Characterization of Two Complete Mitochondrial Genomes of Ledrinae (Hemiptera: Cicadellidae) and Phylogenetic Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. Sequencing, Assembly, Annotation and Bioinformatic Analyses
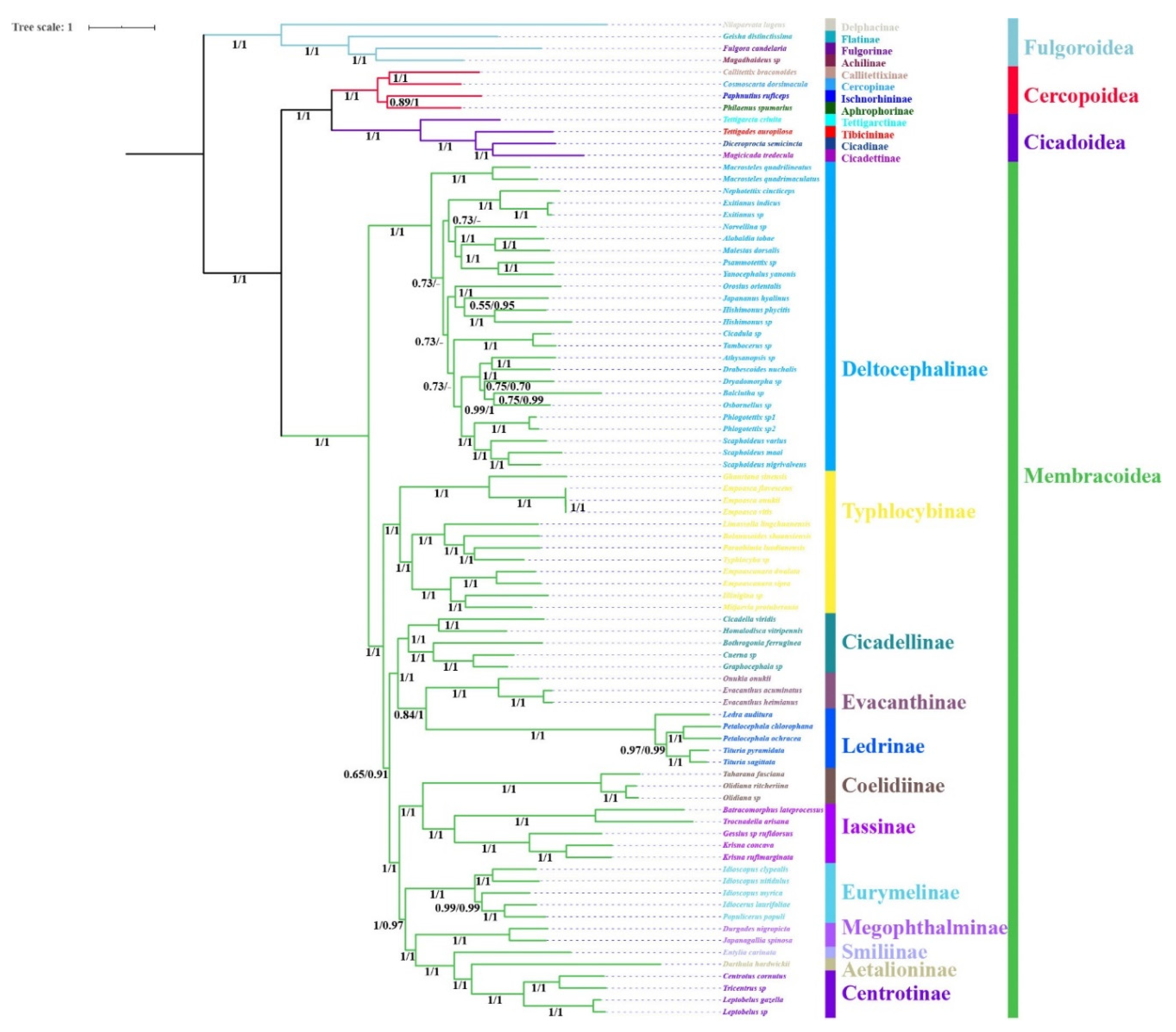
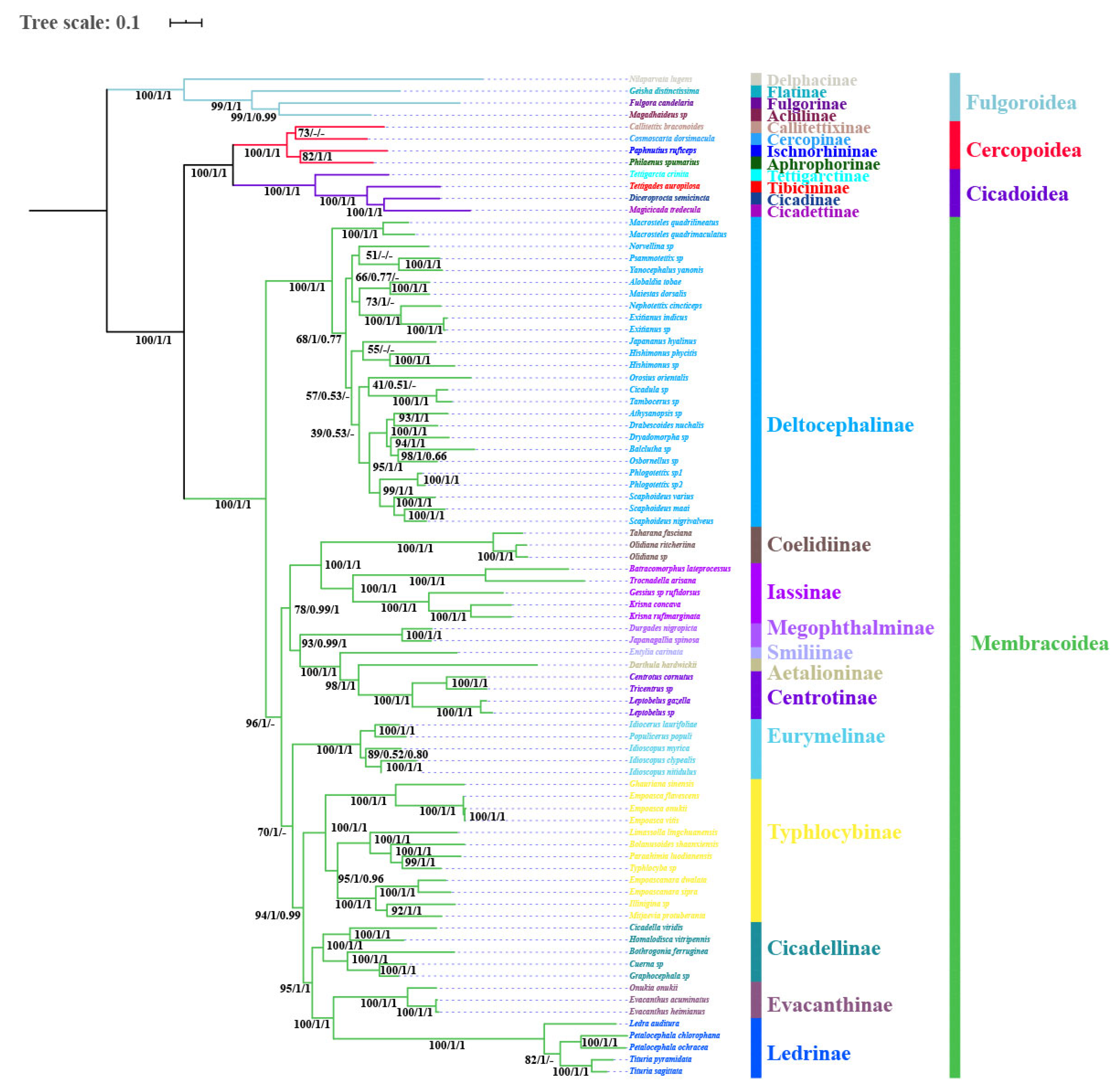
2.3. Phylogenetic Analysis
3. Results and Discussion
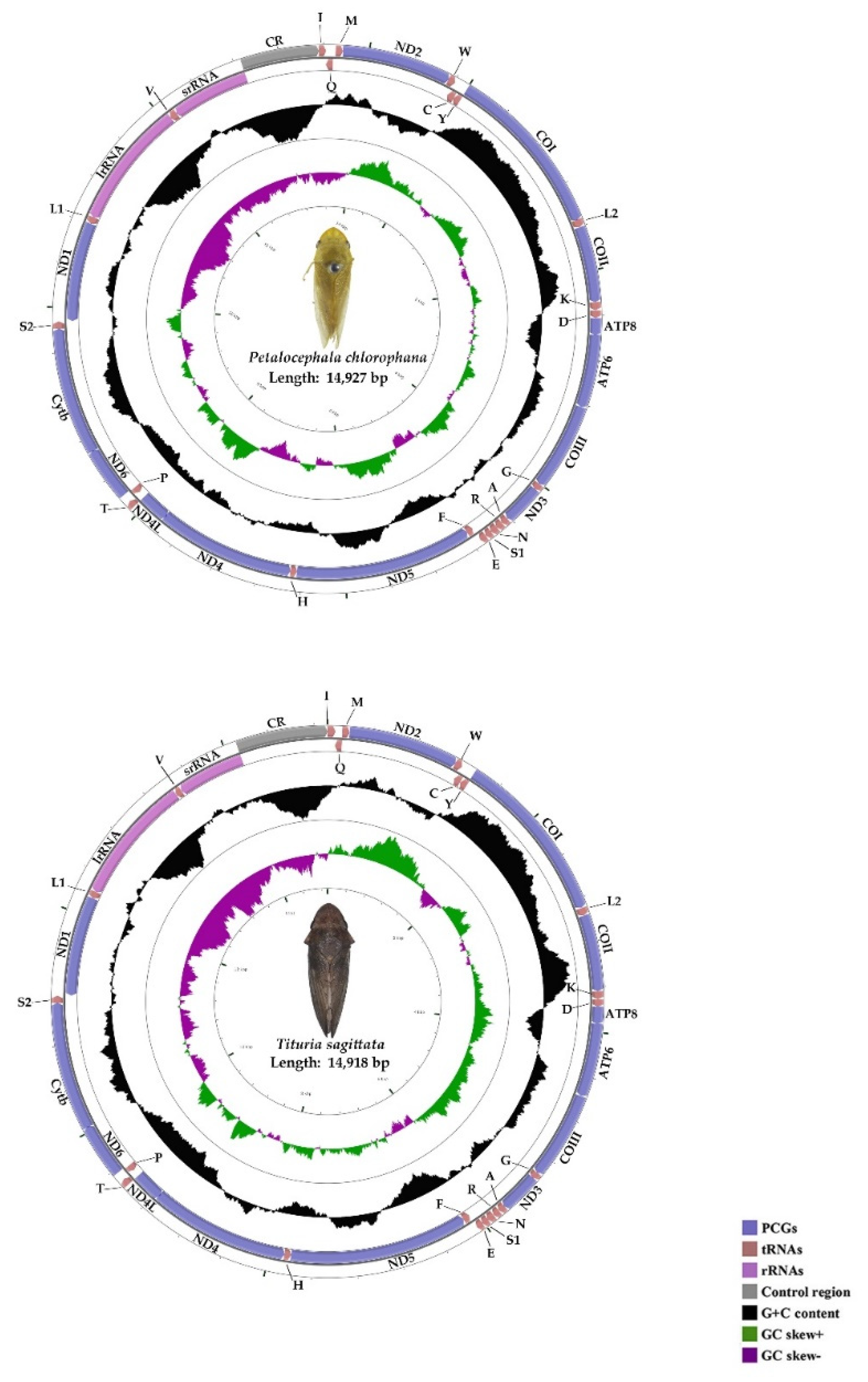
3.1. Genome Organization and Base Composition
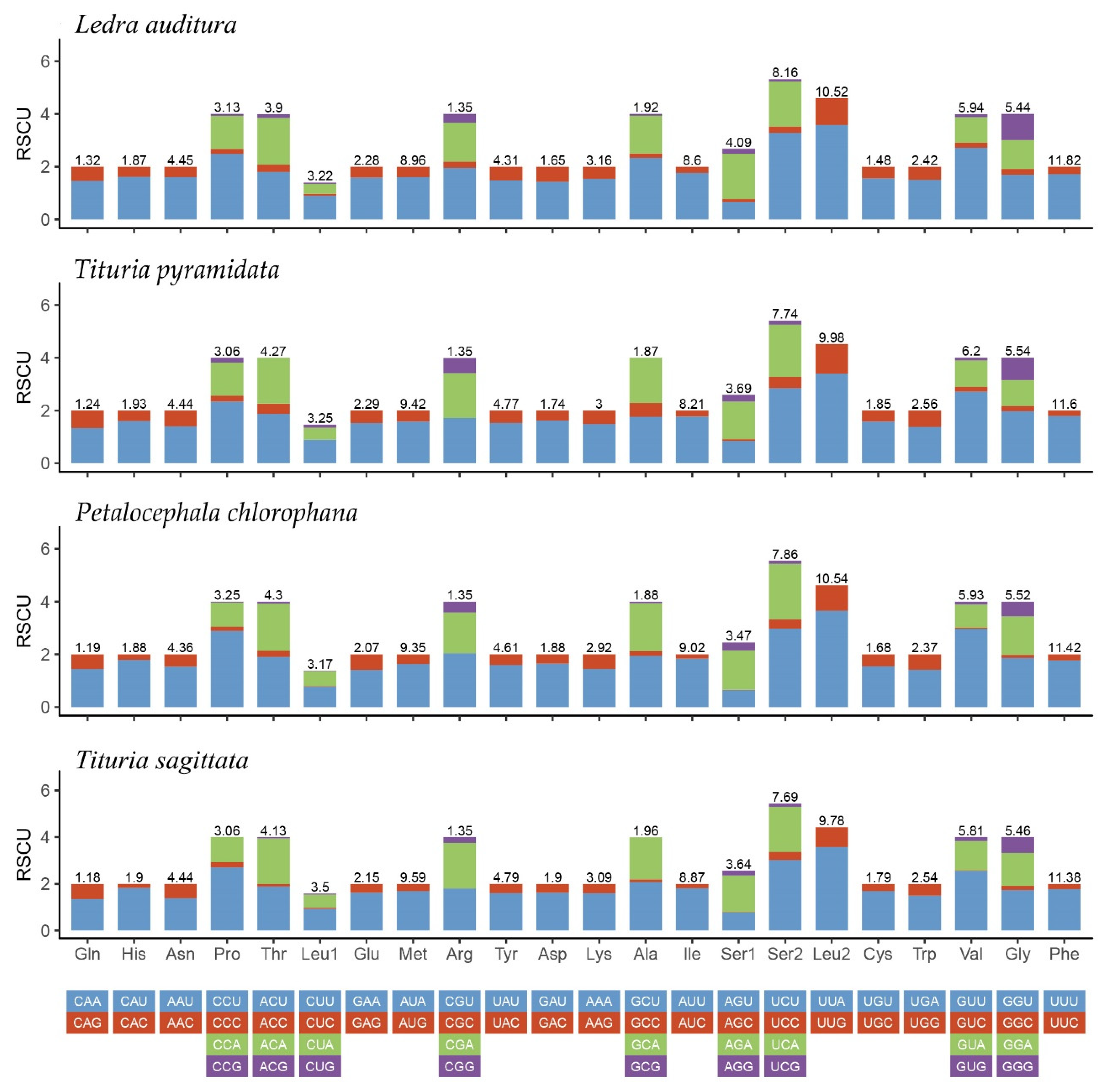
3.2. Protein-Coding Genes and Codon Usage
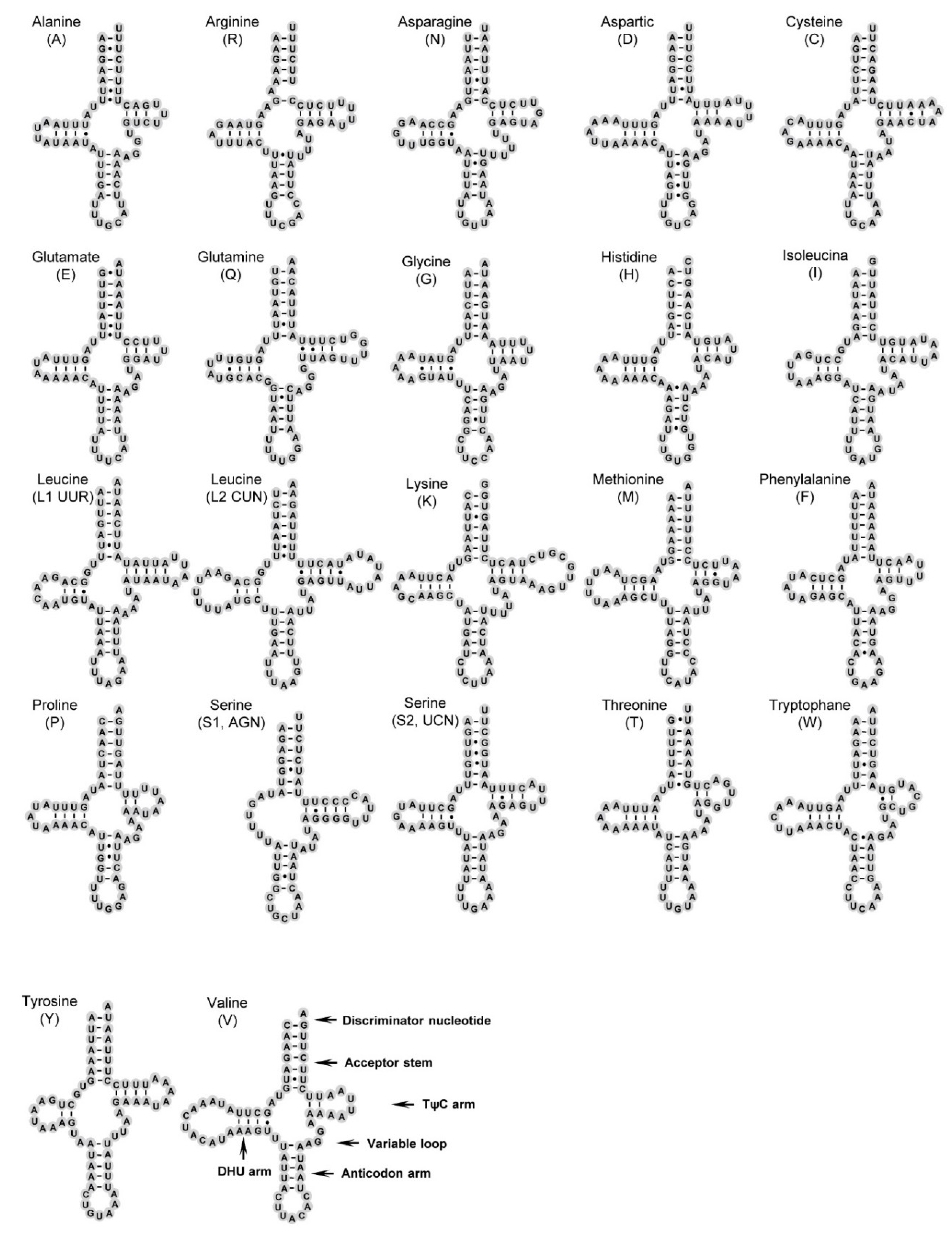
3.3. Transfer and Ribosomal RNA Genes
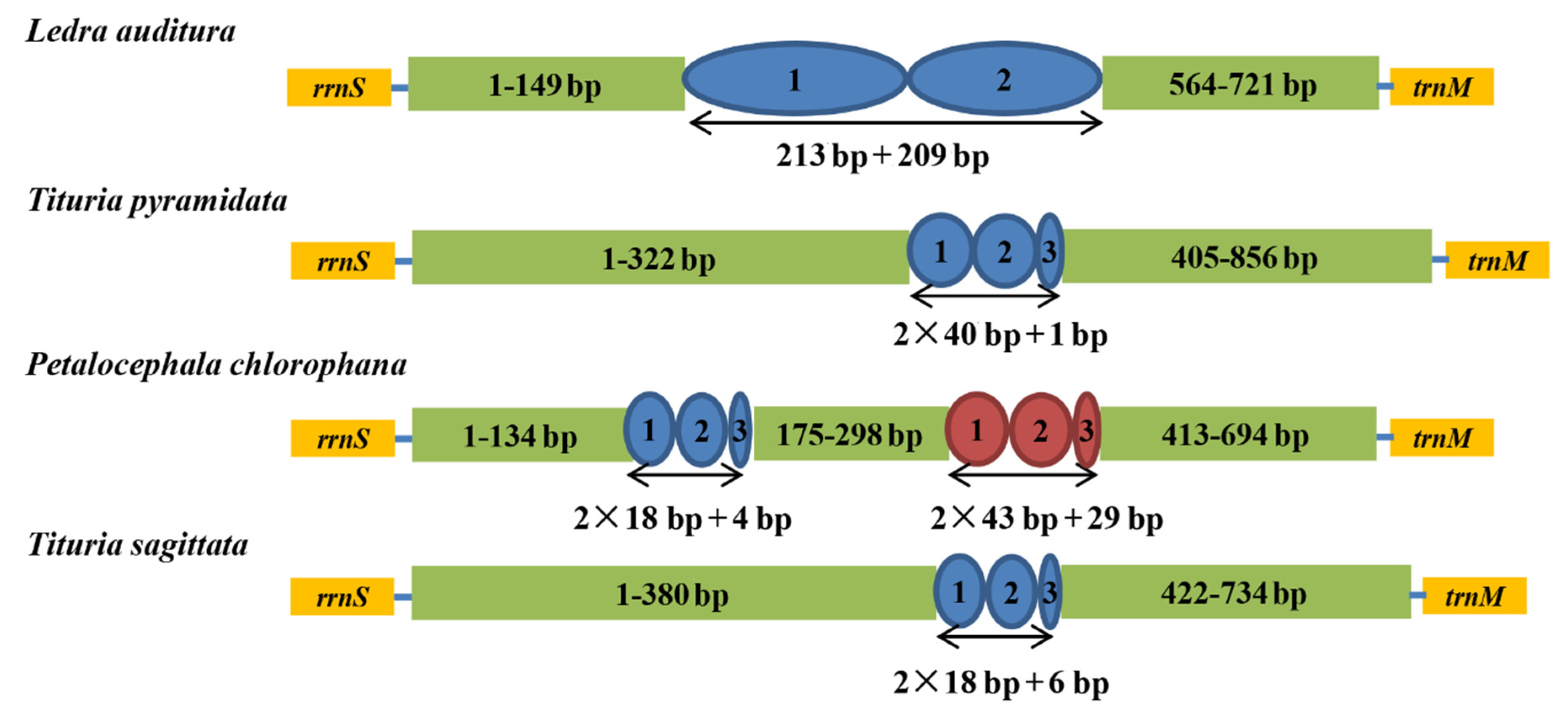
3.4. Overlapping Sequences and Intergenic Spacers
3.5. Control Region
3.6. Nucleotide Diversity and Evolutionary Rate Analysis
3.7. Phylogenetic Relationships
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Hasegawa, H.; Cooley, J.R.; Simon, C.; Yoshimura, J.; Cai, W.; Sota, T.; Li, H. Mitochondrial genomics reveals shared phylogeographic patterns and demographic history among three periodical cicada species groups. Mol. Biol. Evol. 2019, 36, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Leavengood, J.M., Jr.; Chapman, E.G.; Burkhardt, D.; Song, F.; Jiang, P.; Liu, J.; Zhou, X.; Cai, W. Mitochondrial phylogenomics of Hemiptera reveals adaptive innovations driving the diversification of true bugs. Proc. R. Soc. B Biol. Sci. 2017, 284. [Google Scholar] [CrossRef]
- Lv, L.; Peng, X.X.; Jing, S.L.; Liu, B.F.; Zhu, L.L.; He, G.C. Intraspecific and interspecific variations in the Mitochondrial genomes of Nilaparvata (Hemiptera: Delphacidae). J. Econ. Entomol. 2015, 108, 2021–2029. [Google Scholar] [CrossRef]
- Song, N.; Liang, A.P.; Bu, C.P. A molecular phylogeny of Hemiptera inferred from mitochondrial genome sequences. PLoS ONE 2012, 7, e48778. [Google Scholar] [CrossRef]
- Song, N.; Liang, A. The complete mitochondrial genome sequence of Geisha distinctissima (Hemiptera: Flatidae) and comparison with other hemipteran insects. Acta Biochim. Biophys. Sin. 2009, 41, 206–216. [Google Scholar] [CrossRef]
- Song, N.; Cai, W.; Li, H. Insufficient power of mitogenomic data in resolving the auchenorrhynchan monophyly. Zool. J. Linn. Soc. 2018, 183, 776–790. [Google Scholar] [CrossRef]
- Liu, J.; Bu, C.; Wipfler, B.; Liang, A. Comparative analysis of the mitochondrial genomes of Callitettixini Spittlebugs (Hemiptera: Cercopidae) confirms the overall high evolutionary speed of the AT-rich region but reveals the presence of short conservative elements at the tribal level. PLoS ONE 2014, 9, e109140. [Google Scholar] [CrossRef]
- Liu, J.; Liang, A.P. The complete mitochondrial genome of spittlebug Paphnutius ruficeps (Insecta: Hemiptera: Cercopidae) with a fairly short putative control region. Acta Biochim. Biophys. Sin. 2013, 45, 309–319. [Google Scholar] [CrossRef]
- Stewart, J.B.; Beckenbach, A.T. Insect mitochondrial genomics: The complete mitochondrial genome sequence of the meadow spittlebug Philaenus spumarius (Hemiptera: Auchenorrhyncha: Cercopoidae). Genome 2005, 48, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lukasik, P.; Chong, R.A.; Nazario, K.; Matsuura, Y.; Bublitz, A.C.; Campbell, M.A.; Meyer, M.C.; Van Leuven, J.T.; Pessacq, P.; Veloso, C.; et al. One Hundred Mitochondrial Genomes of Cicadas. J. Hered. 2019, 110, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.P.; Gao, J.; Zhao, X. Characterization of the complete mitochondrial genome of the treehopper Darthula hardwickii (Hemiptera: Aetalionidae). Mitochondrial DNA Part A 2016, 27, 3291–3292. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Yang, X.S.; Bennett, G. The complete mitochondrial genome of Entylia carinata (Hemiptera: Membracidae). Mitochondrial DNA Part B Resour. 2016, 1, 662–663. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, A.P. Complete DNA sequence of the mitochondrial genome of the treehopper Leptobelus gazella (Membracoidea: Hemiptera). Mitochondrial DNA Part A 2016, 27, 3318–3319. [Google Scholar] [CrossRef]
- Song, N.; Cai, W.Z.; Li, H. Deep-level phylogeny of Cicadomorpha inferred from mitochondrial genomes sequenced by NGS. Sci. Rep. 2017, 7, 11. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, H.; Dai, R.H. Complete mitochondrial genome of Taharana fasciana (Insecta, Hemiptera: Cicadellidae) and comparison with other Cicadellidae insects. Genetica 2017, 145, 593–602. [Google Scholar] [CrossRef]
- Du, Y.M.; Zhang, C.N.; Dietrich, C.H.; Zhang, Y.L.; Dai, W. Characterization of the complete mitochondrial genomes of Maiestas dorsalis and Japananus hyalinus (Hemiptera: Cicadellidae) and comparison with other Membracoidea. Sci. Rep. 2017, 7, 10. [Google Scholar] [CrossRef]
- Mao, M.; Yang, X.S.; Bennett, G. The complete mitochondrial genome of Macrosteles quadrilineatus (Hemiptera: Cicadellidae). Mitochondrial DNA Part B Resour. 2017, 2, 173–175. [Google Scholar] [CrossRef]
- Du, Y.M.; Dietrich, C.H.; Dai, W. Complete mitochondrial genome of Macrosteles quadrimaculatus (Matsumura) (Hemiptera: Cicadellidae: Deltocephalinae) with a shared tRNA rearrangement and its phylogenetic implications. Int. J. Biol. Macromol. 2019, 122, 1027–1034. [Google Scholar] [CrossRef]
- Yu, P.F.; Wang, M.X.; Cui, L.; Chen, X.X.; Han, B.Y. The complete mitochondrial genome of Tambocerus sp. (Hemiptera: Cicadellidae). Mitochondrial DNA Part A 2017, 28, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Dai, R.H.; Zhan, H.P.; Qu, L. Complete mitochondrial genome of Drabescoides nuchalis (Hemiptera: Cicadellidae). Mitochondrial DNA Part A 2016, 27, 3626–3627. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhang, H.; Zhao, T. Insights into the phylogeny of Hemiptera from increased mitogenomic taxon sampling. Mol. Phylogenet. Evol. 2019, 137, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Yang, M.F.; Dai, R.H.; Li, H. Complete mitochondrial genome of Evacanthus heimianus (Hemiptera: Cicadellidae: Evacanthinae) from China. Mitochondrial DNA Part B Resour. 2019, 4, 284–285. [Google Scholar] [CrossRef]
- Yuan, Z.W.; Yang, X.; Li, C.; Song, Y.H. The complete mitochondrial genome of the leafhopper Evacanthus acuminatus (Hemiptera: Cicadellidae: Evacanthinae). Mitochondrial DNA Part B Resour. 2019, 4, 3866–3867. [Google Scholar] [CrossRef]
- Wang, J.J.; Wu, Y.F.; Dai, R.H.; Yang, M.F. Comparative mitogenomes of six species in the subfamily Iassinae (Hemiptera: Cicadellidae) and phylogenetic analysis. Int. J. Biol. Macromol. 2020, 149, 1294–1303. [Google Scholar] [CrossRef]
- Wang, J.J.; Yang, M.F.; Dai, R.H.; Li, H.; Wang, X.Y. Characterization and phylogenetic implications of the complete mitochondrial genome of Idiocerinae (Hemiptera: Cicadellidae). Int. J. Biol. Macromol. 2018, 120, 2366–2372. [Google Scholar] [CrossRef]
- Choudhary, J.S.; Naaz, N.; Das, B.; Bhatt, B.P.; Prabhakar, C.S. Complete mitochondrial genome of ldioscopus nitidulus (Hemiptera: Cicadellidae). Mitochondrial DNA Part B Resour. 2018, 3, 191–192. [Google Scholar] [CrossRef]
- Dai, R.H.; Wang, J.J.; Yang, M.F. The complete mitochondrial genome of the leafhopper Idioscopus clypealis (Hemiptera: Cicadellidae: Idiocerinae). Mitochondrial DNA Part B Resour. 2018, 3, 32–33. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, D.F.; Li, H.; Yang, M.F.; Dai, R.H. Structural and phylogenetic implications of the complete mitochondrial genome of Ledra Audit. Sci. Rep. 2019, 9, 11. [Google Scholar] [CrossRef]
- Wang, J.J.; Dai, R.H.; Li, H.; Zhan, H.P. Characterization of the complete mitochondrial genome of Japanagallia spinosa and Durgades nigropicta (Hemiptera: Cicadellidae: Megophthalminae). Biochem. Syst. Ecol. 2017, 74, 33–41. [Google Scholar] [CrossRef]
- Liu, J.H.; Sun, C.Y.; Long, J.; Guo, J.J. Complete mitogenome of tea green leafhopper, Empoasca onukii (Hemiptera: Cicadellidae) from Anshun, Guizhou Province in China. Mitochondrial DNA Part B Resour. 2017, 2, 808–809. [Google Scholar] [CrossRef]
- Zhou, N.N.; Wang, M.X.; Cui, L.; Chen, X.X.; Han, B.Y. Complete mitochondrial genome of Empoasca vitis (Hemiptera: Cicadellidae). Mitochondrial DNA Part A 2016, 27, 1052–1053. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, Y.; Chen, C.; Pu, D.Q.; Tang, X.B.; Zhang, H.; Lu, D.H.; Mao, J.H. Characterization of the complete mitochondrial genome of Empoasca sp. (Cicadellidae: Hemiptera). Mitochondrial DNA Part B Resour. 2019, 4, 1477–1478. [Google Scholar] [CrossRef]
- Yuan, X.; Xiong, K.; Li, C.; Song, Y. The complete mitochondrial genome of Limassolla lingchuanensis (Hemiptera: Cicadellidae: Typhlocybinae). Mitochondrial DNA Part B Resour. 2019, 5, 229–230. [Google Scholar] [CrossRef]
- Tan, C.; Chen, X.; Li, C.; Song, Y. The complete mitochondrial genome of Empoascanara sipra (Hemiptera:Cicadellidae:Typhlocybinae) with phylogenetic consideration. Mitochondrial DNA Part B Resour. 2019, 5, 260–261. [Google Scholar] [CrossRef]
- Song, Y.H.; Yuan, X.W.; Li, C. The mitochondrial genome of Paraahimia luodianensis (Hemiptera: Cicadellidae: Typhlocybinae), a new genus and species from China. Mitochondrial DNA Part B Resour. 2020, 5, 1351–1352. [Google Scholar] [CrossRef]
- Yuan, X.W.; Li, C.; Song, Y.H. Characterization of the complete mitochondrial genome of Mitjaevia protuberanta (Hemiptera: Cicadellidae: Typhlocybinae. Mitochondrial DNA Part B Resour 2020, 5, 601–602. [Google Scholar] [CrossRef]
- Shi, R.; Yu, X.F.; Yang, M.F. Complete mitochondrial genome of Ghauriana sinensis (Hemiptera: Cicadellidae: Typhlocybinae). Mitochondrial DNA Part B Resour. 2020, 5, 1367–1368. [Google Scholar] [CrossRef]
- Jones, J.R.; Deitz, L.L. Phylogeny and systematics of the leafhopper subfamily Ledrinae (Hemiptera: Cicadellidae). Zootaxa 2009, 2186, 1–120. [Google Scholar] [CrossRef]
- Sun, J.; Huang, W.; Zhang, Y. Taxonomic studies of two genera, Elongationa gen. nov. and Midoria Kato (Hemiptera: Cicadellidae: Ledrinae), with two new species from China. Zootaxa 2017, 4294, 361. [Google Scholar] [CrossRef]
- Shcherbakov, E.D. The earliest leafhoppers (Hemiptera: Karajassidae n. fam.) from the Jurassic of Karatau. N. Jahrb. Geol. Paläont. Mon. 1992, 1, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, B.; Jones, J.R.; Zheng, Y.; Jiang, H.; Jiang, T.; Zhang, J.; Zhang, H. A representative of the modern leafhopper subfamily Ledrinae in mid-Cretaceous Burmese amber (Hemiptera, Cicadellidae). Cretac. Res. 2019, 95, 252–259. [Google Scholar] [CrossRef]
- Dietrich, C.H.; Rakitov, R.A.; Holmes, J.L.; Black, W.C. Phylogeny of the major lineages of Membracoidea (Insecta: Hemiptera: Cicadomorpha) based on 28S rDNA sequences. Mol. Phylogenet. Evol. 2001, 18, 293–305. [Google Scholar] [CrossRef]
- Dietrich, C.H.; Allen, J.M.; Lemmon, A.R.; Lemmon, E.M.; Takiya, D.M.; Evangelista, O.; Walden, K.K.O.; Grady, P.G.S.; Johnson, K.P. Anchored Hybrid Enrichment-Based Phylogenomics of Leafhoppers and Treehoppers (Hemiptera: Cicadomorpha: Membracoidea). Insect Syst. Divers. 2017, 1, 57–72. [Google Scholar] [CrossRef]
- Skinner, R.K.; Dietrich, C.H.; Walden, K.K.O.; Gordon, E.; Sweet, A.D.; Podsiadlowski, L.; Petersen, M.; Simon, C.; Takiya, D.M.; Johnson, K.P. Phylogenomics of Auchenorrhyncha (Insecta: Hemiptera) using transcriptomes: Examining controversial relationships via degeneracy coding and interrogation of gene conflict. Syst. Entomol. 2020, 45, 85–113. [Google Scholar] [CrossRef]
- Hahn, C.; Bachmann, L.; Chevreux, B. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads-a baiting and iterative mapping approach. Nucleic Acids Res. 2013, 41, 9. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Juhling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Putz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Grant, J.R.; Stothard, P. The CGView Server: A comparative genomics tool for circular genomes. Nucleic Acids Res. 2008, 36, W181–W184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlic, I.; Zhou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2019, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE); Institute of Electrical and Electronics Engineers (IEEE): New Orleans, LA, USA, 2010; Volume 14, pp. 1–8. [Google Scholar]
- Lartillot, N.; Rodrigue, N.; Stubbs, D.; Richer, J. PhyloBayes MPI: Phylogenetic Reconstruction with Infinite Mixtures of Profiles in a Parallel Environment. Syst. Biol. 2013, 62, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Clary, D.O.; Wolstenholme, D.R. The mitochondrial DNA molecular of Drosophila yakuba: Nucleotide sequence, gene organization, and genetic code. J. Mol. Evol. 1985, 22, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Garey, J.R.; Wolstenholme, D.R. Platyhelminth mitochondrial DNA: Evidence for early evolutionary origin of a tRNA(serAGN) that contains a dihydrouridine arm replacement loop, and of serine-specifying AGA and AGG codons. J. Mol. Evol. 1989, 28, 374–387. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.L.; Duan, Y.N. DNA barcoding of Deltocephalus Burmeister leafhoppers (Cicadellidae, Deltocephalinae, Deltocephalini) in China. Zookeys 2019, 867, 55–71. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, Y.L. Two new species in the genus Destinoides (Hemiptera: Cicadellidae, Ledrinae) from China, with DNA barcoding data. Zootaxa 2019, 4711, 571–578. [Google Scholar] [CrossRef]
- Wang, Y.; Nansen, C.; Zhang, Y.L. Integrative insect taxonomy based on morphology, mitochondrial DNA, and hyperspectral reflectance profiling. Zool. J. Linn. Soc. 2016, 177, 378–394. [Google Scholar] [CrossRef]
- Johnson, K.P.; Dietrich, C.H.; Friedrich, F.; Beutel, R.G.; Wipfler, B.; Peters, R.S.; Allen, J.M.; Petersen, M.; Donath, A.; Walden, K.K.O.; et al. Phylogenomics and the evolution of hemipteroid insects. Proc. Natl. Acad. Sci. USA 2018, 115, 12775–12780. [Google Scholar] [CrossRef]


| Superfamily | Family/Subfamily | Species | Accession Number | Reference |
|---|---|---|---|---|
| Outgroup | ||||
| Fulgoroidea | Fulgoridae/Fulgorinae | Fulgora candelaria | NC_019576 | [6] |
| Flatidae/Flatinae | Geisha distinctissima | NC_012617 | [7] | |
| Achilidae/Achilinae | Magadhaideus sp. | MH324928 | Unpublished | |
| Delphacidae/Delphacinae | Nilaparvata lugens | NC_021748 | Unpublished | |
| Cercopoidea | Cercopidae/Cercopinae | Cosmoscarta dorsimacula | NC_040115 | Unpublished |
| Cercopidae/Callitettixinae | Callitettix braconoides | NC_025497 | [9] | |
| Cercopidae/Ischnorhininae | Paphnutius ruficeps | NC_021100 | [10] | |
| Aphrophoridae/Aphrophorinae | Philaenus spumarius | NC_005944 | [11] | |
| Cicadoidea | Cicadidae/Cicadinae | Diceroprocta semicincta | KM000131 | Unpublished |
| Cicadidae/Cicadettinae | Magicicada tredecula | MH937705 | [3] | |
| Cicadidae/Tibicininae | Tettigades auropilosa | KM000129 | Unpublished | |
| Tettigarctidae/Tettigarctinae | Tettigarcta crinita | MG737758 | [12] | |
| Ingroup | ||||
| Membracoidea | Aetalionidae/Aetalioninae | Darthula hardwickii | NC_026699 | [13] |
| Membracidae/Smiliinae | Entylia carinata | NC_033539 | [14] | |
| Membracidae/Centrotinae | Leptobelus gazella | NC_023219 | [15] | |
| Membracidae/Centrotinae | Centrotus cornutus | KX437728 | [8] | |
| Membracidae/Centrotinae | Tricentrus sp. | KY039115 | [16] | |
| Membracidae/Centrotinae | Leptobelus sp. | JQ910984 | [4] | |
| Cicadellidae/Cicadellinae | Bothrogonia ferruginea | KU167550 | Unpublished | |
| Cicadellidae/Cicadellinae | Cuerna sp. | KX437741 | [8] | |
| Cicadellidae/Cicadellinae | Graphocephala sp. | KX437740 | [8] | |
| Cicadellidae/Cicadellinae | Cicadella viridis | KY752061 | Unpublished | |
| Cicadellidae/Cicadellinae | Homalodisca vitripennis | NC_006899 | Unpublished | |
| Cicadellidae/Coelidiinae | Taharana fasciana | NC_036015 | [17] | |
| Cicadellidae/Coelidiinae | Olidiana sp. | KY039119 | Unpublished | |
| Cicadellidae/Coelidiinae | Olidiana_ritcheriina | MK738125 | Unpublished | |
| Cicadellidae/Deltocephalinae | Japananus hyalinus | NC_036298 | [18] | |
| Cicadellidae/Deltocephalinae | Maiestas dorsalis | NC_036296 | [18] | |
| Cicadellidae/Deltocephalinae | Macrosteles quadrilineatus | NC_034781 | [19] | |
| Cicadellidae/Deltocephalinae | Macrosteles quadrimaculatus | NC_039560 | [20] | |
| Cicadellidae/Deltocephalinae | Tambocerus sp. | KT827824 | [21] | |
| Cicadellidae/Deltocephalinae | Nephotettix cincticeps | NC_026977 | Unpublished | |
| Cicadellidae/Deltocephalinae | Hishimonus phycitis | KX437727 | [8] | |
| Cicadellidae/Deltocephalinae | Hishimonus sp. | KX437735 | [8] | |
| Cicadellidae/Deltocephalinae | Psammotettix sp. | KX437742 | [8] | |
| Cicadellidae/Deltocephalinae | Cicadula sp. | KX437724 | [8] | |
| Cicadellidae/Deltocephalinae | Exitianus sp. | KX437722 | [8] | |
| Cicadellidae/Deltocephalinae | Phlogotettix sp. 1 | KY039135 | [16] | |
| Cicadellidae/Deltocephalinae | Phlogotettix sp. 2 | KX437721 | [8] | |
| Cicadellidae/Deltocephalinae | Dryadomorpha sp. | KX437736 | [8] | |
| Cicadellidae/Deltocephalinae | Osbornellus sp. | KX437739 | [8] | |
| Cicadellidae/Deltocephalinae | Balclutha sp. | KX437738 | [8] | |
| Cicadellidae/Deltocephalinae | Scaphoideus varius | KY817245 | [16] | |
| Cicadellidae/Deltocephalinae | Scaphoideus nigrivalveus | KY817244 | [16] | |
| Cicadellidae/Deltocephalinae | Scaphoideus maai | KY817243 | [16] | |
| Cicadellidae/Deltocephalinae | Yanocephalus yanonis | NC_036131 | [16] | |
| Cicadellidae/Deltocephalinae | Alobaldia tobae | KY039116 | [16] | |
| Cicadellidae/Deltocephalinae | Exitianus indicus | KY039128 | [16] | |
| Cicadellidae/Deltocephalinae | Orosius orientalis | KY039146 | [16] | |
| Cicadellidae/Deltocephalinae | Athysanopsis sp. | KX437726 | [8] | |
| Cicadellidae/Deltocephalinae | Norvellina sp. | KY039131 | [16] | |
| Cicadellidae/Deltocephalinae | Drabescoides nuchalis | NC_028154 | [22] | |
| Cicadellidae/Evacanthinae | Onukia onukii | MK251119 | [23] | |
| Cicadellidae/Evacanthinae | Evacanthus heimianus | MG813486 | [24] | |
| Cicadellidae/Evacanthinae | Evacanthus acuminatus | MK948205 | [25] | |
| Cicadellidae/Iassinae | Trocnadella arisana | NC036480 | [26] | |
| Cicadellidae/Iassinae | Krisna rufimarginata | NC046068 | [26] | |
| Cicadellidae/Iassinae | Krisna concava | MN577635 | [26] | |
| Cicadellidae/Iassinae | Gessius rufidorsus | MN577633 | [26] | |
| Cicadellidae/Iassinae | Batracomorphus lateprocessus | MG813489 | [26] | |
| Cicadellidae/Eurymelinae | Populicerus populi | NC_039427 | [27] | |
| Cicadellidae/Eurymelinae | Idioscopus nitidulus | NC_029203 | [28] | |
| Cicadellidae/Eurymelinae | Idiocerus laurifoliae | NC_039741 | [27] | |
| Cicadellidae/Eurymelinae | Idioscopus clypealis | NC_039642 | [29] | |
| Cicadellidae/Eurymelinae | Idioscopus myrica | MH492317 | [27] | |
| Cicadellidae/Ledrinae | Tituria pyramidata | MN920440 | Unpublished | |
| Cicadellidae/Ledrinae | Ledra auditura | MK387845 | [30] | |
| Cicadellidae/Ledrinae | Petalocephala ochracea | KX437734 | [8] | |
| Cicadellidae/Ledrinae | Petalocephala chlorophana | MT610899 | This study | |
| Cicadellidae/Ledrinae | Tituria sagittata | MT610900 | This study | |
| Cicadellidae/Megophthalminae | Japanagallia spinosa | NC_035685 | [31] | |
| Cicadellidae/Megophthalminae | Durgades nigropicta | NC_035684 | [31] | |
| Cicadellidae/Typhlocybinae | Illinigina sp. | KY039129 | [16] | |
| Cicadellidae/Typhlocybinae | Empoasca onukii | NC_037210 | [32] | |
| Cicadellidae/Typhlocybinae | Empoasca vitis | NC_024838 | [33] | |
| Cicadellidae/Typhlocybinae | Typhlocyba sp. | KY039138 | [16] | |
| Cicadellidae/Typhlocybinae | Empoascanara dwalata | MT350235 | Unpublished | |
| Cicadellidae/Typhlocybinae | Empoasca flavescens | MK211224 | [34] | |
| Cicadellidae/Typhlocybinae | Bolanusoides shaanxiensis | MN661136 | Unpublished | |
| Cicadellidae/Typhlocybinae | Limassolla lingchuanensis | NC046037 | [35] | |
| Cicadellidae/Typhlocybinae | Empoascanara sipra | MN604278 | [36] | |
| Cicadellidae/Typhlocybinae | Paraahimia luodianensis | NC047464 | [37] | |
| Cicadellidae/Typhlocybinae | Mitjaevia protuberanta | NC047465 | [38] | |
| Cicadellidae/Typhlocybinae | Ghauriana sinensis | MN699874 | [39] |
| Name | Location | Size (bp) | Intergenic | Codon | Strand | ||
|---|---|---|---|---|---|---|---|
| From | To | Nucleotides | Start | Stop | |||
| trnI | 1/1 | 64/69 | 64/69 | J/J | |||
| trnQ | 62/67 | 126/130 | 65/64 | −3/−3 | N/N | ||
| trnM | 150/130 | 215/194 | 65/65 | 23/−1 | J/J | ||
| nad2 | 215/195 | 1189/1169 | 975/975 | -/- | ATT/ATT | TAA/TAA | J/J |
| trnW | 1188/1169 | 1250/1232 | 63/64 | −2/−1 | J/J | ||
| trnC | 1243/1225 | 1307/1288 | 65/64 | −8/−1 | N/N | ||
| trnY | 1307/1292 | 1370/1354 | 64/63 | −1/3 | N/N | ||
| cox1 | 1372/1354 | 2910/2889 | 1539/1536 | 1/−1 | ATG/ATG | TAA/TAG | J/J |
| trnL2(UUR) | 2906/2892 | 2977/2960 | 72/69 | −5/2 | J/J | ||
| cox2 | 2978/2961 | 3656/3639 | 679/679 | -/- | ATT/ATT | T/T | J/J |
| trnK | 3657/3640 | 3727/3710 | 71/71 | -/- | J/J | ||
| trnD | 3731/3713 | 3796/3775 | 66/63 | 3/2 | J/J | ||
| atp8 | 3797/3776 | 3949/3928 | 153/153 | -/- | ATT/ATT | TAA/TAA | J/J |
| atp6 | 3946/3925 | 4599/4578 | 654/654 | −4/−4 | ATG/ATA | TAA/TAA | J/J |
| cox3 | 4600/4580 | 5379/5357 | 780/778 | -/1 | ATG/ATG | TAA/T | J/J |
| trnG | 5379/5358 | 5439/5418 | 61/61 | −1/- | J/J | ||
| nad3 | 5437/5416 | 5793/5772 | 357/357 | −3/−3 | ATA/ATA | TAG/TAG | J/J |
| trnA | 5797/5771 | 5856/5830 | 60/60 | 3/−2 | J/J | ||
| trnR | 5857/5833 | 5917/5892 | 61/60 | -/2 | J/J | ||
| trnN | 5917/5894 | 5980/5957 | 64/64 | −1/1 | J/J | ||
| trnS1(ACN) | 5981/5957 | 6038/6016 | 58/60 | -/−1 | J/J | ||
| trnE | 6040/6016 | 6099/6074 | 60/59 | 1/−1 | J/J | ||
| trnF | 6099/6073 | 6160/6136 | 62/64 | −2/−2 | N/N | ||
| nad5 | 6160/6137 | 7819/7799 | 1660/1663 | −1/- | TTG/TTG | T/T | N/N |
| trnH | 7820/7800 | 7880/7861 | 61/62 | -/- | N/N | ||
| nad4 | 7880/7861 | 9169/9156 | 1290/1296 | −1/−1 | ATC/ATC | TAA/TAA | N/N |
| nad4L | 9160/9147 | 9498/9487 | 276/276 | −10/−10 | ATG/ATG | TAA/TAAS | N/N |
| trnT | 9438/9425 | 9498/9487 | 61/63 | 2/2 | J/J | ||
| trnP | 9499/9488 | 9559/9549 | 61/62 | -/- | N/N | ||
| nad6 | 9574/9564 | 10,056/10,049 | 483/486 | 14/14 | ATT/ATT | TAA/TAA | J/J |
| cytb | 10,049/10,042 | 11,185/11,178 | 1137/1137 | −8/−8 | ATG/ATG | TAA/TAG | J/J |
| trnS2(UCN) | 11,185/11,177 | 11,248/11,240 | 64/64 | −1/−2 | J/J | ||
| nad1 | 11,251/11,245 | 12,180/12,172 | 930/928 | 2/4 | ATA/ATA | TAG/T | N/N |
| trnL1(CUN) | 12,181/12,173 | 12,245/12,238 | 65/66 | -/- | N/N | ||
| rrnL | 12,246/12,239 | 13,431/13,418 | 1186/1180 | -/- | N/N | ||
| trnV | 13,432/13,419 | 13,499/13,478 | 68/60 | -/- | N/N | ||
| rrnS | 13,500/13,479 | 14,233/14,183 | 734/705 | -/- | N/N | ||
| CR | 14,234/14,184 | 14,927/14,917 | 694/734 | -/- | J/J | ||
| Feature | Length (bp) | T% | C% | A% | G% | A + T% | AT-Skew | GC-Skew |
|---|---|---|---|---|---|---|---|---|
| PCGs | 10,911 | 45.8 | 11.4 | 29.5 | 13.3 | 75.3 | −0.217 | 0.076 |
| Control region | 694 | 43.2 | 6.6 | 42.2 | 7.9 | 85.4 | −0.012 | 0.089 |
| tRNAs | 1401 | 40.8 | 9.1 | 36.5 | 13.6 | 77.3 | −0.054 | 0.195 |
| rRNAs | 1920 | 34.5 | 8.9 | 46.1 | 10.5 | 80.6 | 0.145 | 0.081 |
| Whole genome | 14,927 | 47 | 9.8 | 29.6 | 13.5 | 76.6 | −0.227 | 0.157 |
| Feature | Length (bp) | T% | C% | A% | G% | A + T% | AT-Skew | GC-Skew |
|---|---|---|---|---|---|---|---|---|
| PCGs | 10,914 | 45.3 | 11.4 | 30.2 | 13.1 | 75.5 | −0.2 | 0.068 |
| Control region | 734 | 43.1 | 8.9 | 39.2 | 8.9 | 82.3 | −0.046 | 0 |
| tRNAs | 1398 | 41.6 | 8.9 | 36.1 | 13.4 | 77.7 | −0.071 | 0.203 |
| rRNAs | 1885 | 34.2 | 9.2 | 44.9 | 11.7 | 79.1 | 0.136 | 0.117 |
| Whole genome | 14,918 | 46.7 | 10.5 | 29.8 | 13 | 76.5 | −0.222 | 0.109 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Zhang, Y. Characterization of Two Complete Mitochondrial Genomes of Ledrinae (Hemiptera: Cicadellidae) and Phylogenetic Analysis. Insects 2020, 11, 609. https://doi.org/10.3390/insects11090609
Huang W, Zhang Y. Characterization of Two Complete Mitochondrial Genomes of Ledrinae (Hemiptera: Cicadellidae) and Phylogenetic Analysis. Insects. 2020; 11(9):609. https://doi.org/10.3390/insects11090609
Chicago/Turabian StyleHuang, Weijian, and Yalin Zhang. 2020. "Characterization of Two Complete Mitochondrial Genomes of Ledrinae (Hemiptera: Cicadellidae) and Phylogenetic Analysis" Insects 11, no. 9: 609. https://doi.org/10.3390/insects11090609
APA StyleHuang, W., & Zhang, Y. (2020). Characterization of Two Complete Mitochondrial Genomes of Ledrinae (Hemiptera: Cicadellidae) and Phylogenetic Analysis. Insects, 11(9), 609. https://doi.org/10.3390/insects11090609






