Host Specificity of the Parasitic Wasp Anaphes flavipes (Hymenoptera: Mymaridae) and a New Defence in Its Hosts (Coleoptera: Chrysomelidae: Oulema spp.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasitic Wasps
2.2. Host Species
2.3. Laboratory Experiments
2.3.1. Host Defence
2.3.2. Host Specificity
3. Results
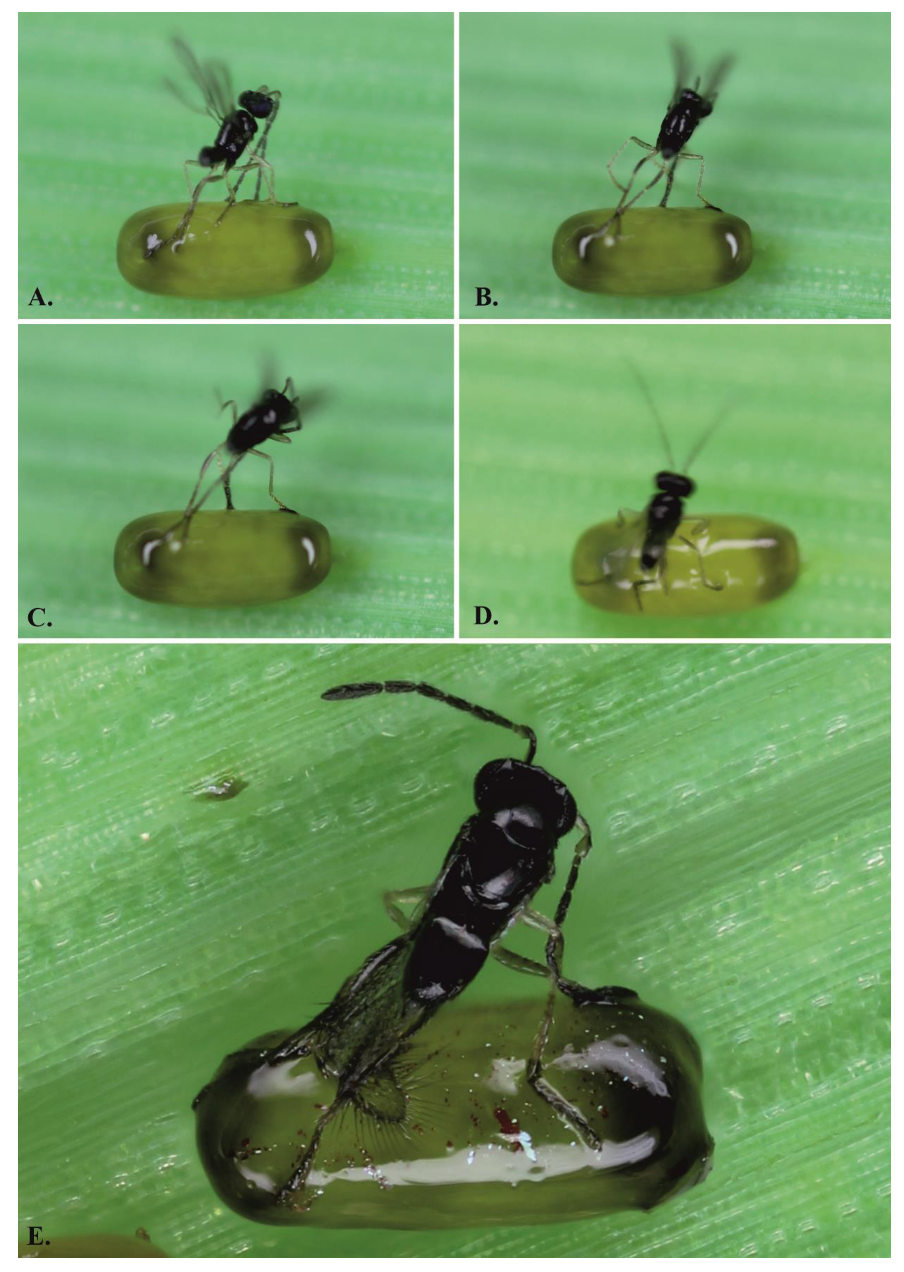
3.1. Host Defence
- (1)
- The female adheres to the sticky layer and is unable to either parasitize or release herself;
- (2)
- The wasp is able to parasitize the egg but cannot release herself from the egg surface;
- (3)
- The wasp successfully parasitizes the eggs and leaves the host, but afterwards, she must clean herself.
3.2. Host Specificity
4. Discussion
- (1)
- The female adheres to the sticky layer and is unable to either parasitize or release herself. First, before parasitization, the female needs to examine the suitability of the host eggs with her antennae (25; on average, for 12 s, n = 19 (Samková, unpubl.)). During this behavior, the wasp can adhere to the surface of the host egg before laying the eggs. In this case, the dark sticky layer succeeds in protecting the specific host egg.
- (2)
- The wasp is able to parasitize the egg but cannot unstick herself from the egg surface. Both of these host defence situations could be considered interspecific because other host eggs in the vicinity are protected against parasitization by this particular A. flavipes female.
- (3)
- The wasp successfully parasitizes and leaves the host, but afterwards, she must clean herself. At first, this defence may seem ineffective, because the sticky layer does not protect the egg from parasitization. However, this third observed behavior could lead to specialization in the wasps with such ‘experience’, which might afterwards prefer eggs without the dark sticky layer (such as those of O. duftschmidi and O. melanopus). It is known that, the choice of a host is related to the individual behavior and previous experiences of the female; flexible females could thus respond to a changing environment [19]. However, we must be careful in interpreting this claim, because scenario of wasps specialisation to host without defense againts parasitation is only our idea and future experiments are needed for it.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strand, M.R.; Obrycki, J.J. Host specifity of insect parasitoids and predators. BioScience 1996, 46, 422–429. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Gross, P. Insect behavioral and morphological defenses against parasitoids. Annu. Rev. Entomol. 1993, 38, 251–273. [Google Scholar] [CrossRef]
- Tylikinais, J.M.; Tscharntke, T.; Klein, A.M. Diversity, ecosystem function and stability of parasitoid—Host interactions across a tropical habitat gradient. Ecology 2006, 87, 3047–3057. [Google Scholar]
- Giunti, G.; Canale, A.; Messing, R.H.; Donati, E.; Stefanini, C.; Michaud, J.P.; Benelli, G. Parasitoid learning: Current knowledge and implications for biological control. Biol. Control. 2015, 90, 208–219. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of Arthropod pests in Agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Van Lenteren, J.C. Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Cock, M.J.; van Lenteren, J.C.; Brodeur, J.; Barratt, B.I.; Bigler, F.; Bolckmans, K.; Cônsoli, F.L.; Haas, F.; Mason, P.G.; Parra, J.R.P. Do new access and benefit sharing procedures under the convention on biological diversity threaten the future of biological control? BioControl 2010, 55, 199–218. [Google Scholar] [CrossRef]
- González, D.; Cervenka, V.; Moratorio, M.; Pickett, C.; Wilson, T.L. Longterm control of variegated leafhopper in grape IPM programs will depem on fading, rearing, and releasing effective natural enemies. Calif. Agric. 1988, 42, 23–25. [Google Scholar]
- Altieri, M.A. Agroecological foundations of alternative agriculture in California. Agric. Ecosyst. Environ. 1992, 39, 23–53. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Biodiversity, Ecosystems Function, and Insect pest Management in Agricultural Systems. In Biodiversity in Agroecosystems; Collins, W.W., Qualset, C.O., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 69–84. [Google Scholar]
- Van Lenteren, J.C.; Bueno, V.H. Augmentative biological control of arthropods in Latin America. BioControl 2003, 48, 123–139. [Google Scholar] [CrossRef]
- Grandgirard, J.; Hoddle, M.S.; Petit, J.N.; Roderick, G.K.; Davies, N. Classical biological control of the glassy-winged sharpshooter, Homalodisca vitripennis, by the egg parasitoid Gonatocerus ashmeadi in the Society, Marqesas and Austral archipelagos of French Polynesia. Biol. Control. 2009, 48, 155–163. [Google Scholar] [CrossRef]
- Rivera, A.C.; Carbone, S.S.; Andrés, J.A. Life cycle and biological control of the Eucalyptus snout beetle [Coleoptera, Curculionidae] by Anaphes nitens [Hymenoptera, Mymaridae] in north-west Spain. Agric. Forest Entomol. 1999, 1, 103–109. [Google Scholar] [CrossRef]
- Rivera, A.C.; Carbone, S. The effect of three species of eukalyptus on growth and fecundity of the Eucalyptus snout beetle. Forestry 2000, 73, 21–29. [Google Scholar] [CrossRef]
- Hoelmer, K.A.; Kirk, A.A. Selecting arthropod biological control agents against arthropod pests: Can the science be improved to decrease the risk of releasing ineffective agents? Biol. Control. 2005, 34, 255–264. [Google Scholar] [CrossRef]
- McEvoy, P.B. Host specificity and biological pest control. BioScience 1996, 46, 401–405. [Google Scholar] [CrossRef][Green Version]
- Krombein, K.V.; Hurd, P.D.; Smith, D.R.; Burks, B.D. Catalog of Hymenoptera in America north of Mexico. Vol. 1; Smithsonian Institution Press: Washington, WA, USA, 1979. [Google Scholar]
- Vinson, S.B. The general host selection behavior of parasitoid Hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol. Control. 1998, 11, 79–96. [Google Scholar] [CrossRef]
- Dawkins, R.; Krebs, J.R. Arms races between and within species. Proc. R. Soc. Lond. B 1979, 205, 489–511. [Google Scholar] [PubMed]
- Kraaijeveld, A.R.; Van Alphen, J.J.M.; Godfray, H.C.J. The coevolution of host resistance and parasitoid virulence. Parasitology 1998, 116, S29–S45. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Paschke, J.D. The biology and ecology of Anaphes flavipes [Hymenoptera: Mymaridae], an exotic egg parasite of the cereal leaf beetle. Ann. Entomol. Soc. Am. 1968, 61, 1–5. [Google Scholar] [CrossRef]
- Samková, A.; Janšta, P.; Huber, J.T. Anaphes flavipes [Foester, 1841] redescription, neotype designation, and comparison with A. nipponicus Kuwayama, 1932 [Hymenoptera: Chalcidoidea: Mymaridae]. Acta Ent. Mus. Nat. Pra. 2017, 57, 677–711. [Google Scholar]
- Samková, A.; Hadrava, J.; Skuhrovec, J.; Janšta, P. Reproductive strategy as a major factor determining female body size and fertility of a gregarious parasitoid. J. Appl. Entomol. 2019, 143, 441–450. [Google Scholar] [CrossRef]
- Dysart, R.J.; Maltby, H.L.; Brunson, M.H. Larval parasites of Oulema melanopus in Europe and their colonization in the United States. Entomophaga 1973, 18, 133–167. [Google Scholar] [CrossRef]
- Skuhrovec, J.O.; Douda, M.; Zouhar, M.; Maňasová, P.; Nový, P.; Božik, M.; Klouček, P. Insecticidal activity of two formulations of essential oils against the cereal leaf beetle. Acta Agric. Scand. B-Soil Plant 2018, 68, 489–495. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Paschke, J.D. Factors affecting the postrelease dispersal of Anaphes flavipes [Hymenoptera: Mymaridae], with notes on its postrelease development, efficiency, and emergence. Ann. Entomol. Soc. Am. 1970, 63, 820–828. [Google Scholar] [CrossRef]
- Maltby, H.L.; Stehr, F.W.; Anderson, R.C.; Moorehead, G.E.; Barton, L.C.; Paschke, J.D. Establishment in the United States of Anaphes flavipes, an egg parasite of the cereal leaf beetle. J. Econ. Entomol. 1971, 64, 693–697. [Google Scholar] [CrossRef]
- Horváth, L.; Szabolcs, J. Parasitoids of cereal leaf beetles, Oulema Goeze spp., in Hungary. Int. St. Crop. 1992, 57, 585–589. [Google Scholar]
- Maltby, H.L.; Burger, T.L.; Holmes, M.C.; Dewitt, P.R. The use of an unnatural host, Lema trilineata trivittata, for rearing the exotic egg parasite Anaphes flavipes. Ann. Entomol. Soc. Am. 1973, 66, 298–301. [Google Scholar] [CrossRef]
- Bezděk, J.; Baselga, A. Revision of western Palaearctic species of the Oulema melanopus group, with description of two new species from Europe [Coleoptera: Chrysomelidae: Criocerinae]. Acta Ent. Mus. Nat. Pra. 2015, 55, 273–304. [Google Scholar]
- R. Core Team. R. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Van de Vijver, E.; Landschoot, S.; Van Roie, M.; Temmerman, F.; Dillen, J.; De Ceuleners, K.; Smagghe, G.; De Baets, B.; Haesaert, G. Inter-and Intrafield Distribution of Cereal Leaf Beetle Species [Coleoptera: Chrysomelidae] in Belgian Winter Wheat. Environ. Entomol. 2019, 48, 276–283. [Google Scholar] [CrossRef]
- Strand, M.R.; Pech, L.L. Immunological basis for compatibility in parasitoid-host relationships. Annu. Rev. Entomol. 1995, 40, 31–56. [Google Scholar] [CrossRef]
- Schaefer, P.W. Ivela auripes Butler in Hokkaido: Behavior and morphology of females; host egg defense mechanism against parasitism by Trichogramma sp. nov. Kontyu 1983, 51, 298–307. [Google Scholar]
- Damman, H.; Cappuccino, N. Two forms of egg defence in chrysomelid beetle: Egg clumping and excrement cover. Ecol. Entomol. 1991, 16, 163–167. [Google Scholar] [CrossRef]
- Chabo, C.S. Biology and phylogeny of the Cassidinae Gyllenhal sensu lato [tortoise and leaf-mining beetles] [Coleoptera: Chrysomelidae]. Bull. Am. Mus. Nat. Hist. 2007, 305, 1–250. [Google Scholar] [CrossRef]
- Hoffman, G.D.; Rao, S. Oviposition site selection on oats: The effect of plant architecture, plant and leaf age, tissue toughness, and hardness on cereal leaf beetle, Oulema melanopus. Entomol. Exp. Appl. 2011, 141, 232–244. [Google Scholar] [CrossRef]
- Stone, G.N.; Cook, J.M. The structure of cynipid oak galls: Patterns in the evolution of an extended phenotype. Proc. R. Soc. Lond. B Biol. 1998, 265, 979–988. [Google Scholar] [CrossRef]
- Stone, G.N.; Schönrogge, K. The adaptive significance of insect gall morphology. Trends. Ecol. Evol. 2003, 18, 512–522. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samková, A.; Hadrava, J.; Skuhrovec, J.; Janšta, P. Host Specificity of the Parasitic Wasp Anaphes flavipes (Hymenoptera: Mymaridae) and a New Defence in Its Hosts (Coleoptera: Chrysomelidae: Oulema spp.). Insects 2020, 11, 175. https://doi.org/10.3390/insects11030175
Samková A, Hadrava J, Skuhrovec J, Janšta P. Host Specificity of the Parasitic Wasp Anaphes flavipes (Hymenoptera: Mymaridae) and a New Defence in Its Hosts (Coleoptera: Chrysomelidae: Oulema spp.). Insects. 2020; 11(3):175. https://doi.org/10.3390/insects11030175
Chicago/Turabian StyleSamková, Alena, Jiří Hadrava, Jiří Skuhrovec, and Petr Janšta. 2020. "Host Specificity of the Parasitic Wasp Anaphes flavipes (Hymenoptera: Mymaridae) and a New Defence in Its Hosts (Coleoptera: Chrysomelidae: Oulema spp.)" Insects 11, no. 3: 175. https://doi.org/10.3390/insects11030175
APA StyleSamková, A., Hadrava, J., Skuhrovec, J., & Janšta, P. (2020). Host Specificity of the Parasitic Wasp Anaphes flavipes (Hymenoptera: Mymaridae) and a New Defence in Its Hosts (Coleoptera: Chrysomelidae: Oulema spp.). Insects, 11(3), 175. https://doi.org/10.3390/insects11030175




