Consuming Parasitized Aphids Alters the Life History and Decreases Predation Rate of Aphid Predator
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Colonies
2.2. Life Table Analyses
2.3. Life Table Data Analysis
3. Results
3.1. Effect of Parasitized Aphids on the Survival of A. aphidimyza
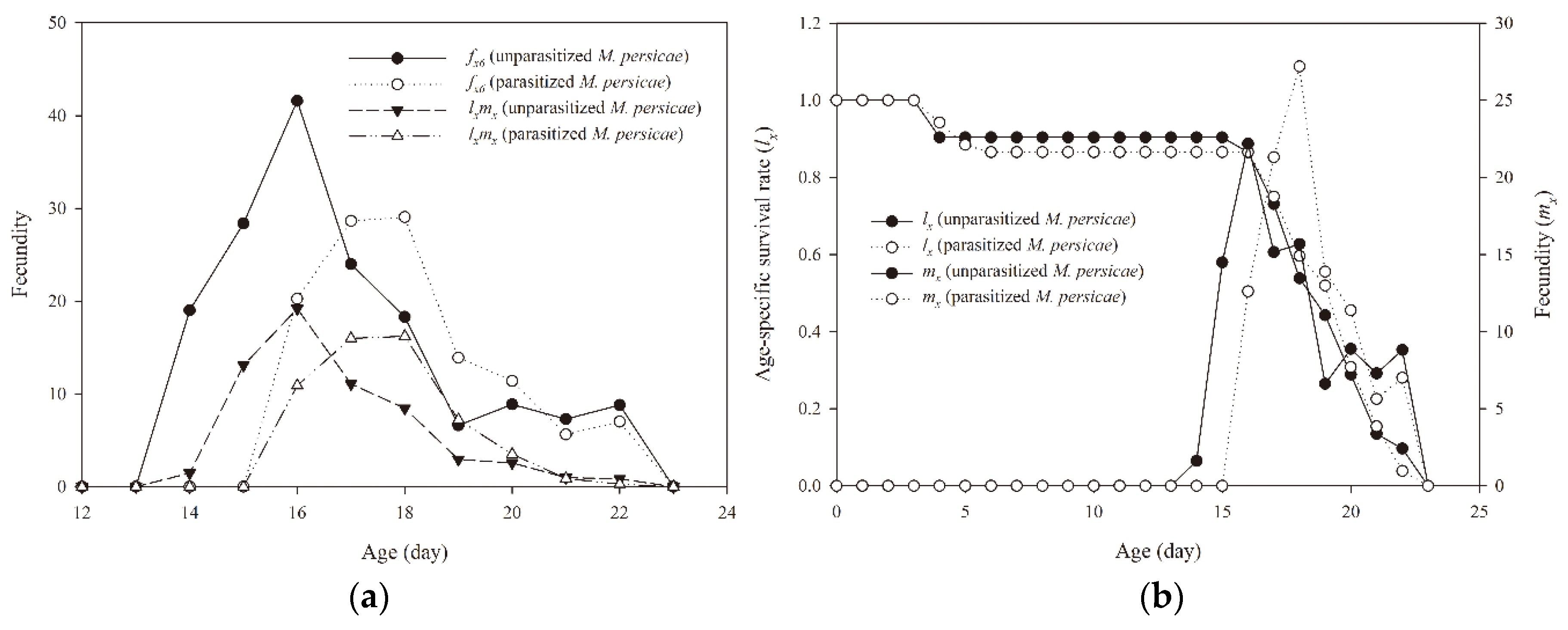
3.2. Effect of Parasitized Aphids on Developmental Time, Longevity, and Fecundity of A. aphidimyza
3.3. Population Parameters
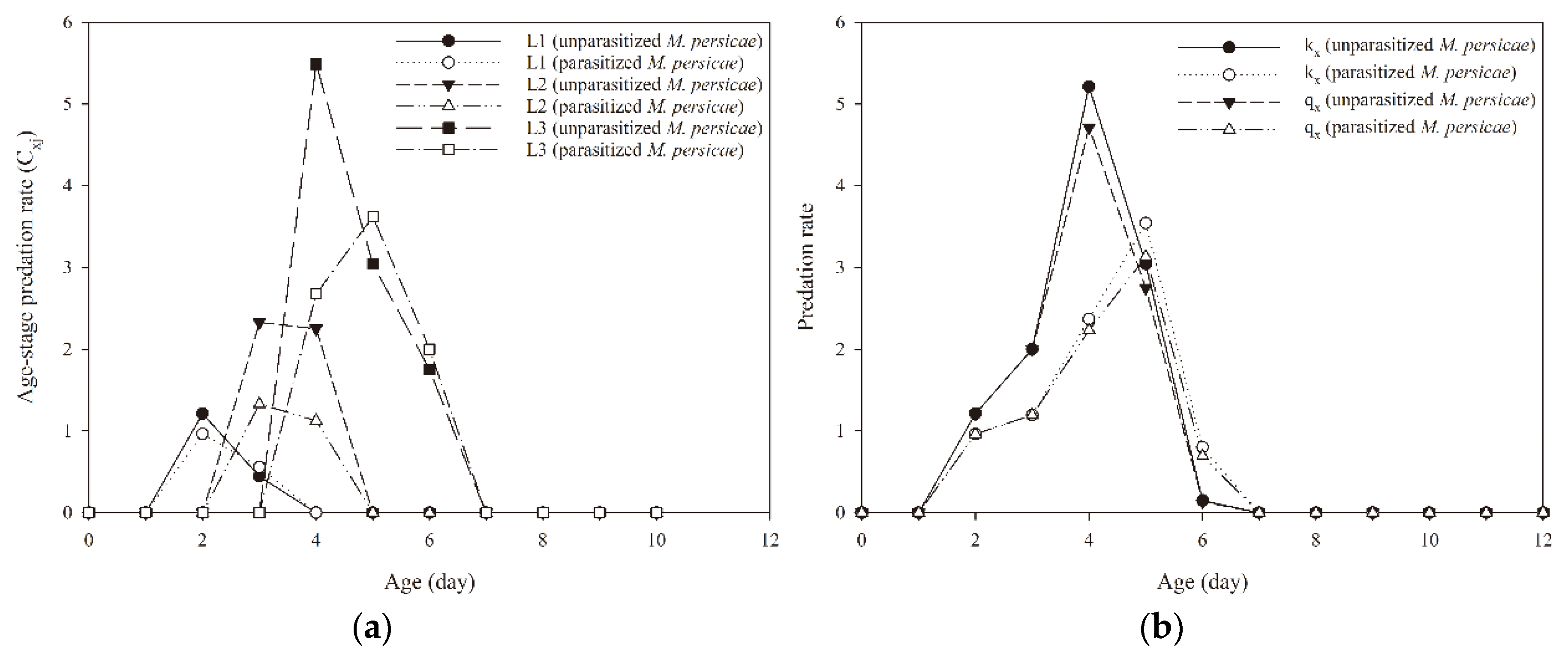
3.4. Predation Rate
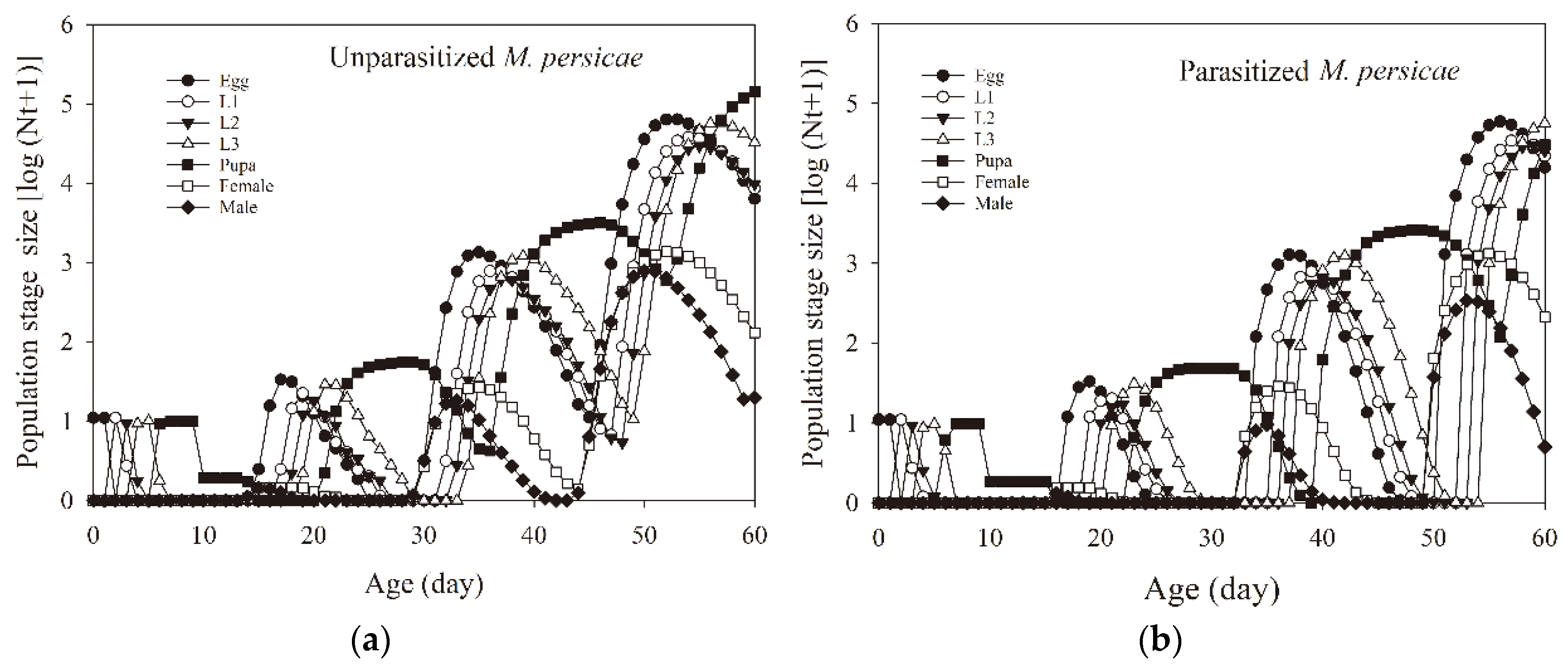
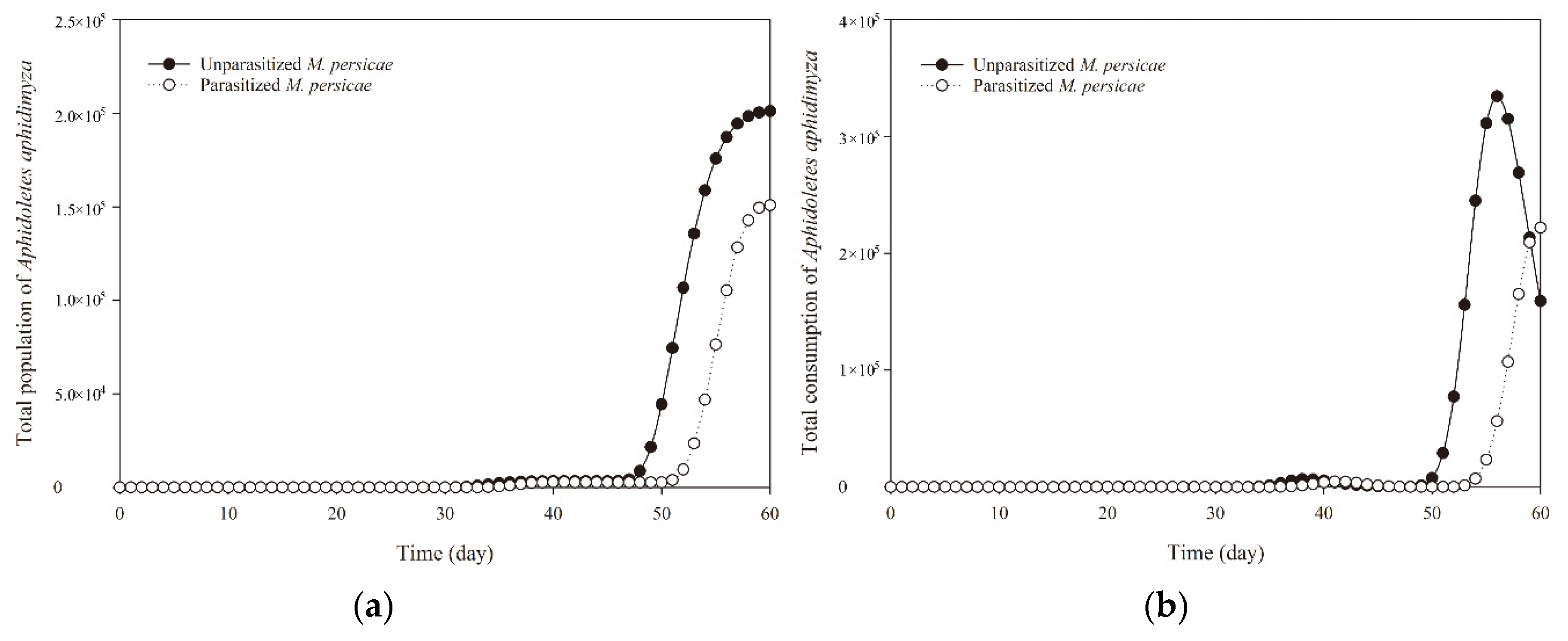
3.5. Population Projection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Polis, G.A.; Myers, C.A.; Holt, R.D. The ecology and evolution of intraguild predation potential competitors that eat each other. Annu. Rev. Ecol. Syst. 1989, 20, 297–330. [Google Scholar] [CrossRef]
- Arim, M.; Marquet, P.A. Intraguild predation: A widespread interaction related to species biology. Ecol. Lett. 2004, 7, 557–564. [Google Scholar] [CrossRef]
- Mansfield, S. Intraguild predation and prey preferences influence biological control of Paropsis charybdis by the southern ladybird, Cleobora mellyi. Biol. Control 2019, 129, 164–170. [Google Scholar] [CrossRef]
- Rocca, M.; Messelink, G.J. Combining lacewings and parasitoids for biological control of foxglove aphids in sweet pepper. J. Appl. Entomol. 2017, 141, 402–410. [Google Scholar] [CrossRef]
- Meyhofer, R.; Klug, T. Intraguild predation on the aphid parasitoid Lysiphlebus fabarum (Marshall) (Hymenoptera: Aphidiidae): Mortality risks and behavioral decisions made under the threats of predation. Biol. Control 2002, 25, 239–248. [Google Scholar] [CrossRef]
- Fu, W.; Yu, X.; Ahmed, N.; Zhang, S.; Liu, T. Intraguild predation on the aphid parasitoid Aphelinus asychis by the ladybird Harmonia axyridis. BioControl 2017, 62, 61–70. [Google Scholar] [CrossRef]
- Royer, T.A.; Giles, K.L.; Lebusa, M.M.; Payton, M.E. Preference and suitability of greenbug, Schizaphis graminum (Hemiptera: Aphididae) mummies parasitized by Lysiphlebus testaceipes (Hymenoptera: Aphidiidae) as food for Coccinella septempunctata and Hippodamia convergens (Coleoptera: Coccinellidae). Biol. Control 2008, 47, 82–88. [Google Scholar] [CrossRef]
- Bilu, E.; Coll, M. The importance of intraguild interactions to the combined effect of a parasitoid and a predator on aphid population suppression. BioControl 2007, 52, 753–763. [Google Scholar] [CrossRef]
- Toosi, M.; Rasekh, A.; Osawa, N. Effects of intraguild predation on the life history traits and progeny of the ladybird beetle Hippodamia variegata. Bull. Insectol. 2019, 72, 161–168. [Google Scholar]
- Yu, X.L.; Feng, Y.; Feng, Z.J.; Chana, P.; Zhu, G.X.; Xia, P.L.; Liu, T.X. Effects of mummy consumption on fitness and oviposition site selection on Harmonia axyridis. Insect Sci. 2020, 27, 1101–1110. [Google Scholar] [CrossRef]
- Wiethoff, J.; Meyhöfer, R.; Poehling, H.M. Einsatz von Nützlingskombinationen bei der biologischen Bekämpfung von Myzus persicae (Sulzer) (Hom.: Aphididae) an Paprika im Unterglasanbau. Gesunde Pflanz. 2002, 54, 126–137. [Google Scholar] [CrossRef]
- Boulanger, F.X.; Jandricic, S.; Bolckmans, K.; Wäckers, F.L.; Pekas, A. Optimizing aphid biocontrol with the predator Aphidoletes aphidimyza, based on biology and ecology. Pest Manag. Sci. 2019, 75, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, M.V.; Korndörfer, A.P.; Pujade-Villar, J.; Hubaide, J.E.A.; Ferreira, S.E.; Arantes, S.O.; Bortoletto, D.M.; Guimarães, C.M.; Sánchez-Espigares, J.A.; Caballero-López, B. Brassica aphid (Hemiptera: Aphididae) populations are conditioned by climatic variables and parasitism level: A study case of Triângulo Mineiro, Brazil. Bull. Entomol. Res. 2017, 107, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Han, H.L.; Pu, P.; Wei, D.; Wang, J.; Liu, Y. Effects of five host plant species on the life history and population growth parameters of Myzus persicae (Hemiptera: Aphididae). J. Insect Sci. 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.-Q. Current status about resistance of Myzus persicae (Sulzer) to insecticides and its integrated management. Agrochem. Res. Appl. 2011, 15, 1–5. [Google Scholar]
- Yang, S.; Yang, S.Y.; Zhang, C.P.; Wei, J.N.; Kuang, R.P. Population dynamics of Myzus persicae on tobacco in Yunnan Province, China, before and after augmentative releases of Aphidius gifuensis. Biocontrol Sci. Technol. 2009, 19, 219–228. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bloemhard, C.M.J.; Vellekoop, R. Biological control of aphids by the predatory midge Aphidoletes aphidimyza in the presence of intraguild predatory bugs and thrips. Acta Hortic. 2011, 915, 171–178. [Google Scholar] [CrossRef]
- Yukawa, J.; Yamaguchi, D.; Mizota, K.; Setokuchi, O. Distribution and host range of an aphidophagous species of Cecidomyiidae, Aphidoletes aphidimyza (Diptera), in Japan. Appl. Entomol. Zool. 1998, 33, 185–193. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Ou, H.; Yu, X.; Gou, J.; Liu, J.; Shen, X.; Liu, M.; Yang, M. Predation ability of the Gall Midge Aphidoletes aphidimyza (Rondani) on tobacco aphid Myzus persicae (Sulzer). Chin. Tob. Sci. 2020, 41, 79–86. [Google Scholar]
- Shang, S.; Huang, C.; Shen, X.; Yu, X.; Cao, Y.; Liu, M.; Yang, M. Field control effect of Aphidoletes aphidimyza on tobacco Myzus persicae. Guizhou Agric. Sci. 2019, 47, 41–44. [Google Scholar]
- Sun, H.; Song, Y. Establishment of a wheat banker plant system for the parasitoid Aphidius gifuensis against Myzus persicae in greenhouse chili pepper. Appl. Entomol. Zool. 2019, 54, 339–347. [Google Scholar] [CrossRef]
- Pan, M.Z.; Liu, T.X. Suitability of three aphid species for Aphidius gifuensis (Hymenoptera: Braconidae): Parasitoid performance varies with hosts of origin. Biol. Control 2014, 69, 90–96. [Google Scholar] [CrossRef]
- Yang, S.; Wei, J.; Yang, S.; Kuang, R. Current status and future trends of augmentative release of aphidius gifuensis for control of Myzus persicae in China’s Yunnan Province. J. Entomol. Res. Soc. 2011, 13, 87–99. [Google Scholar]
- Brodeur, J.; Rosenheim, J.A. Intraguild interactions in aphid parasitoids. Entomol. Exp. Appl. 2000, 97, 93–108. [Google Scholar] [CrossRef]
- Harizanova, V.; Ekbom, B. An evaluation of the parasitoid, Aphidius colemani Viereck (Hymenoptera: Braconidae) and the predator Aphidoletes aphidimyza Rondani (Diptera: Cecidomyiidae) for biological control of Aphis gossypii Glover (Homoptera: Aphididae) on cucumber. J. Entomol. Sci. 1997, 32, 17–24. [Google Scholar] [CrossRef]
- Özgökçe, M.S.; Chi, H.; Atlıhan, R.; Kara, H. Demography and population projection of Myzus persicae (Sulz.) (Hemiptera: Aphididae) on five pepper (Capsicum annuum L.) cultivars. Phytoparasitica 2018, 46, 153–167. [Google Scholar] [CrossRef]
- Huang, H.W.; Chi, H.; Smith, C.L. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum (Solanales: Solanaceae): With a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 2018, 111, 1–9. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin 1985, 24, 225–240. [Google Scholar]
- Liu, J.F.; Zhang, Z.Q.; Beggs, J.R. Tri-partite complexity: Odour from a psyllid’s mutualist ant increased predation by a predatory mite on the psyllid. Pest Manag. Sci. 2019, 75, 1317–1327. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2018. [Google Scholar]
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): With additional comments on life table research criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef]
- Chi, H. CONSUME-MSChart: Computer Program for Consumption Rate Analysis Based on the Age Stage, Two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan, 2018. [Google Scholar]
- Chi, H. TIMING-MSChart:Computerprogramfor the Population Projection Based on the Age Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2018. [Google Scholar]
- Takizawa, T.; Yasuda, H.; Agarwala, B.K. Effect of parasitized aphids (Homoptera: Aphididae) as food on larval performance of three predatory ladybirds (Coleoptera: Coccinellidae). Appl. Entomol. Zool. 2000, 35, 467–472. [Google Scholar] [CrossRef]
- Bilu, E.; Coll, M. Parasitized aphids are inferior prey for a coccinellid predator: Implications for intraguild predation. Environ. Entomol. 2009, 38, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Almohamad, R.; Verheggen, F.J.; Francis, F.; Hance, T.; Haubruge, E. Discrimination of parasitized aphids by a hoverfly predator: Effects on larval performance, foraging, and oviposition behavior. Entomol. Exp. Appl. 2008, 128, 73–80. [Google Scholar] [CrossRef]
- Pineda, A.; Morales, I.; Marcos-García, M.A.; Fereres, A. Oviposition avoidance of parasitized aphid colonies by the syrphid predator Episyrphus balteatus mediated by different cues. Biol. Control 2007, 42, 274–280. [Google Scholar] [CrossRef]
- Gao, X.; Luo, J.; Zhu, X.; Wang, L.; Ji, J.; Zhang, L.; Zhang, S.; Cui, J. Growth and fatty acid metabolism of Aphis gossypii parasitized by the parasitic wasp Lysiphlebia japonica. J. Agric. Food Chem. 2019, 67, 8756–8765. [Google Scholar] [CrossRef]


| Parameters | Stage | Unparasitized M. persicae | Parasitized M. persicae | p-Value | ||
|---|---|---|---|---|---|---|
| N | Mean ± SE | N | Mean ± SE | |||
| Egg | 52 | 100.0 ± 0 | 52 | 100.0 ± 0 | - | |
| 1st instar larva | 47 | 92.1 ± 3.7 | 48 | 90.4 ± 4.1 | 0.7276 | |
| Survival rates (%) | 2nd instar larva | 47 | 100.0 ± 0 | 45 | 93.8 ± 3.5 | 0.0909 |
| 3rd instar larva | 47 | 100.0 ± 0 | 45 | 100.0 ± 0 | - | |
| Pupa | 47 | 100.0 ± 0 | 45 | 100.0 ± 0 | - | |
| Proportion of female Nf/N (%) | 46.2 ± 6.9 | 55.8 ± 6.8 | 0.3178 | |||
| Development time (d) | Egg | 52 | 2.0 ± 0.0 | 52 | 2.0 ± 0.0 | - |
| 1st instar larva | 47 | 1.1 ± 0.0 | 48 | 1.1 ± 0.0 | 0.7775 | |
| 2nd instar larva | 47 | 1.0 ± 0.0 | 45 | 1.0 ± 0.0 | 0.5524 | |
| 3rd instar larva | 47 | 2.0 ± 0.0 | 45 | 2.3 ± 0.1 | <0.001 | |
| Pupa | 47 | 8.7 ± 0.1 | 45 | 9.6 ± 0.1 | <0.001 | |
| Total immature stages | 47 | 14.8 ± 0.1 | 45 | 16.0 ± 0.0 | <0.001 | |
| Reproduction parameters | Female adult longevity (d) | 24 | 6.3 ± 0.3 | 29 | 4.8 ± 0.2 | <0.001 |
| Male adult longevity (d) | 23 | 3.0 ± 0.2 | 16 | 1.8 ± 0.2 | <0.001 | |
| Adult preoviposition period (APOP, d) | 24 | 0.3 ± 0.1 | 29 | 0.7 ± 0.5 | 0.2692 | |
| Total preoviposition period (TPOP, d) | 24 | 15.2 ± 0.1 | 29 | 16.6 ± 0.2 | <0.001 | |
| Oviposition period (d) | 24 | 5.4 ± 0.3 | 29 | 3.8 ± 0.3 | <0.001 | |
| Fecundity (eggs per female) | 24 | 131.3 ± 5.9 | 29 | 98.5 ± 5.8 | <0.001 | |
| Parameter | M. persicae | Parasitized M. persicae | p-Value |
|---|---|---|---|
| Net reproduction rate, R0 (offspring/individual) | 60.58 ± 9.46 | 54.94 ± 7.54 | 0.6427 |
| Intrinsic rate of increase, rm (d−1) | 0.24 ± 0.01 | 0.22 ± 0.01 | 0.1202 |
| Finite rate of increase, λ (d−1) | 1.27 ± 0.01 | 1.24 ± 0.01 | 0.1197 |
| Mean generation time, T (d) | 17.40 ± 0.11 | 18.48 ± 0.12 | <0.001 |
| Sex/Stage | Unparasitized M. persicae | Parasitized M. persicae | p-Value |
|---|---|---|---|
| Female | |||
| L1 | 1.29 ± 0.09 | 1.00 ± 0.00 | 0.0023 |
| L2 | 2.38 ± 0.12 | 1.41 ± 0.12 | <0.001 |
| L3 | 8.62 ± 0.41 | 7.10 ± 0.34 | 0.0036 |
| Male | |||
| L1 | 1.35 ± 0.12 | 1.19 ± 0.10 | 0.2941 |
| L2 | 2.26 ± 0.21 | 1.38 ± 0.12 | 0.0006 |
| L3 | 7.78 ± 0.33 | 6.25 ± 0.48 | 0.0079 |
| Net predation rate, C0 | 10.8077 ± 0.5182 | 8.2115 ± 0.4285 | <0.001 |
| Stable predation rate, ψ | 0.9827 ± 0.0325 | 0.7129 ± 0.0231 | <0.001 |
| Finite predation rate, λ | 1.2441 ± 0.0458 | 0.8855 ± 0.0314 | <0.001 |
| Transformation rate, Qp | 0.1784 ± 0.0289 | 0.1495 ± 0.0191 | 0.3376 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-F.; Wang, X.-Q.; Beggs, J.R.; Ou, H.-D.; Yu, X.-F.; Shen, X.-X.; Yang, M.-F. Consuming Parasitized Aphids Alters the Life History and Decreases Predation Rate of Aphid Predator. Insects 2020, 11, 889. https://doi.org/10.3390/insects11120889
Liu J-F, Wang X-Q, Beggs JR, Ou H-D, Yu X-F, Shen X-X, Yang M-F. Consuming Parasitized Aphids Alters the Life History and Decreases Predation Rate of Aphid Predator. Insects. 2020; 11(12):889. https://doi.org/10.3390/insects11120889
Chicago/Turabian StyleLiu, Jian-Feng, Xiu-Qin Wang, Jacqueline R. Beggs, Hou-Ding Ou, Xiao-Fei Yu, Xiu-Xian Shen, and Mao-Fa Yang. 2020. "Consuming Parasitized Aphids Alters the Life History and Decreases Predation Rate of Aphid Predator" Insects 11, no. 12: 889. https://doi.org/10.3390/insects11120889
APA StyleLiu, J.-F., Wang, X.-Q., Beggs, J. R., Ou, H.-D., Yu, X.-F., Shen, X.-X., & Yang, M.-F. (2020). Consuming Parasitized Aphids Alters the Life History and Decreases Predation Rate of Aphid Predator. Insects, 11(12), 889. https://doi.org/10.3390/insects11120889






