New Data, Old Story: Molecular Data Illuminate the Tribal Relationships among Rove Beetles of the Subfamily Staphylininae (Coleoptera: Staphylinidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Taxon Sampling and Sequence Alignment
2.2. Outgroup Selection
2.3. Model Selection, Data Partitioning and Phylogenetic Analyses
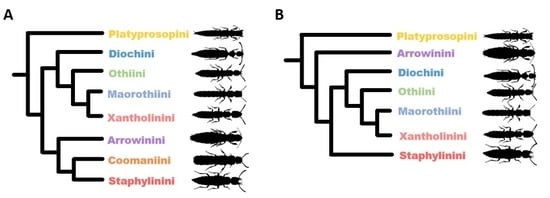
3. Results
3.1. Monophyly of Staphylininae and Paederinae
3.2. Phylogeny of Staphylininae
3.2.1. Position of Arrowinini
3.2.2. Are Arrowinus + Platyprosopus Monophyletic?
3.2.3. Systematic Position of Coomania
3.2.4. Unresolved Nodes and Remaining Questions in Staphylininae Phylogeny
4. Discussion
4.1. Sources of Phylogenetic Incongruence
4.2. Taxonomic Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newton, A.F. Beetles (Coleoptera) of Peru: A Survey of the Families. Staphylinidae Latreille, 1802. J. Kans. Entomol. Soc. 2015, 88, 283–304. [Google Scholar] [CrossRef]
- Thayer, M.K. Staphylinidae Latreille, 1802. In Handbook of Zoology; Arthropoda: Insecta, Coleoptera, Beetles. Vol. 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga Partim); De Gruyter: Berlin/Heidelberg, Germany, 2016; pp. 394–442. [Google Scholar]
- Cai, C.-Y.; Wang, Y.-L.; Lü, L.; Yin, Z.-W.; Thayer, M.K.; Newton, A.F.; Zhou, Y.-L. Congruence of morphological and molecular phylogenies of the rove beetle subfamily Staphylininae (Coleoptera: Staphylinidae). Sci. Rep. 2019, 9, 15137. [Google Scholar] [CrossRef] [PubMed]
- Brunke, A.J.; Smetana, A. A new genus of Staphylinina and a review of major lineages (Staphylinidae: Staphylininae: Staphylinini). Syst. Biodivers. 2019, 17, 745–758. [Google Scholar] [CrossRef]
- Herman, L.H. Catalog of the Staphylinidae (Insecta: Coleoptera). 1758 to the end of the second millennium. I. Introduction, history, biographical sketches, and omaliine group. Bull. Am. Mus. Nat. Hist. 2001, 265, 1–659. [Google Scholar] [CrossRef]
- Kasule, F.K. The larvae of Paederinae and Staphylininae (Coleoptera: Staphylinidae) with keys to the known British genera. Trans. R. Entomol. Soc. Lond. 1970, 122, 49–80. [Google Scholar] [CrossRef]
- Newton, A.F.; Thayer, M.K. Staphylinidae Latreille, 1802. In American Beetles (Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia); CRC Press: London, UK, 2000; Volume 1, pp. 272–418. [Google Scholar]
- Grebennikov, V.V.; Newton, A.F. Good-bye Scydmaenidae, or why the ant-like stone beetles should become megadiverse Staphylinidae sensu latissimo (Coleoptera). Eur. J. Entomol. 2013, 106, 275–301. [Google Scholar] [CrossRef]
- Newton, A.F.; Thayer, M.K. Current classification and family-group names in Staphyliniformia (Coleoptera). Fieldiana Zool. 1992, 67, 1–92. [Google Scholar]
- Solodovnikov, A.Y.; Newton, A.F. Phylogenetic placement of Arrowinini trib. n. within the subfamily Staphylininae (Coleoptera: Staphylinidae), with revision of the relict South African genus Arrowinus and description of its larva. Syst. Entomol. 2005, 30, 398–441. [Google Scholar] [CrossRef]
- McKenna, D.D.; Wild, A.L.; Kanda, K.; Bellamy, C.L.; Beutel, R.G.; Caterino, M.S.; Farnum, C.W.; Hawks, D.C.; Ivie, M.A.; Jameson, M.L.; et al. The beetle tree of life reveals that Coleoptera survived end-Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst. Entomol. 2015, 40, 835–880. [Google Scholar] [CrossRef]
- Chatzimanolis, S.; Cohen, I.M.; Schomann, A.; Solodovnikov, A. Molecular phylogeny of the mega-diverse rove beetle tribe Staphylinini (Insecta, Coleoptera, Staphylinidae). Zool. Scr. 2010, 39, 436–449. [Google Scholar] [CrossRef]
- McKenna, D.D.; Farrell, B.D.; Caterino, M.S.; Farnum, C.W.; Hawks, D.C.; Maddison, D.R.; Seago, A.E.; Short, A.E.Z.; Newton, A.F.; Thayer, M.K. Phylogeny and evolution of Staphyliniformia and Scarabaeiformia: Forest litter as a stepping stone for diversification of nonphytophagous beetles. Syst. Entomol. 2015, 40, 35–60. [Google Scholar] [CrossRef]
- Hansen, M. Evolutionary trends in “staphyliniform” beetles (Coleoptera). Steenstrupia 1997, 23, 43–86. [Google Scholar]
- Welch, R.C. Ovariole development in Staphylinidae (Coleoptera). Invertebr. Reprod. Dev. 1993, 23, 225–234. [Google Scholar] [CrossRef]
- Kasule, F.K. The subfamilies of the larvae of Staphylinidae (Coleoptera) with keys to the larvae of the British genera of Steninae and Proteininae. Trans. R. Entomol. Soc. Lond. 1966, 118, 261–283. [Google Scholar] [CrossRef]
- Brunke, A.J.; Chatzimanolis, S.; Schillhammer, H.; Solodovnikov, A. Early evolution of the hyperdiverse rove beetle tribe Staphylinini (Coleoptera: Staphylinidae: Staphylininae) and a revision of its higher classification. Cladistics 2016, 32, 427–451. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, H. Aedeagus evolution promotes speciation? A primary pattern in rove beetle phylogeny. Zool. Syst. 2018, 43, 125–138. [Google Scholar]
- Schomann, A.M.; Solodovnikov, A. Phylogenetic placement of the austral rove beetle genus Hyperomma triggers changes in classification of Paederinae (Coleoptera: Staphylinidae). Zool. Scr. 2017, 46, 336–347. [Google Scholar] [CrossRef]
- Kypke, J.L.; Solodovnikov, A.; Brunke, A.; Yamamoto, S.; Żyła, D. The past and the present through phylogenetic analysis: The rove beetle tribe Othiini now and 99 Ma. Syst. Entomol. 2019, 44, 1–18. [Google Scholar] [CrossRef]
- Żyła, D.; Solodovnikov, A. Multilocus phylogeny defines a new classification of Staphylininae (Coleoptera, Staphylinidae), a rove beetle group with high lineage diversity. Syst. Entomol. 2019, 45, 114–127. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucl. Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Lin, C.-P.; Danforth, B.N. How do insect nuclear and mitochondrial gene substitution patterns differ? Insights from Bayesian analyses of combined datasets. Mol. Phylogenet. Evol. 2004, 30, 686–702. [Google Scholar] [CrossRef]
- Li, T.; Hua, J.; Wright, A.M.; Cui, Y.; Xie, Q.; Bu, W.; Hillis, D.M. Long-branch attraction and the phylogeny of true water bugs (Hemiptera: Nepomorpha) as estimated from mitochondrial genomes. BMC Evol. Biol. 2014, 14, 99. [Google Scholar] [CrossRef]
- Uribe, J.E.; Irisarri, I.; Templado, J.; Zardoya, R. New patellogastropod mitogenomes help counteracting long-branch attraction in the deep phylogeny of gastropod mollusks. Mol. Phylogenet. Evol. 2019, 133, 12–23. [Google Scholar] [CrossRef]
- Young, A.D.; Gillung, J.P. Phylogenomics—Principles, opportunities and pitfalls of big-data phylogenetics. Syst. Entomol. 2019. [Google Scholar] [CrossRef]
- Lartillot, N.; Lepage, T.; Blanquart, S. PhyloBayes 3: A Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics 2009, 25, 2286–2288. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Bernhauer, M. Zwei Neue Staphylinidengattungen (Col.) Aus Afrika. Proc. R. Entomol. Soc. Lond. Ser. B Taxon. 1935, 4, 213–216. [Google Scholar] [CrossRef]
- Solodovnikov, A.; Yue, Y.; Tarasov, S.; Ren, D. Extinct and extant rove beetles meet in the matrix: Early Cretaceous fossils shed light on the evolution of a hyperdiverse insect lineage (Coleoptera: Staphylinidae: Staphylininae). Cladistics 2013, 29, 360–403. [Google Scholar] [CrossRef]
- Bordoni, A. Revision of the Oriental Platyprosopus (Coleoptera Staphylinidae Platyprosopini). 255th Contribution to the knowledge of the Staphylinidae. Redia 2016, 98, 57–75. [Google Scholar]
- Cameron, M. New species of Staphylinidae from Tonkin. Rev. Fr. Entomol. 1939, 6, 22–26. [Google Scholar]
- De Rougemont, G. Rediscovery of Coomania Cameron (Coleoptera Staphylinidae: Diochini). Entomol. Mon. Mag. 2018, 154, 309. [Google Scholar] [CrossRef]
- Shaw, J.J.; Żyła, D.; Solodovnikov, A. A spectacular new genus of Staphylinini rove beetle from the tropical Andes and its phylogenetic assessment (Coleoptera: Staphylinidae). Invert. Syst. 2017, 31, 713–722. [Google Scholar] [CrossRef]
- Lartillot, N.; Philippe, H. A Bayesian mixture model for across-site heterogeneities in the amino-acid replacement process. Mol. Biol. Evol. 2004, 21, 1095–1109. [Google Scholar] [CrossRef]
- Bernt, M.; Bleidorn, C.; Braband, A.; Dambach, J.; Donath, A.; Fritzsch, G.; Golombek, A.; Hadrys, H.; Jühling, F.; Meusemann, K.; et al. A comprehensive analysis of bilaterian mitochondrial genomes and phylogeny. Mol. Phylogenet. Evol. 2013, 69, 352–364. [Google Scholar] [CrossRef]
- Hosner, P.A.; Faircloth, B.C.; Glenn, T.C.; Braun, E.L.; Kimball, R.T. Avoiding missing data biases in phylogenomic inference: An empirical study in the Landfowl (Aves: Galliformes). Mol. Biol. Evol. 2016, 33, 1110–1125. [Google Scholar] [CrossRef]
- Lartillot, N.; Brinkmann, H.; Philippe, H. Suppression of long-branch attraction artefacts in the animal phylogeny using a site-heterogeneous model. BMC Evol. Biol. 2007, 7, S4. [Google Scholar] [CrossRef] [PubMed]
- Blanquart, S.; Lartillot, N. A Site- and time-heterogeneous model of amino acid replacement. Mol. Biol. Evol. 2008, 25, 842–858. [Google Scholar] [CrossRef]
- Heath, T.A.; Hedtke, S.M.; Hillis, D.M. Taxon sampling and the accuracy of phylogenetic analyses. J. Syst. Evol. 2008, 46, 239–257. [Google Scholar]
- Brunke, A.J.; Chatzimanolis, S.; Metscher, B.D.; Wolf-Schwenninger, K.; Solodovnikov, A. Dispersal of thermophilic beetles across the intercontinental Arctic forest belt during the early Eocene. Sci. Rep. 2017, 7, 12972. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.V.; Beutel, R.G.; Lawrence, J.F. Whirling in the late Permian: Ancestral Gyrinidae show early radiation of beetles before Permian-Triassic mass extinction. BMC Evol. Biol. 2018, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, Y.; Ren, D.; Yang, X. New fossil species of ommatids (Coleoptera: Archostemata) from the Middle Mesozoic of China illuminating the phylogeny of Ommatidae. BMC Evol. Biol. 2012, 12, 113. [Google Scholar] [CrossRef]
- McKenna, D.D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.J.; Donath, A.; Escalona, H.E.; Friedrich, F.; Letsch, H.; et al. The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef]
- Toussaint, E.F.A.; Seidel, M.; Arriaga-Varela, E.; Hájek, J.; Král, D.; Sekerka, L.; Short, A.E.Z.; Fikáček, M. The peril of dating beetles. Syst. Entomol. 2017, 42, 1–10. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Che, L.-H.; Li, Y.; Liang, D.; Pang, H.; Ślipiński, A.; Zhang, P. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat. Commun. 2018, 9, 205. [Google Scholar] [CrossRef]
- Brunke, A.J.; Solodovnikov, A. Alesiella gen.n. and a newly discovered relict lineage of Staphylinini (Coleoptera: Staphylinidae). Syst. Entomol. 2013, 38, 689–707. [Google Scholar] [CrossRef]
- Smetana, A. The nearctic genus Beeria Hatch. Taxonomy, distribution and ecology (Coleoptera: Staphylinidae). Insect Syst. Evol. 1977, 8, 177–190. [Google Scholar] [CrossRef]
- Smetana, A. Contributions to the Knowledge of the Quediina (Coleoptera, Staphylinidae, Staphylinini) of China: Part 14. Quelaestrygon puetzi gen. nov., sp. nov. from Sichuan. Elytra 1999, 27, 241–248. [Google Scholar]


| Żyła and Solodovnikov [21] | Present Paper |
|---|---|
| “former subfamily Staphylininae” | |
| XANTHOLININAE Erichson, 1839 Diochini Casey, 1906 Othiini Thomson, 1859 Maorothiini Assing, 2000 Xantholinini Erichson, 1839 PLATYPROSOPINAE Lynch, 1884 †Thayeralinini Solodovnikov & Yue, 2013 Arrowinini Solodovnikov & Newton, 2005 Platyprosopini Lynch, 1884 COOMANIINAE Żyła & Solodovnikov, 2019 STAPHYLININAE Latreille, 1802 †Baltognathini Brunke, Żyła & Solodovnikov, 2019 Afroquediini Brunke, Żyła & Solodovnikov, 2019 Antimerini Brunke, Żyła & Solodovnikov, 2019 Tanygnathinini Reitter, 1909 Hyptiomini Casey, 1906 Amblyopinini Seevers 1944 Erichsoniini Brunke & Solodovnikov, 2016 Acylophorini Outerelo & Gamarra 1985 Cyrtoquediini Brunke & Solodovnikov 2016 Indoquediini Brunke & Solodovnikov, 2016 Quediini Kraatz, 1857 Staphylinini Latreille, 1802 Algonina Schillhammer & Brunke, 2017 Anisolinina Hayashi, 1993 Philonthina Kirby, 1837 Philothalpina Chatzimanolis & Brunke, 2017 Staphylinina Latreille, 1802 Xanthopygina Sharp, 1884 | STAPHYLININAE Latreille, 1802 stat. res. †Thayeralinini Solodovnikov & Yue, 2013 Platyprosopini Lynch, 1884 stat. res. Diochini Casey, 1906 Othiini Thomson, 1859 Maorothiini Assing, 2000 Xantholinini Erichson, 1839 stat. res. Arrowinini Solodovnikov & Newton, 2005 Coomaniini Żyła & Solodovnikov, 2019 stat. nov. Staphylinini Latreille, 1802 stat. res. †Baltognathina Brunke, Żyła & Solodovnikov, 2019 stat. res. Antimerina Brunke, Żyła & Solodovnikov, 2019 stat. res. Afroquediina Brunke, Żyła & Solodovnikov, 2019 stat. res. Amblyopinina Seevers, 1944 stat. res. Cyrtoquediina Brunke & Solodovnikov, 2016 stat. res. Acylophorina Outerelo & Gamarra, 1985 stat. res. Erichsoniina Brunke & Solodovnikov, 2016 stat. res. Indoquediina Brunke & Solodovnikov, 2016 stat. res. Quediina Kraatz, 1857 stat. res. Hyptiomina Casey, 1906 stat. res. Tanygnathinina Reitter, 1909 stat. res. Staphylinini propria Algonina Schillhammer & Brunke, 2017 Anisolinina Hayashi, 1993 Philonthina Kirby, 1837 Philothalpina Chatzimanolis & Brunke, 2017 Staphylinina Latreille, 1802 Xanthopygina Sharp, 1884 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tihelka, E.; Thayer, M.K.; Newton, A.F.; Cai, C. New Data, Old Story: Molecular Data Illuminate the Tribal Relationships among Rove Beetles of the Subfamily Staphylininae (Coleoptera: Staphylinidae). Insects 2020, 11, 164. https://doi.org/10.3390/insects11030164
Tihelka E, Thayer MK, Newton AF, Cai C. New Data, Old Story: Molecular Data Illuminate the Tribal Relationships among Rove Beetles of the Subfamily Staphylininae (Coleoptera: Staphylinidae). Insects. 2020; 11(3):164. https://doi.org/10.3390/insects11030164
Chicago/Turabian StyleTihelka, Erik, Margaret K. Thayer, Alfred F. Newton, and Chenyang Cai. 2020. "New Data, Old Story: Molecular Data Illuminate the Tribal Relationships among Rove Beetles of the Subfamily Staphylininae (Coleoptera: Staphylinidae)" Insects 11, no. 3: 164. https://doi.org/10.3390/insects11030164
APA StyleTihelka, E., Thayer, M. K., Newton, A. F., & Cai, C. (2020). New Data, Old Story: Molecular Data Illuminate the Tribal Relationships among Rove Beetles of the Subfamily Staphylininae (Coleoptera: Staphylinidae). Insects, 11(3), 164. https://doi.org/10.3390/insects11030164