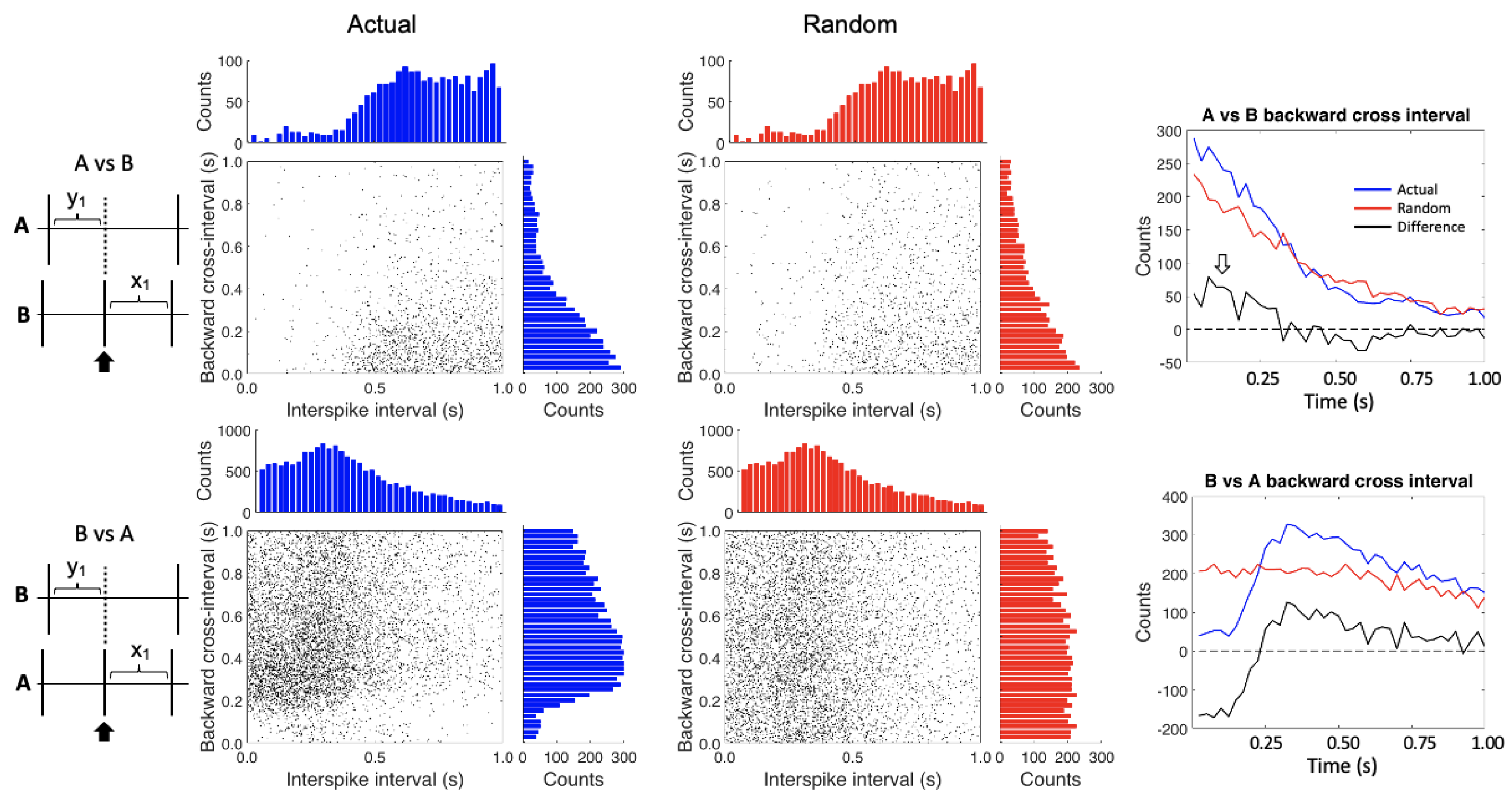
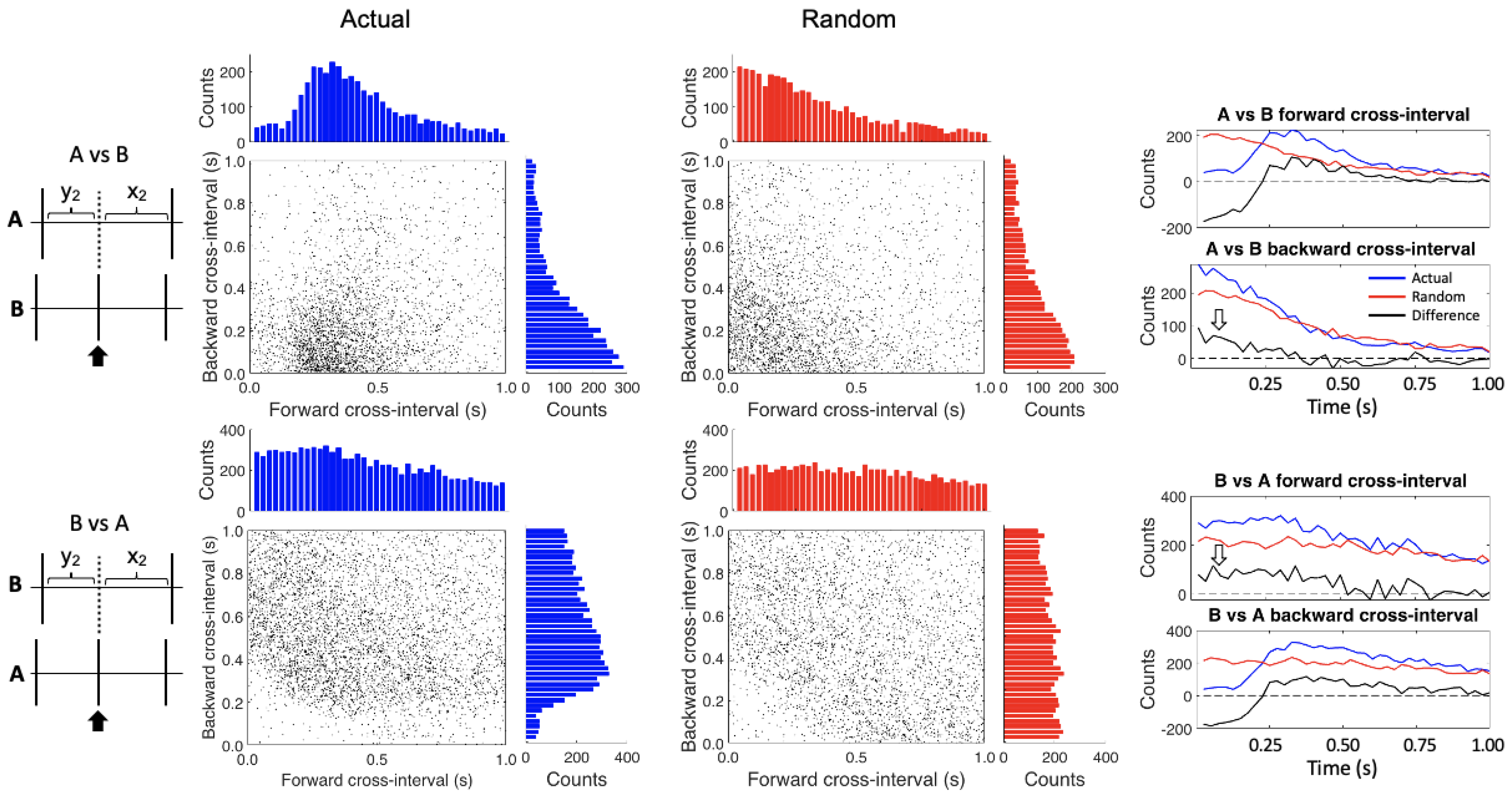
2.5. Conditional Interspike Interval and Conditional Cross-Interval Plots
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gaffin, D.D.; Brownell, P.H. Response properties of chemosensory peg sensilla on the pectines of scorpions. J. Comp. Physiol. A 1997, 181, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Gaffin, D.D.; Brownell, P.H. Electrophysiological evidence of synaptic interactions within chemosensory sensilla of scorpion pectines. J. Comp. Physiol. A 1997, 181, 301–307. [Google Scholar] [CrossRef]
- Foelix, R.F.; Müller-Vorholt, G. The fine structure of scorpion sensory organs. II. Pecten sensilla. Bull. Br. Arachnol. Soc. 1983, 6, 68–74. [Google Scholar]
- Ivanov, V.; Balashov, Y. The structural and functional organization of the pectine in a scorpion Buthus eupeus Koch (Scorpiones, Buthidae) studied by electron microscopy. In The Fauna and Ecology of Arachnida; Trudy Zoological Institute: Leningrad, Russia, 1979; Volume 85, pp. 73–87. [Google Scholar]
- Knowlton, E.D.; Gaffin, D.D. Functionally redundant peg sensilla on the scorpion pecten. J. Comp. Physiol. A 2011, 197, 895. [Google Scholar] [CrossRef] [PubMed]
- Krapf, D. Contact chemoreception of prey in hunting scorpions (Arachnida: Scorpiones). Zool. Anz. 1986, 217, 119–129. [Google Scholar]
- Gaffin, D.D.; Brownell, P.H. Evidence of chemical signaling in the sand scorpion, Paruroctonus Mesaensis (Scorpionida: Vaejovidae). Ethology 1992, 91, 59–69. [Google Scholar] [CrossRef]
- Taylor, M.S.; Cosper, C.R.; Gaffin, D.D. Behavioral evidence of pheromonal signaling in desert grassland scorpions Paruroctonus Utahensis. J. Arachnol. 2012, 40, 240–244. [Google Scholar] [CrossRef]
- Gaffin, D.D.; Brayfield, B.P. Exploring the chemo-textural familiarity hypothesis for scorpion navigation. J. Arachnol. 2017, 45, 265–270. [Google Scholar] [CrossRef]
- Musaelian, A.; Gaffin, D.D. High-throughput simulations indicate feasibility of navigation by familiarity with a local sensor such as scorpion pectines. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gaffin, D.D. Analysis of sensory processing in scorpion peg sensilla. J. Arachnol. 2010, 38, 1–8. [Google Scholar] [CrossRef]
- Gaffin, D.D. Electrophysiological analysis of synaptic interactions within peg sensilla of scorpion pectines. Microsc. Res. Tech. 2002, 58, 325–334. [Google Scholar] [CrossRef]
- Chapman, R.F.; Ascoli-Christensen, A.; White, P.R. Sensory coding for feeding deterrence in the grasshopper Schistocerca. Am. J. Exp. Biol. 1991, 158, 241–259. [Google Scholar] [CrossRef]
- Su, C.Y.; Menuz, K.; Reisert, J.; Carlson, J.R. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature 2012, 492, 66–71. [Google Scholar] [CrossRef]
- Foelix, R.F.; Troyer, D. Giant neurons and associated synapses in the peripheral nervous system of whip spiders. J. Neurocytol. 1980, 9, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Foelix, R.F. Occurrence of synapses in peripheral sensory nerves of arachnids. Nature 1975, 254, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Foelix, R.; Troyer, D.; Igelmund, P. Peripheral synapses and giant neurons in whip spiders. Microsc. Res. Tech. 2002, 58, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Hartline, H.K.; Ratliff, F. Spatial summation of inhibitory influences in the eye of Limulus, Mutual Interact. Recept. Units. J. Gen. Physiol. 1958, 41, 1049–1066. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, S.W. Discharge patterns and functional organization of mammalian retina. J. Neurophysiol. 1953, 16, 37–68. [Google Scholar] [CrossRef] [PubMed]
- Urban, N.N. Lateral inhibition in the olfactory bulb and in olfaction. Physiol. Behav. 2002, 77, 607–612. [Google Scholar] [CrossRef]
- Melville, J.M.; Tallarovic, S.K.; Brownell, P.H. Evidence of mate trailing in the giant hairy desert scorpion, Hadrurus Arizonensis (Scorpionida, Iuridae). J. Insect Behav. 2003, 16, 97–115. [Google Scholar] [CrossRef]
- Skutelsky, O. Flexibility in foraging tactics of Buthus Occ. Scorpions A Response Above-Ground Act. Termit. J. Arachnol. 1995, 23, 46–47. [Google Scholar]
- Tam, D.C.; Ebner, T.J.; Knox, C.K. Conditional cross-interval correlation analyses with applications to simultaneously recorded cerebellar Purkinje neurons. J. Neurosci. Methods 1988, 23, 23–33. [Google Scholar] [CrossRef]
- Knowlton, E.D.; Gaffin, D.D. Electrophysiology of scorpion peg sensilla. JoVE (J. Vis. Exp.) 2011, 50, e2642. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, E.D.; Gaffin, D.D. A new approach to examining scorpion peg sensilla: The mineral oil flood technique. J. Arachnol. 2009, 37, 379–382. [Google Scholar] [CrossRef]
- Perkel, D.H.; Gerstein, G.L.; Moore, G.P. Neuronal spike trains and stochastic point processes: I. The single spike train. Biophys. J. 1967, 7, 391–418. [Google Scholar] [CrossRef]
- Perkel, D.H.; Gerstein, G.L.; Smith, M.S.; Tatton, W.G. Nerve-impulse patterns: A quantitative display technique for three neurons. Brain Res. 1975, 100, 271–296. [Google Scholar] [CrossRef]
- Perkel, D.H.; Gerstein, G.L.; Moore, G.P. Neuronal spike trains and stochastic point processes: II. Simultaneous spike trains. Biophys. J. 1967, 7, 419–440. [Google Scholar] [CrossRef]
- Brownell, P.H. Glomerular cytoarchitectures in chemosensory cystems of arachnids. Ann. N. Y. Acad. Sci. 1998, 855, 502–507. [Google Scholar] [CrossRef]
- Brownell, P. Sensory ecology and orientational behaviors. In Scorpion Biology and Research; Oxford University Press: Oxford, UK, 2001; pp. 159–183. [Google Scholar]
- Drozd, D.; Wolf, H.; Stemme, T. Structure of the pecten neuropil pathway and its innervation by bimodal peg afferents in two scorpion species. PLoS ONE 2020, 15, e0243753. [Google Scholar] [CrossRef]
- Hughes, K.L.; Gaffin, D.D. Investigating sensory processing in the pectines of the striped bark scorpion, Centruroides vittatus. Invertebr. Neurosci. 2019, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, E.D.; Gaffin, D.D. A new tip-recording method to test scorpion pecten chemoresponses to water-soluble stimulants. J. Neurosci. Methods 2010, 193, 264–270. [Google Scholar] [CrossRef]
- Whitmire, C.; Stanley, G. Rapid sensory adaptation redux: A circuit perspective. Neuron 2016, 92, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Miriyala, A.; Kessler, S.; Rind, F.C.; Wright, G.A. Burst firing in fee gustatory neurons prevents adaptation. Curr. Biol. 2018, 28, 1585–1594.e3. [Google Scholar] [CrossRef] [PubMed]
- Gaffin, D.D.; Walvoord, M.E. Scorpion peg sensilla: Are they the same or are they different? Euscorpius 2004, 17, 7–15. [Google Scholar]
- Cohen, R.A. Lateral inhibition. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 1436–1437. [Google Scholar] [CrossRef]
- Ratliff, F.; Knight, B.W.; Milkman, N. Superposition of excitatory and inhibitory influences in the retina of Limulus: Eff. Delayed Inhib. Proc. Natl. Acad. Sci. USA 1970, 67, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, J.A.; Johansson, R.S. Edge-orientation processing in first-order tactile neurons. Nat. Neurosci. 2014, 17, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.M. The Pectines of Scorpions: Analysis of Structure and Function. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2000. [Google Scholar]
- Prévost, E.D.; Stemme, T. Non-visual homing and the current status of navigation in scorpions. Anim. Cogn. 2020, 23, 1215–1234. [Google Scholar] [CrossRef]
- Gaffin, D.D.; Curry, C.M. Arachnid navigation—A review of classic and emerging models. J. Arachnol. 2020, 48, 1–25. [Google Scholar] [CrossRef]
- Hebets, E.A.; Chapman, R.F. Electrophysiological studies of olfaction in the whip spider Phrynus parvulus (Arachnida, Amblypygi). J. Insect Physiol. 2000, 46, 1441–1448. [Google Scholar] [CrossRef]
- Hebets, E.A.; Aceves-Aparicio, A.; Aguilar-Argüello, S.; Bingman, V.P.; Escalante, I.; Gering, E.J.; Nelsen, D.R.; Rivera, J.; Sánchez-Ruiz, J.; Segura-Hernández, L.; et al. Multimodal sensory reliance in the nocturnal homing of the amblypygid Phrynus pseudoparvulus (Class Arachnida, Order Amblypygi)? Behav. Process. 2014, 108, 123–130. [Google Scholar] [CrossRef][Green Version]
- Hebets, E.A.; Gering, E.J.; Bingman, V.P.; Wiegmann, D.D. Nocturnal homing in the tropical amblypygid Phrynus pseudoparvulus (Class Arachnida, Order Amblypygi). Anim. Cogn. 2014, 17, 1013–1018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hebets, E.A. Relating the unique sensory system of amblypygids to the ecology and behavior of Phrynus parvulus Costa Rica (Arachnida, Amblypygi). Can. J. Zool. Ott. 2002, 80, 286–295. [Google Scholar] [CrossRef]
- Bingman, V.P.; Graving, J.M.; Hebets, E.A.; Wiegmann, D.D. Importance of the antenniform legs, but not vision, for homing by the neotropical whip spider Paraphrynus laevifrons. J. Exp. Biol. 2017, 220, 885–890. [Google Scholar] [CrossRef]
- Foelix, R.; Hebets, E. Sensory biology of whip spiders (Arachnida, Amblypygi). Andrias 2001, 15, 129–140. [Google Scholar]
- Fabian-Fine, R.; Meinertzhagen, I.A.; Seyfarth, E.A. Organization of efferent peripheral synapses at mechanosensory neurons in spiders. J. Comp. Neurol. 2000, 420, 195–210. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaffin, D.D.; Shakir, S.F. Synaptic Interactions in Scorpion Peg Sensilla Appear to Maintain Chemosensory Neurons within Dynamic Firing Range. Insects 2021, 12, 904. https://doi.org/10.3390/insects12100904
Gaffin DD, Shakir SF. Synaptic Interactions in Scorpion Peg Sensilla Appear to Maintain Chemosensory Neurons within Dynamic Firing Range. Insects. 2021; 12(10):904. https://doi.org/10.3390/insects12100904
Chicago/Turabian StyleGaffin, Douglas D., and Safra F. Shakir. 2021. "Synaptic Interactions in Scorpion Peg Sensilla Appear to Maintain Chemosensory Neurons within Dynamic Firing Range" Insects 12, no. 10: 904. https://doi.org/10.3390/insects12100904
APA StyleGaffin, D. D., & Shakir, S. F. (2021). Synaptic Interactions in Scorpion Peg Sensilla Appear to Maintain Chemosensory Neurons within Dynamic Firing Range. Insects, 12(10), 904. https://doi.org/10.3390/insects12100904





