Relationships between Hyalesthes obsoletus, Its Herbaceous Hosts and Bois Noir Epidemiology in Northern Italian Vineyards
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Influence of the Host Plant on Adult Phenology and Distribution
2.2. Adults’ Survival and Adaptation on Different Development Host Plants
2.3. Adult Populations and Bois Noir Incidence
2.4. Molecular Identification and Characterization of “Ca. P. solani” in Newly Symptomatic Grapevines
3. Results
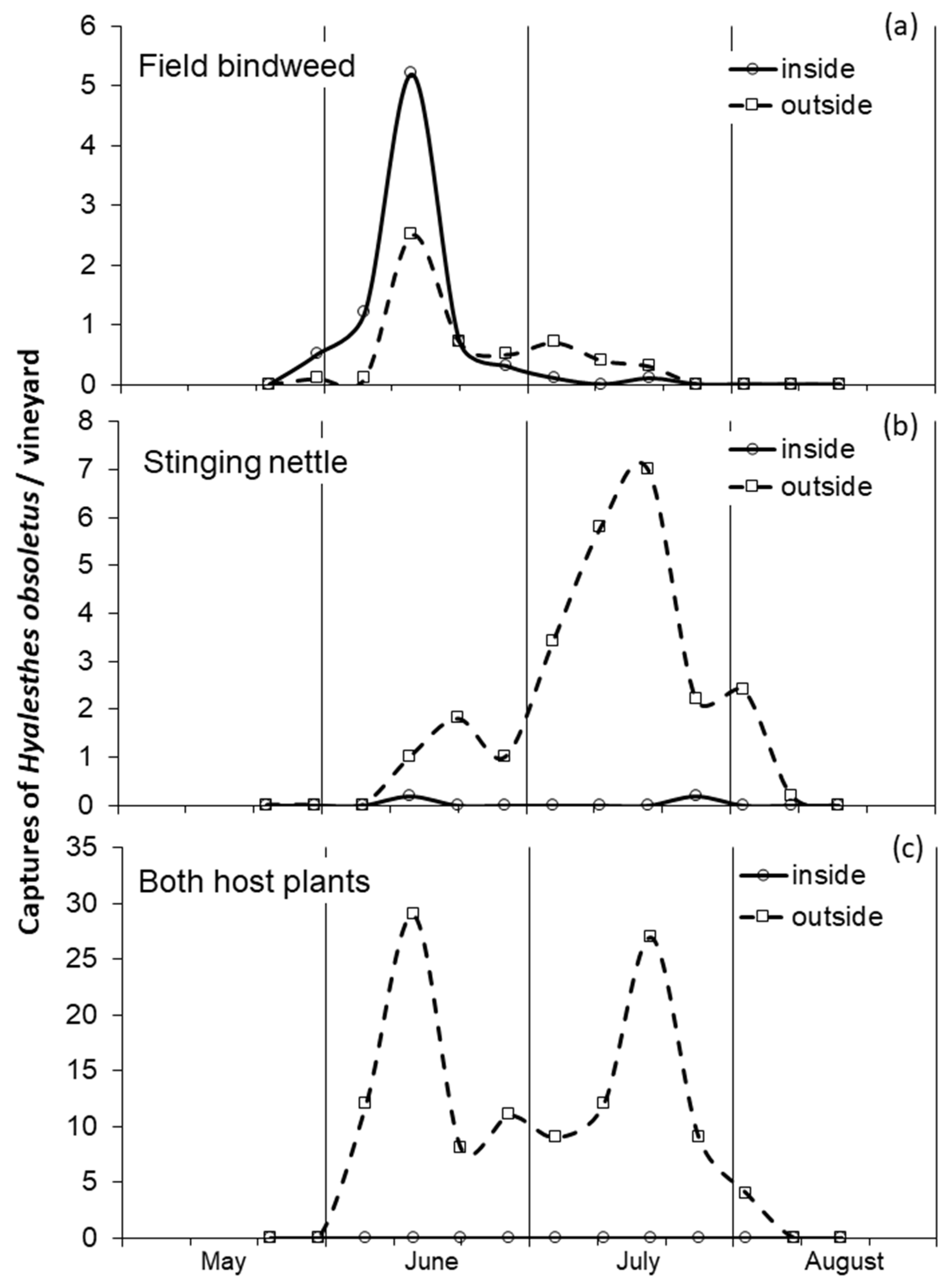
3.1. Influence of Host Plant on the Adult Phenology and Distribution
3.2. Adults’ Survival and Adaptation on Different Development Host Plants
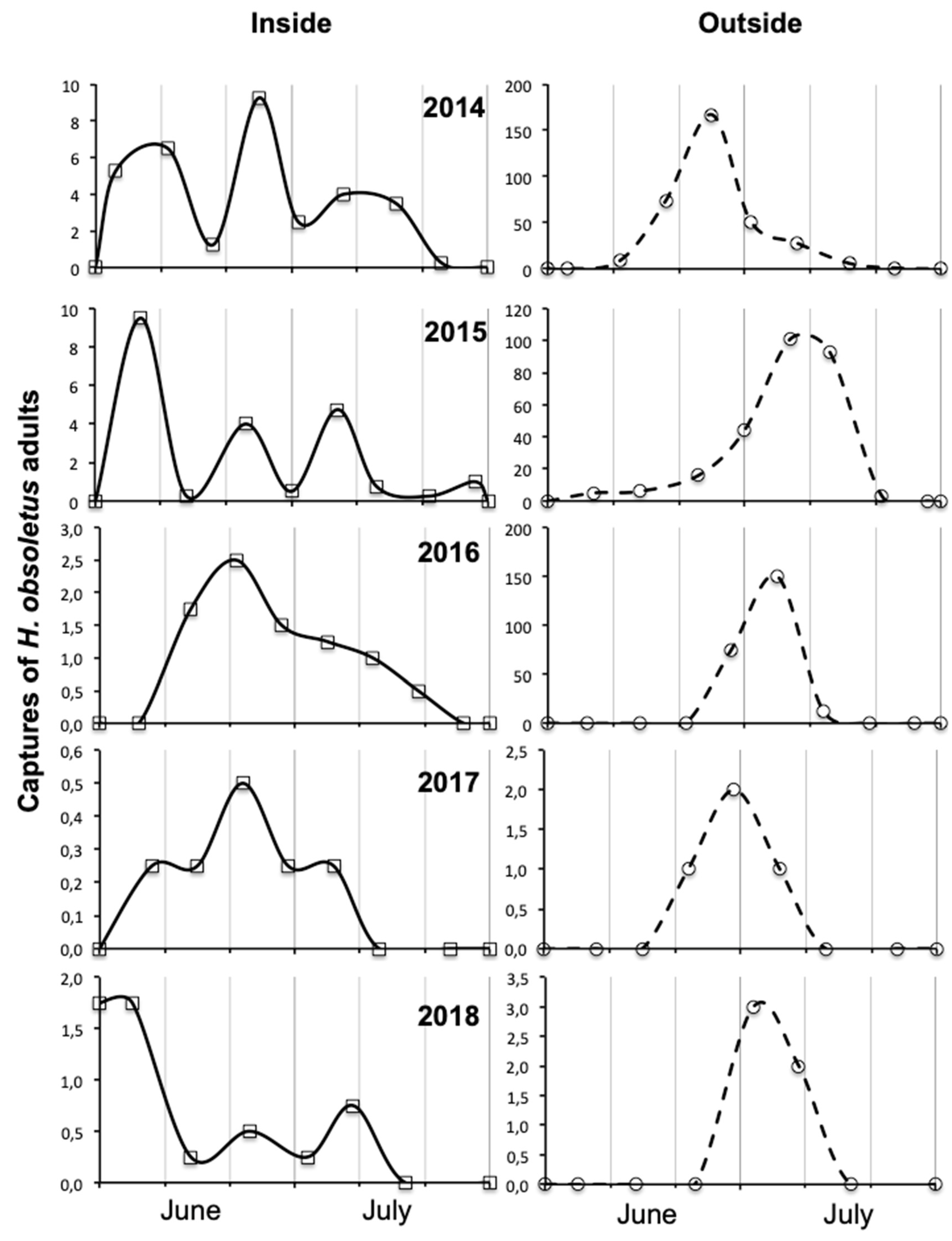
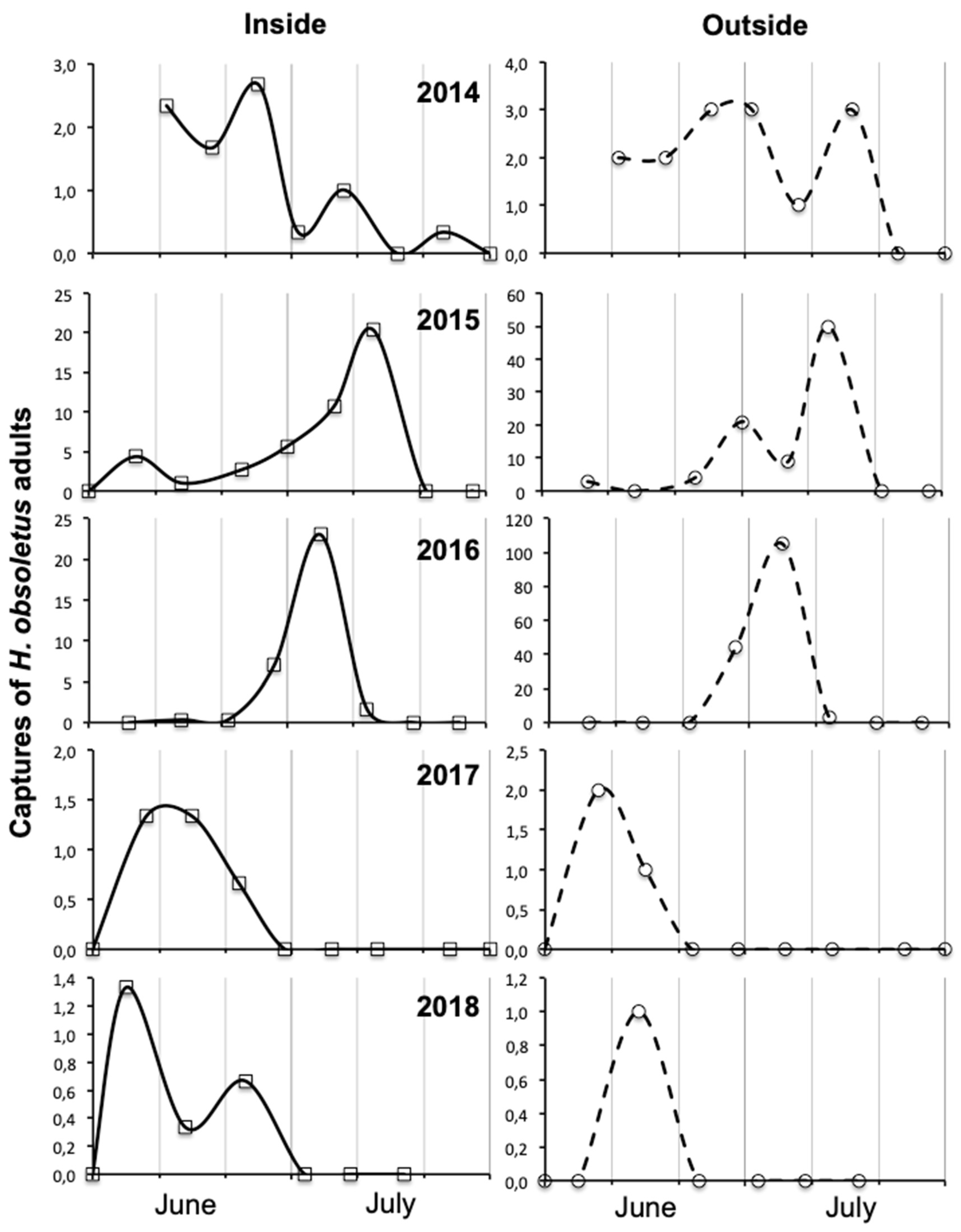
3.3. Factors Influencing Adult Abundance and Phenology in Vineyard Habitats
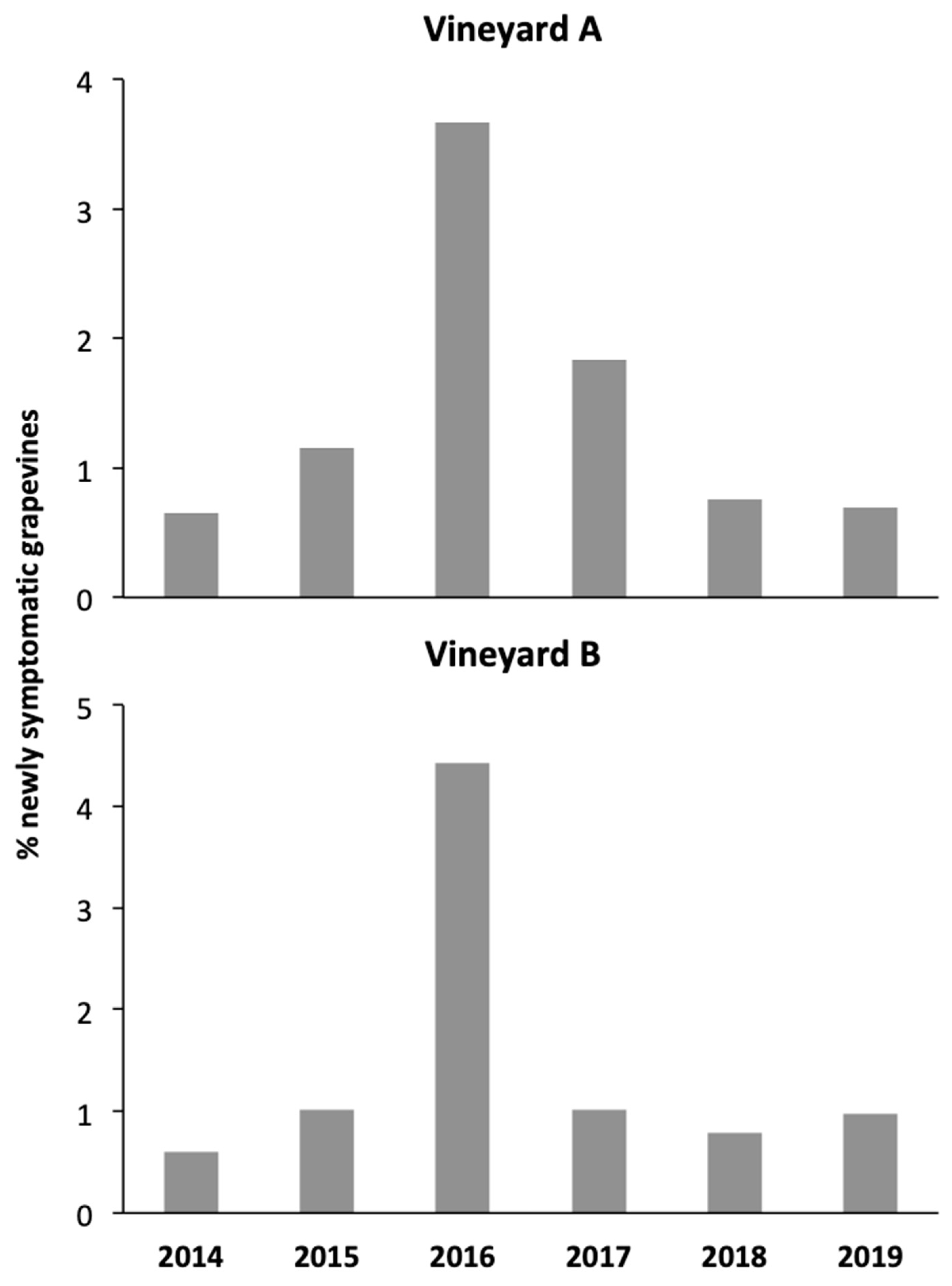
3.4. Bois Noir Incidence and Its Relationship with Adult Abundance
4. Discussion
4.1. Phenology of Adults on Field Bindweed and Stinging Nettle
4.2. Survival and Adaptation of Adults on Field Bindweed and Stinging Nettle
4.3. Influence of Year on Amount, Spatial Distribution and Phenology of Adult Captures
4.4. Relationship between Adult Populations and Newly Symptomatic Grapevines
4.5. Relationship between Adult Population and “Ca. P. solani” Tuf-Type
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belli, G.; Bianco, P.A.; Conti, M. Grapevine yellows in Italy: Past, present and future. J. Plant Pathol. 2010, 92, 303–326. [Google Scholar] [CrossRef]
- Pavan, F.; Mori, N.; Bressan, S.; Mutton, P. Control strategies for grapevine phytoplasma diseases: Factors influencing the profitability of replacing symptomatic plants. Phytopathol. Mediterr. 2012, 51, 11–22. [Google Scholar] [CrossRef]
- Quaglino, F.; Zhao, Y.; Casati, P.; Bulgari, D.; Bianco, P.A.; Wei, W.; Davis, R.E. ‘Candidatus Phytoplasma solani’, a novel taxon associated with stolbur-and bois noir-related diseases of plants. Int. J. Syst. Evol. Microbiol. 2013, 63, 2879–2894. [Google Scholar] [CrossRef] [PubMed]
- Angelini, E.; Constable, F.; Duduk, B.; Fiore, N.; Quaglino, F.; Bertaccini, A. Grapevine phytoplasmas. In Phytoplasmas: Plant Pathogenic Bacteria—I, Characterisation and Epidemiology of Phytoplasma—Associated Diseases; Rao, G.P., Bertaccini, A., Fiore, N., Liefting, L.W., Eds.; Springer: Singapore, 2018; pp. 123–152. [Google Scholar]
- Maixner, M. Transmission of German grapevine yellows (Vergilbungskrankheit) by the planthopper Hyalesthes obsoletus (Auchenorrhyncha: Cixiidae). Vitis 1994, 33, 103–104. [Google Scholar] [CrossRef]
- Maixner, M. Phytoplasmas epidemiological systems with multiple plant hosts. In Phytoplasmas: Genomes, Plant Hosts and Vectors; Weintraub, P.G., Jones, P., Eds.; CABI Publishing: Wallingford, UK, 2010; pp. 213–232. [Google Scholar]
- Sforza, R.; Clair, D.; Daire, X.; Larrue, J.; Boudon-Padieu, E. The role of Hyalesthes obsoletus (Hemiptera: Cixiidae) in the occurrence of bois noir of grapevines in France. J. Phytopathol. 1998, 146, 549–556. [Google Scholar] [CrossRef]
- Alma, A.; Soldi, G.; Tedeschi, R.; Marzachì, C. Ruolo di Hyalesthes obsoletus Signoret (Homoptera, Cixiidae) nella trasmissione del Legno nero della vite in Italia. Petria 2002, 12, 411–412. [Google Scholar]
- Alma, A.; Lessio, F.; Nickel, H. Insects as phytoplasma vectors: Ecological and epidemiological aspects. In Phytoplasmas: Plant Pathogenic Bacteria—II, Transmission and Management of Phytoplasma—Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 1–25. [Google Scholar]
- Bressan, A.; Turata, R.; Maixner, M.; Spiazzi, S.; Boudon-Padieu, E.; Girolami, V. Vector activity of Hyalesthes obsoletus living on nettles and transmitting a stolbur phytoplasma to grapevines: A case study. Ann. Appl. Biol. 2007, 150, 331–339. [Google Scholar] [CrossRef]
- Kosovac, A.; Radonjić, S.; Hrnčić, S.; Krstić, O.; Toševski, I.; Jović, J. Molecular tracing of the transmission routes of bois noir in Mediterranean vineyards of Montenegro and experimental evidence for the epidemiological role of Vitex agnus-castus (Lamiaceae) and associated Hyalesthes obsoletus (Cixiidae). Plant Pathol. 2016, 65, 285–298. [Google Scholar] [CrossRef]
- Kosovac, A.; Jakovljević, M.; Krstić, O.; Cvrković, T.; Mitrović, M.; Toševski, I.; Jović, J. Role of plant-specialized Hyalesthes obsoletus associated with Convolvulus arvensis and Crepis foetida in the transmission of ‘Candidatus Phytoplasma solani’-inflicted bois noir disease of grapevine in Serbia. Eur. J. Plant Pathol. 2019, 153, 183–195. [Google Scholar] [CrossRef]
- Alma, A.; Arnò, C.; Arzone, A.; Vidano, C. New biological reports on Auchenorrhyncha in vineyards. In Proceedings of 6th Auchenorrhyncha Meeting, Turin, Italy, 7–11 September 1987; Vidano, C., Arzone, A., Eds.; CNR-IPRA: Turin, Italy, 1988; pp. 509–516. [Google Scholar]
- Weber, A.; Maixner, M. Habitat requirements of Hyalesthes obsoletus Signoret (Auchenorrhyncha: Cixiidae) and approaches to control this planthopper in vineyards. IOBC/WPRS Bull. 1998, 21, 77–78. [Google Scholar]
- Langer, M.; Maixner, M. Molecular characterisation of grapevine yellows associated phytoplasmas of the stolbur-group based on RFLP-analysis of non-ribosomal DNA. Vitis 2004, 43, 191–199. [Google Scholar] [CrossRef]
- Sharon, R.; Soroker, V.; Wesley, S.D.; Zahavi, T.; Harari, A.; Weintraub, P.G. Vitex agnus-castus is a preferred host plant for Hyalesthes obsoletus. J. Chem. Ecol. 2005, 31, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Pavan, F.; Bondavalli, R.; Reggiani, N.; Paltrinieri, S.; Bertaccini, A. Factors affecting the spread of “Bois Noir” disease in north Italy vineyards. Vitis 2008, 47, 65–72. [Google Scholar] [CrossRef]
- Mori, N.; Mitrović, J.; Smiljković, M.; Duduk, N.; Paltrinieri, S.; Bertaccini, A.; Duduk, B. Hyalesthes obsoletus in Serbia and its role in the epidemiology of corn reddening. Bull. Insectol. 2013, 66, 245–250. [Google Scholar]
- Cargnus, E.; Pavan, F.; Mori, N.; Martini, M. Identification and phenology of Hyalesthes obsoletus (Hemiptera: Auchenorrhyncha: Cixiidae) nymphal instars. Bull. Entomol. Res. 2012, 102, 504–514. [Google Scholar] [CrossRef]
- Kosovac, A.; Johannesen, J.; Krstić, O.; Mitrović, M.; Cvrković, T.; Maixner, M.; Toševski, I.; Jović, J. Microsatellite and mtDNA evidence of genetic differentiation in Hyalesthes obsoletus populations associated with a new major host, stinking hawk’s-beard (Crepis foetida), in Southeast Europe. In Proceedings of 3rd European Bois Noir Workshop, Barcelona, Spain, 20–21 March 2013; Torres, E., Lavina, A., Batlle, A., Eds.; 2013; pp. 18–19. Available online: https://www.researchgate.net/profile/Jelena_Jovic/publication/258032154_Microsatellite_and_mtDNA_evidence_for_genetic_differentiation_of_Hyalesthes_obsoletus_populations_associated_with_a_new_major_host_stinking_hawk’s-beard_Crepis_foetida_in_Southeast_Europe/links/02e7e526ac84c08e1c000000/Microsatellite-and-mtDNA-evidence-for-genetic-differentiation-of-Hyalesthes-obsoletus-populations-associated-with-a-new-major-host-stinking-hawks-beard-Crepis-foetida-in-Southeast-Europe.pdf (accessed on 6 September 2020).
- Jović, J.; Riedle-Bauer, M.; Chuche, J. Vector role of cixiids and other planthopper species. In Phytoplasmas: Plant Pathogenic Bacteria—II, Transmission and Management of Phytoplasma—Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 79–113. [Google Scholar]
- Cvrković, T.; Jović, J.; Mitrović, M.; Krstić, O.; Toševski, I. Experimental and molecular evidence of Reptalus panzeri as a natural vector of bois noir. Plant Pathol. 2014, 63, 42–53. [Google Scholar] [CrossRef]
- Riedle-Bauer, M.; Sára, A.; Regner, F. Transmission of a stolbur phytoplasma by the Agalliinae leafhopper Anaceratagallia ribauti (Homoptera, Auchenorrhyncha, Cicadellidae). J. Phytopathol. 2008, 156, 687–690. [Google Scholar] [CrossRef]
- Chuche, J.; Danet, J.-L.; Salar, P.; Foissac, X.; Thiéry, D. Transmission of ‘Candidatus Phytoplasma solani’ by Reptalus quinquecostatus (Hemiptera: Cixiidae). Ann. Appl. Biol. 2016, 169, 214–223. [Google Scholar] [CrossRef]
- Quaglino, F.; Sanna, F.; Moussa, A.; Faccincani, M.; Passera, A.; Casati, P.; Bianco, P.A.; Mori, N. Identification and ecology of alternative insect vectors of ‘Candidatus Phytoplasma solani’ to grapevine. Sci. Rep. 2019, 9, 19522. [Google Scholar] [CrossRef]
- Maixner, M. Biology of Hyalesthes obsoletus and approaches to control this soil-borne vector of Bois noir disease. IOBC/WPRS Bull. 2007, 30, 3–9. [Google Scholar]
- Riolo, P.; Landi, L.; Nardi, S.; Isidoro, N. Relationship among Hyalesthes obsoletus, its herbaceous host plant and “bois noir” phytoplasma strains in vineyard ecosystems in Marche region (central-eastern Italy). Bull. Insectol. 2007, 60, 353–354. [Google Scholar]
- Mori, N.; Quaglino, F.; Tessari, F.; Pozzebon, A.; Bulgari, D.; Casati, P.; Bianco, P.A. Investigation on ‘bois noir’ epidemiology in north-eastern Italian vineyards through a multidisciplinary approach. Ann. Appl. Biol. 2015, 166, 75–89. [Google Scholar] [CrossRef]
- Aryan, A.; Brader, G.; Mörtel, J.; Pastar, M.; Riedle-Bauer, M. An abundant 'Candidatus Phytoplasma solani' tuf b phytoplasma strain is associated with grapevine, stinging nettle and Hyalesthes obsoletus. Eur. J. Plant Pathol. 2014, 140, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Lessio, F.; Tedeschi, R.; Alma, A. Population dynamics, host plants and infection rate with stolbur phytoplasma of Hyalesthes obsoletus Signoret in northwestern Italy. J. Plant Pathol. 2007, 89, 97–102. [Google Scholar] [CrossRef]
- Forte, V.; Angelini, E.; Maixner, M.; Borgo, M. Preliminary results on population dynamics and host plants of Hyalesthes obsoletus in North-Eastern Italy. Vitis 2010, 49, 39–42. [Google Scholar] [CrossRef]
- Maixner, M.; Johannesen, J. Optimized monitoring of host population of the Bois noir, Hyalesthes obsoletus, based on flight phenology observations. IOBC/WPRS Bull. 2014, 105, 151–157. [Google Scholar]
- Langer, M.; Darimont, H.; Maixner, M. Control of phytoplasma vectors in organic viticulture. IOBC/WPRS Bull. 2003, 26, 197–202. [Google Scholar]
- Maixner, M.; Langer, M. Prediction of the flight of Hyalesthes obsoletus, vector of stolbur phytoplasma, using temperature sums. IOBC/WPRS Bull. 2006, 29, 161–166. [Google Scholar]
- Kessler, S.; Schaerer, S.; Delabays, N.; Turlings, T.C.J.; Trivellone, V.; Kehrli, P. Host plant preferences of Hyalesthes obsoletus, the vector of the grapevine yellows disease ‘bois noir’, in Switzerland. Entomol. Exp. Appl. 2011, 139, 60–67. [Google Scholar] [CrossRef]
- Johannesen, J.; Albert, A.; Imo, M.; Maixner, M. Stolbur phytoplasma interaction with vector longevity in alternative plants. Bull. Insectol. 2011, 64, S147–S148. [Google Scholar]
- Maixner, M.; Albert, A.; Johannesen, J. Survival relative to new and ancestral host plants, phytoplasma infection, and genetic constitution in host races of a polyphagous insect disease vector. Ecol. Evol. 2014, 4, 3082–3092. [Google Scholar] [CrossRef] [PubMed]
- Imo, M.; Maixner, M.; Johannesen, J. Sympatric diversification vs. immigration: Deciphering host-plant specialization in a polyphagous insect, the stolbur phytoplasma vector Hyalesthes obsoletus (Cixiidae). Mol. Ecol. 2013, 22, 2188–2203. [Google Scholar] [CrossRef] [PubMed]
- Kosovac, A.; Johannesen, J.; Krstić, O.; Mitrović, M.; Cvrković, T.; Toševski, I.; Jović, J. Widespread plant specialization in the polyphagous planthopper Hyalesthes obsoletus (Cixiidae), a major vector of stolbur phytoplasma: Evidence of cryptic speciation. PLoS ONE 2018, 13, e0196969. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, W.E.; Kammerlander, I.; Nickel, H. The Auchenorrhyncha of Central Europe. Die Zikaden Mitteleuropas. Volume 1. Fulgoromoropha, Cicadomorpha excl. Cicadellidae; Brill Academic Publishers: Leiden, The Netherlands, 2003. [Google Scholar]
- Bertin, S.; Picciau, L.; Àcs, Z.; Alma, A.; Bosco, D. Molecular identification of the Hyalesthes species (Hemiptera: Cixiidae) occurring in vineyard agroecosystems. Ann. Appl. Biol. 2010, 157, 435–445. [Google Scholar] [CrossRef]
- Martini, M.; Musetti, R.; Grisan, S.; Polizzotto, R.; Borselli, S.; Pavan, F.; Osler, R. DNA-dependent detection of the grapevine fungal endophytes Aureobasidium pullulans and Epicoccum nigrum. Plant Dis. 2009, 93, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Saccardo, F.; Martini, M.; Palmano, S.; Ermacora, P.; Scortichini, M.; Loi, N.; Firrao, G. Genome drafts of four phytoplasma strains of the ribosomal group 16SrIII. Microbiology 2012, 158, 2805–2814. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Pavan, F.; Bianchi, G.L.; Loi, N.; Ermacora, P. Recent spread of the “flavescence dorée” disease in north-eastern Italy. Phytopathog. Mollicutes 2019, 9, 207–208. [Google Scholar] [CrossRef]
- Fialová, R.; Válová, P.; Balakishiyeva, G.; Danet, J.L.; Šafárová, D.; Foissac, X.; Navrátil, M. Genetic variability of stolbur phytoplasma in annual crop and wild plant species in South Moravia. J. Plant Pathol. 2009, 91, 411–416. [Google Scholar] [CrossRef]
- Pavan, F. Possibilità di controllo dei potenziali vettori dell'agente della flavescenza dorata. Inf. Agrar. 1989, 45, 55–61. [Google Scholar]
- Pavan, F.; Carraro, L.; Girolami, V.; Osler, R.; Refatti, E. Nuove risultanze sperimentali sulla Flavescenza dorata della vite acquisite nelle ricerche condotte nella Regione Friuli-Venezia Giulia durante il 1988. Not. ERSA 1989, 2, 4–17. [Google Scholar]
- Mori, N.; Martini, M.; Malagnini, V.; Fontana, P.; Bressan, A.; Girolami, V.; Bertaccini, A. Vettori dei giallumi della vite: Diffusione e strategie di lotta. Inf. Agrar. 1999, 55, 53–56. [Google Scholar]
- Cavallini, G.; Castiglioni, A.; Bortolotti, P.; Mori, N.; Nicoli Aldini, R.; Botti, S.; Malossi, A.; Bertaccini, A. Flavescenza dorata e legno nero in vigneti del Modenese. Inf. Agrar. 2003, 59, 69–71. [Google Scholar]
- Sforza, R.; Boudon-Padieu, E. Le principal vecteur de la maladie du Bois noir. Phytoma 1998, 510, 33–37. [Google Scholar]
- Mori, N.; Pavan, F.; Maixner, M. Control of Hyalesthes obsoletus nymphs based on chemical weeding and insecticides applied on Urtica dioica. Vitis 2014, 53, 103–109. [Google Scholar] [CrossRef]
- Mori, N.; Pozzebon, A.; Duso, C.; Reggiani, N.; Pavan, F. Vineyard colonization by Hyalesthes obsoletus (Hemiptera: Cixiidae) induced by stinging nettle cut along surrounding ditches. J. Econ. Entomol. 2016, 109, 49–56. [Google Scholar] [CrossRef]
- Panassiti, B.; Breuer, M.; Marquardt, S.; Biedermann, R. Influence of environment and climate on occurrence of the cixiid planthopper Hyalesthes obsoletus, the vector of the grapevine disease ‘bois noir’. Bull. Entomol. Res. 2013, 103, 621–633. [Google Scholar] [CrossRef]
- Panassiti, B.; Hartig, F.; Breuer, M.; Biedermann, R. Bayesian inference of environmental and biotic factors determining the occurrence of the grapevine disease ‘bois noir’. Ecosphere 2015, 6, 143. [Google Scholar] [CrossRef]
- Cicogna, A.; Tonello, P. Gelate primavera 2017: Analisi climatologica e danni sulle produzioni. Not. ERSA 2017, 2017, 8–13. [Google Scholar]
- Maixner, M.; Reinert, W. Monitoring of planthopper vectors in vineyards: An aid for grapevine yellows management decisions. IOBC/WPRS Bull. 2000, 23, 123–124. [Google Scholar]
- Darimont, H.; Maixner, M. Actual distribution of Hyalesthes obsoletus Signoret (Auchenorrhyncha: Cixiidae) in German viticulture and its significance as a vector of Bois noir. IOBC/WPRS Bull. 2001, 24, 199–202. [Google Scholar]
- Mori, N.; Pavan, F.; Reggiani, N.; Bacchiavini, M.; Mazzon, L.; Paltrinieri, S.; Bertaccini, A. Correlation of bois noir disease with nettle and vector abundance in northern Italy vineyards. J. Pest Sci. 2012, 85, 23–28. [Google Scholar] [CrossRef]
- Panassiti, B.; Hartig, F.; Fahrentrapp, J.; Breuer, M.; Biedermann, R. Identifying local drivers of a vector-pathogen disease system using Bayesian modeling. Basic Appl. Ecol. 2017, 18, 75–85. [Google Scholar] [CrossRef]
- Brčak, J. Leafhopper and planthopper vectors of plant disease agents in central and southern Europe. In Leafhopper Vectors and Plant Disease Agents in Central and Southern Europe; Maramorosch, K., Harris, K.F., Eds.; Academic Press: New York, NY, USA, 1979; pp. 97–154. [Google Scholar] [CrossRef]
- Caudwell, A.; Kuszala, C.; Bachelier, J.C.; Larrue, J. Transmission de la flavescence dorée de la vigne aux plantes herbacées par l’allongement du temps d’utilisation de la cicadelle Scaphoideus titanus Ball et l’etude de sa survie sur un grand nombre d’especes végétales. Ann. Phytopathol. 1970, 2, 415–428. [Google Scholar]
- Caudwell, A. Epidemiology and characterization of Flavescence dorée (FD) and other grapevine yellows. Agronomie 1990, 10, 655–663. [Google Scholar] [CrossRef]
- Osler, R.; Carraro, L.; Loi, N.; Refatti, E. Symptom expression and disease occurrence of a yellows disease of grapevine in northeastern Italy. Plant Dis. 1993, 77, 496–498. [Google Scholar] [CrossRef]
- Mutton, P.; Boccalon, W.; Bressan, S.; Coassin, C.; Colautti, M.; Del Cont Bernard, D.; Floreani, A.; Zucchiatti, D.; Pavan, F.; Mucignat, D.; et al. Legno nero della vite in vigneti di Chardonnay del Friuli-Venezia Giulia. Inf. Fitopatol. 2002, 52, 52–59. [Google Scholar]


| Source of Variation | Levels | Mean ± SE | F | Degree of Freedom | p |
|---|---|---|---|---|---|
| Year | 2014 | 13.07 ± 6.10 c | 30.36 | 4, 118 | <0.0001 |
| 2015 | 10.76 ± 5.15 c | ||||
| 2016 | 6.13 ± 2.49 b | ||||
| 2017 | 0.196 ± 0.085 a | ||||
| 2018 | 0.241 ± 0.180 a | ||||
| Period | 1–25 June | 0.750 ± 0.147 | 61.15 | 1, 118 | <0.0001 |
| 26 June to 20 July | 11.41 ± 3.31 | ||||
| Position | Outside | 11.09 ± 3.32 | 25.38 | 1, 118 | <0.0001 |
| Inside | 1.08 ± 0.191 | ||||
| Replicate | 3.30 | 6, 118 | 0.005 | ||
| Year × period | 9.26 | 4, 118 | <0.0001 | ||
| Year × position | 2.86 | 4, 118 | 0.026 | ||
| Period × position | 45.22 | 1, 118 | <0.0001 |
| Source of Variation | Levels | Mean ± SE | F | Degree of Freedom | p |
|---|---|---|---|---|---|
| Year | 2014 | 0.961 ± 0.278 bc | 18.67 | 4, 118 | <0.0001 |
| 2015 | 4.07 ± 1.20 d | ||||
| 2016 | 4.45 ± 2.26 d | ||||
| 2017 | 0.214 ± 0.084 ab | ||||
| 2018 | 0.083 ± 0.033 a | ||||
| Period | 1–25 June | 0.610 ± 0.123 | 13.31 | 1, 118 | <0.0001 |
| 26 June to 20 July | 3.303 ± 1.036 | ||||
| Position | Outside | 3.033 ± 1.035 | 2.98 | 1, 118 | 0.087 |
| Inside | 0.880 ± 0.191 | ||||
| Replicate | 2.10 | 6, 118 | 0.059 | ||
| Year × period | 17.34 | 4, 118 | <0.0001 | ||
| Year × position | 2.02 | 4, 118 | 0.096 | ||
| Period × position | 12.54 | 1, 118 | 0.001 |
| % Newly Symptomatic Grapevines | Vineyard | Equation | P | R2 |
|---|---|---|---|---|
| In the same year | A | Y = −0.009X + 2.17 | 0.48 | 0.18 |
| B | Y = 0.016X + 1.01 | 0.60 | 0.10 | |
| In the next year | A | Y = 0.014X + 0.71 | 0.19 | 0.49 |
| B | Y = 0.047X + 0.15 | 0.032 | 0.83 |
| Vineyard/Year | Total Newly Symptomatic Grapevines | Newly Symptomatic Grapevines Analysed | Tuf-Type a | Tuf-Type b | P at Binomial Test | ||
|---|---|---|---|---|---|---|---|
| N. | N. | N. | % | N. | % | ||
| Vineyard A | |||||||
| 2016 | 56 | 21 | 21 | 100 | 0 | 0.0 | >0.0001 |
| 2017 | 20 | 8 | 5 | 62.5 | 3 | 37.5 | NS |
| 2018–2019 1 | 15 | 6 | 2 | 33.3 | 5 | 83.3 | NS |
| Vineyard B | |||||||
| 2015–2016 | 90 | 20 | 19 | 95.0 | 1 | 5.0 | >0.0001 |
| 2017 | 16 | 13 | 10 | 76.9 | 3 | 23.1 | 0.046 |
| 2018–2019 | 27 | 14 | 13 | 92.9 | 1 | 7.1 | 0.0009 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, N.; Cargnus, E.; Martini, M.; Pavan, F. Relationships between Hyalesthes obsoletus, Its Herbaceous Hosts and Bois Noir Epidemiology in Northern Italian Vineyards. Insects 2020, 11, 606. https://doi.org/10.3390/insects11090606
Mori N, Cargnus E, Martini M, Pavan F. Relationships between Hyalesthes obsoletus, Its Herbaceous Hosts and Bois Noir Epidemiology in Northern Italian Vineyards. Insects. 2020; 11(9):606. https://doi.org/10.3390/insects11090606
Chicago/Turabian StyleMori, Nicola, Elena Cargnus, Marta Martini, and Francesco Pavan. 2020. "Relationships between Hyalesthes obsoletus, Its Herbaceous Hosts and Bois Noir Epidemiology in Northern Italian Vineyards" Insects 11, no. 9: 606. https://doi.org/10.3390/insects11090606
APA StyleMori, N., Cargnus, E., Martini, M., & Pavan, F. (2020). Relationships between Hyalesthes obsoletus, Its Herbaceous Hosts and Bois Noir Epidemiology in Northern Italian Vineyards. Insects, 11(9), 606. https://doi.org/10.3390/insects11090606





