Effects of Larval Density on Plutella xylostella Resistance to Granulosis Virus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Test Subjects
2.3. Mortality Exposure to Entomopathogenic Granulosis Virus
2.4. Immune Assays
2.4.1. Hemolymph Sampling
2.4.2. Phenoloxidase Activity
2.4.3. Lysozyme Activity
2.4.4. Antibacterial Activity
2.5. Total Hemocyte Counts
2.6. Data Analyses
3. Results
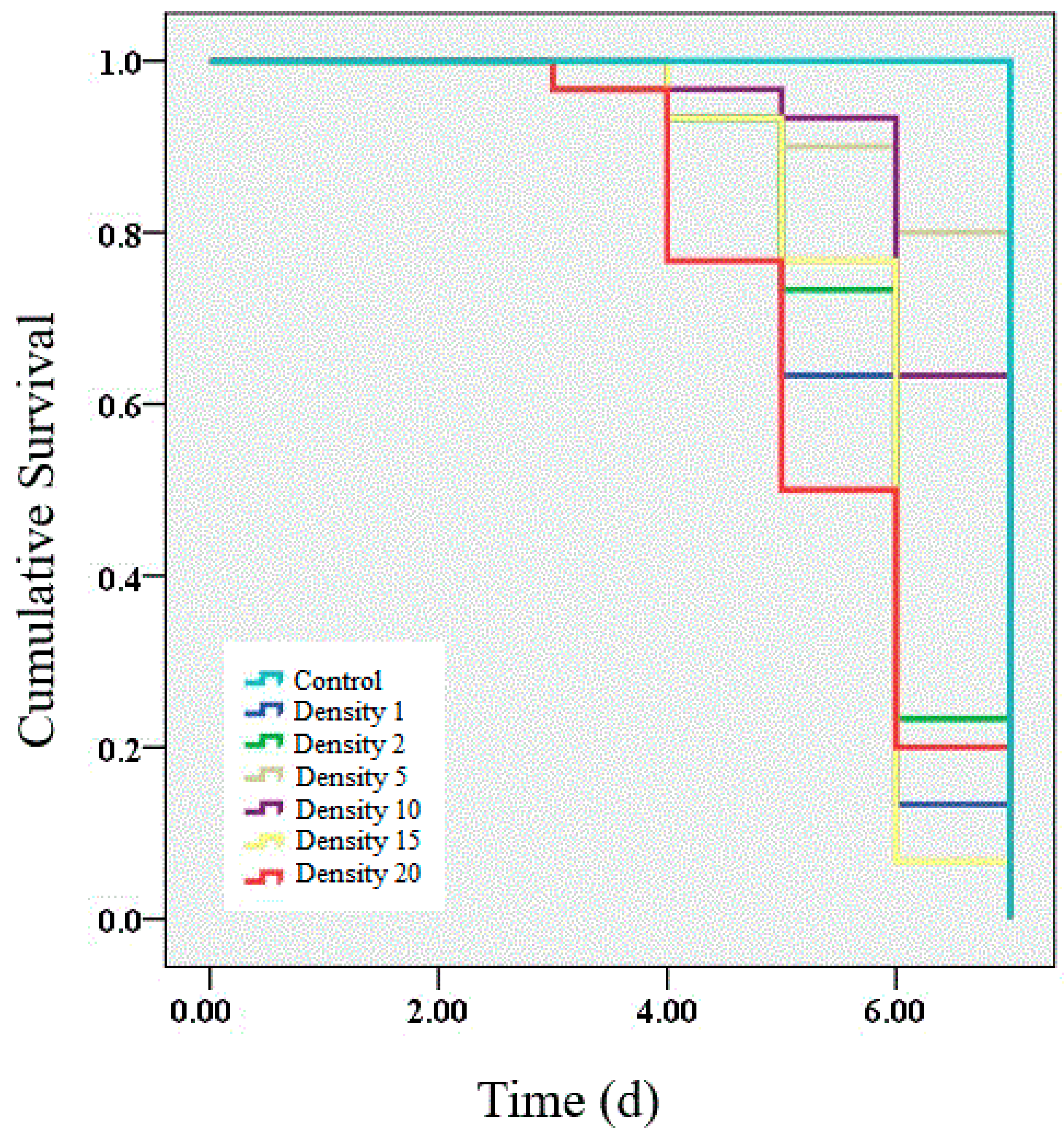
3.1. Survival after Challenge with Entomopathogenic Granulosis Virus
3.2. Enzyme Activity
3.2.1. Phenoloxidase (PO) Activity
3.2.2. Lysozyme Activity
3.2.3. Antibacterial Activity
3.3. Total Hemocyte Counts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kong, H.L.; Dong, C.L.; Tian, Z.; Mao, N.; Wang, C.; Cheng, Y.X.; Zhang, L.; Jiang, X.F.; Luo, L.Z. Altered immunity in crowded Mythimna separata is mediated by octopamine and dopamine. Sci. Rep. 2018, 8, e3215. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. The population dynamics of microparasites and their invertebrate hosts. Philos. Trans. R. Soc. B 1981, 291, 451–524. [Google Scholar]
- Wilson, K.; Reeson, A.F. Density-dependent prophylaxis: Evidence from Lepidoptera-baculovirus interactions? Ecol. Entomol. 1998, 23, 100–101. [Google Scholar] [CrossRef]
- Wilson, K.; Cotter, S.C.; Reeson, A.F.; Pell, J.K. Melanism and disease resistance in insects. Ecol. Lett. 2001, 4, 637–649. [Google Scholar] [CrossRef]
- Wang, Y.D.; Yang, P.C.; Cui, F.; Kang, L. Altered immunity in crowded Locust reduced fungal (Metarhizium anisopliae) pathogenesis. PLoS Pathog. 2013, 9, e1003102. [Google Scholar] [CrossRef]
- Piesk, M.; Karl, I.; Franke, K.; Fischer, K. High larval density dose not induce a prophylactic immune response in a butterfly. Ecol. Entomol. 2013, 38, 346–354. [Google Scholar] [CrossRef]
- Lindsey, E.; Metha, M.; Dhulipala, V.; Oberhauser, K.; Altizer, S. Crowding and disease: Effects of host density on response to infection in a butterfy-parasite interation. Ecol. Entomol. 2009, 34, 551–561. [Google Scholar] [CrossRef]
- Goulson, D.; Cory, J.S. Responses of Mamestra brassicae (Lepidoptera, Noctuidae) to crowding-interactions with disease resistance, color phase and growth. Oecologia 1995, 104, 416–423. [Google Scholar] [CrossRef]
- Adamo, S.A. The emergency life-history stage and immunity in the cricket, Gryllus texensis. Anim. Behav. 2006, 72, 235–244. [Google Scholar] [CrossRef]
- Reilly, J.R.; Hajek, A.E. Density-dependent resistance of the gypsy moth Lymantria dispar to its nudeopolyhedrovirus, and the consequences for population dynamics. Oecologia 2008, 154, 691–701. [Google Scholar] [CrossRef]
- Stavely, F.J.L.; Pell, J.K.; Chapman, B.; Glare, T.R.; Yeo, H.; Suckling, D.M.; Walter, M. Insect pathogens for biological control of the diamondback moth with particular emphasis on the fuguns Zoophthora radicans in New Zealand. In Proceedings of the 4th international Workshop, Melboume, Australia, 26–29 November 2001. [Google Scholar]
- Talekar, N.S.; Shelton, A.M. Biology, ecology, and management of the diamond back moth. Annu. Rev. Entomol. 1993, 38, 275–301. [Google Scholar] [CrossRef]
- Sun, D.; Guo, Z.J.; Liu, Y.; Zhang, Y.J. Progress and prospects of CRISPR/Cas systems in insects and other arthropods. Front. Physiol. 2017, 8, 608. [Google Scholar] [CrossRef]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Furlong, M.J. Predicting outbreaks of a migratorypest: An analysis of DBM distribution and abundance revisited. In Management of the Diamondback Moth and Other Crucifer Insect Pests: Proceedings of the Sixth International Workshop; Srinivasan, R., Shelton, A.M., Collins, H.L., Eds.; Shanhua: Taiwan, China, 2011; pp. 8–14. [Google Scholar]
- Sarfraz, M.; Keddie, A.B.; Dosdall, L.M. Biological control of the diamondback moth, Plutella xylostella: A review. Biocontrol. Sci. Technol. 2005, 15, 763–789. [Google Scholar] [CrossRef]
- Guo, Z.J.; Kang, S.; Sun, D.; Gong, L.J.; Zhou, J.L.; Qin, J.Y.; Guo, L.; Zhu, L.H.; Bai, Y.; Ye, F.; et al. MAPK- dependent hormonal signaling plasticity contributes to overcoming Bacillus thuringiensis toxin action in an insect host. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Haseeb, M.; Kobori, Y.; Amano, H.; Nemoto, H. Population density of Plutella xylostella (Lepidoptera: Plutellidae) and its parasitoid Cotesia plutellae (Hymenoptera: Braconidae) on two varieties of cabbage in an urban environment. Appl. Entomol. Zool. 2001, 36, 353–360. [Google Scholar] [CrossRef]
- Meng, X.L.; Ye, L.B. Research on synergistic factor of Plutella xylostella granulosis virus. J. Wuhan Univ. 1996, 42, 519–522. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wilson, K.; Thomas, M.B.; Blanford, S.; Doggett, M.; Simpson, S.J.; Moore, S.L. Coping with crowds: Density-dependent disease resistance in desert locusts. Proc. Natl. Acad. Sci. USA 2002, 99, 5471–5475. [Google Scholar] [CrossRef]
- Landau, S.; Everitt, B.S. A Handbook of Statistical Analyses Using Spss; A CRC Press Company: Bosa Raton, FL, USA; Lodon, UK; New York, NY, USA; Washington, DC, USA, 2004. [Google Scholar]
- Kong, H.L.; Zhang, Y.X.; Zhu, S.D.; Kong, Y.; Wu, L.; Hu, R.L. Effects of larval density on growth, development and reproduction of diamondback moth (DBM), Plutella xylostella (L.). Chin. J. Eco Agric. 2013, 21, 474–479. [Google Scholar] [CrossRef]
- Feng, X.; Li, Z.Y.; Wu, Q.J.; Shen, A.D.; Wu, Y.D.; Hou, Y.M.; He, Y.R.; Li, J.H.; Xie, S.H.; Zhang, J.M.; et al. Research progress of the resistance management and sustainable control of diamondback moth (Plutella xylostella) in China. J. Appl. Entomol. 2011, 48, 247–253. [Google Scholar]
- Nappi, A.J.; Vass, E. Melanogenesis and the generation of cytotoxic molecules during insect cellular immune-reactions. Pigment Cell Res. 1993, 6, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.J.; Shikano, I.; Felton, G.W.; Liu, T.X.; Hoover, K. Host permissiveness to baculovirus influences time-dependent immune responses and fitness costs. Insect Sci. 2020, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Zhao, X.F.; Wang, J.X. Molecular characterization and expression analysis of a chicken-type lysozyme gene from housefly (Musca domestica). J. Genet. Genom. 2009, 36, 7–16. [Google Scholar] [CrossRef]
- Kong, H.L.; Lv, M.; Mao, N.; Wang, C.; Cheng, Y.X.; Zhang, L.; Jiang, X.F.; Luo, L.Z. Molecular characterization of a lysozyme gene and its altered expression profile in crowded Beet Webworm (Loxostege sticticalis). PLoS ONE 2016, 11, e161384. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.L.; Dong, C.L.; Jing, W.H.; Zheng, M.Y.; Tian, Z.; Hou, Q.L.; Wang, C.; Cheng, Y.X.; Zhang, L.; Jiang, X.F.; et al. Transcriptomic insight into antimicrobial peptide factors involved in the prophylactic immunity of crowded Mythimna separata larvae. Dev. Comp. Immunol. 2019, 98, 34–41. [Google Scholar] [CrossRef]
- Lü, P.; Pan, Y.; Yang, Y.H.; Zhu, F.F.; Li, C.J.; Guo, Z.J.; Yao, Q.; Chen, K.P. Discovery of anti-viral molecules and their vital functions in Bombyx mori. J. Invertebr. Pathol. 2018, 154, 12–18. [Google Scholar] [CrossRef]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- De Andrade, F.G.; De Negreiro, M.C.C.; Levy, S.M.; De Batista Fonesca, I.C.; Moscardi, F.; Falleiros, Â.M.F. Haemocyte quantitative changes in Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae infected by AgMNPV. Braz. Arch. Biol. Technol. 2010, 53, 279–284. [Google Scholar] [CrossRef]
- Shaurub, E.S.H.; El-Meguid, A.A.; Abd El-Aziz, N.M. Quantitative and ultrastructural changes in the haemocytes of Spodoptera littoralis (Boisd.) treated individually or in combination with Spodoptera littoralis multicapsid nucleopolyhedrovirus (SpliMNPV) and azadirachtin. Micron 2014, 65, 62–68. [Google Scholar] [CrossRef]
- Silva, F.W.S.; Viol, D.L.; Faria, S.V.; Lima, E.; Valicente, F.H.; Elliot, S.L. Two’s a crowd: Phenotypic adjustments and prophylaxis in Anticarsia gemmatalis larvae are triggered by the presence of conspecifics. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Silva, F.W.S.; Elliot, S.L. Temperature and population density: Interactional effects of environmental factors on phenotypic plasticity, immune defenses, and disease resistance in an insect pest. Ecol. Evol. 2016, 6, 3672–3683. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Cheng, Y.X.; Luo, L.Z.; Kong, H.L.; Zhang, L.; Lei, C.L. Effects of larval density on the number and composition of hemocytes in the beet webworm, Loxostege sticticalis (Lepidoptera: Pyralidae). Acta Entomol. Sin. 2013, 56, 630–637. [Google Scholar]
- Kiran Kumar, K.P.; Singh, G.P. Hemocyte and biochemical changes of Antheraea mylitta D. infected with Antheraea mylitta cytoplasmic polyhedrosis virus (AmCPV). IJSR 2015, 12, 276–279. [Google Scholar]
- Kong, H.L.; Cheng, Y.X.; Luo, L.Z.; Sappington, T.W.; Jiang, X.F.; Zhang, L. Density-dependent prophylaxis in crowded Beet Webworm, Loxostege sticticalis (Lepidoptera: Pyralidae) larvae to a parasitoid and a fungal pathogen. Int. J. Pest Manag. 2013, 59, 174–179. [Google Scholar] [CrossRef]
- Prokkola, J.; Roff, D.; Karkkainen, T.; Krams, I.; Rantala, M.J. Genetic and phenotypic relationships between immune defense, melanism and life-history traits at different temperatures and sexes in Tenebrio molitor. Heredity 2013, 111, 89–96. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, H.; Liu, Z.; Yang, P.; Yuan, L.; Jing, W.; Dong, C.; Zheng, M.; Tian, Z.; Hou, Q.; Zhu, S. Effects of Larval Density on Plutella xylostella Resistance to Granulosis Virus. Insects 2020, 11, 857. https://doi.org/10.3390/insects11120857
Kong H, Liu Z, Yang P, Yuan L, Jing W, Dong C, Zheng M, Tian Z, Hou Q, Zhu S. Effects of Larval Density on Plutella xylostella Resistance to Granulosis Virus. Insects. 2020; 11(12):857. https://doi.org/10.3390/insects11120857
Chicago/Turabian StyleKong, Hailong, Zhonglin Liu, Pingjun Yang, Lin Yuan, Wanghui Jing, Chuanlei Dong, Minyuan Zheng, Zhen Tian, Qiuli Hou, and Shude Zhu. 2020. "Effects of Larval Density on Plutella xylostella Resistance to Granulosis Virus" Insects 11, no. 12: 857. https://doi.org/10.3390/insects11120857
APA StyleKong, H., Liu, Z., Yang, P., Yuan, L., Jing, W., Dong, C., Zheng, M., Tian, Z., Hou, Q., & Zhu, S. (2020). Effects of Larval Density on Plutella xylostella Resistance to Granulosis Virus. Insects, 11(12), 857. https://doi.org/10.3390/insects11120857



