Identification of Plant DNA in Adults of the Phytoplasma Vector Cacopsylla picta Helps Understanding Its Feeding Behavior
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
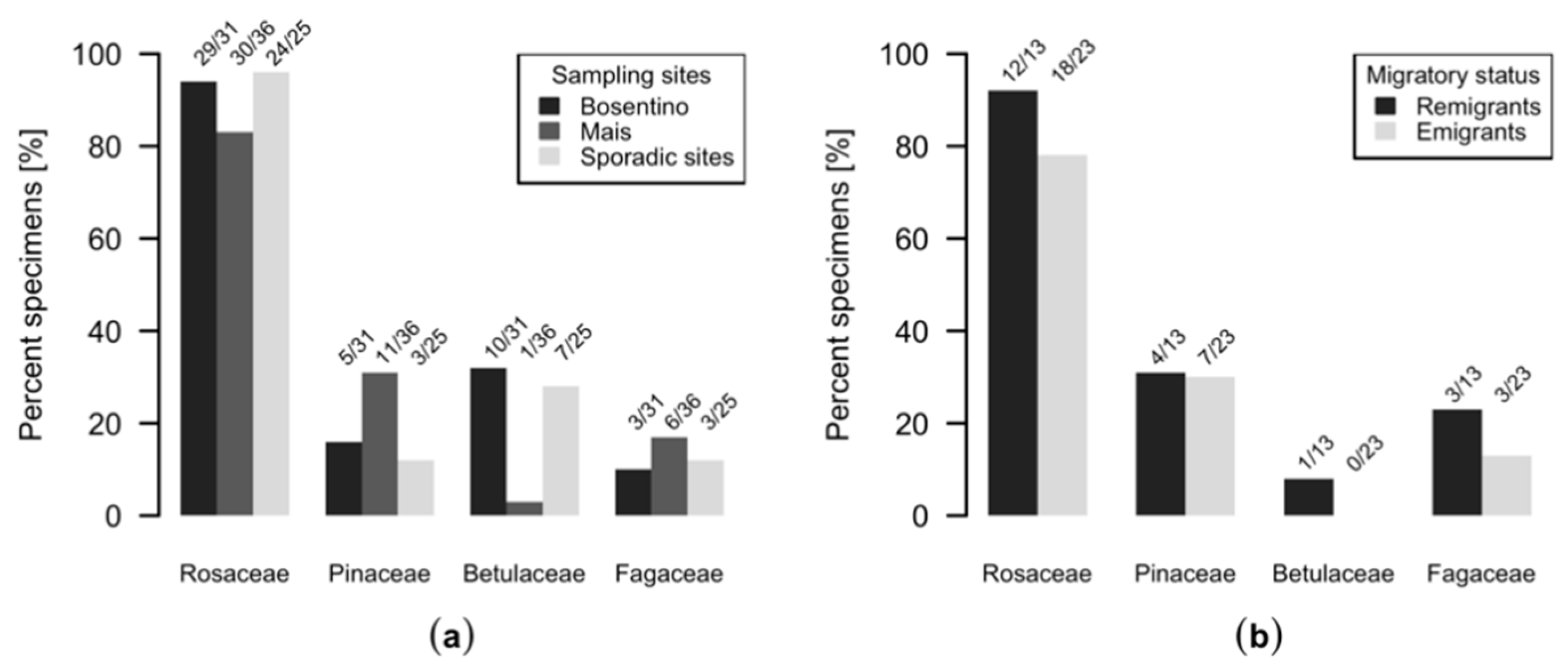
2.1. Insect Sampling and Morphological and Molecular Species Identification
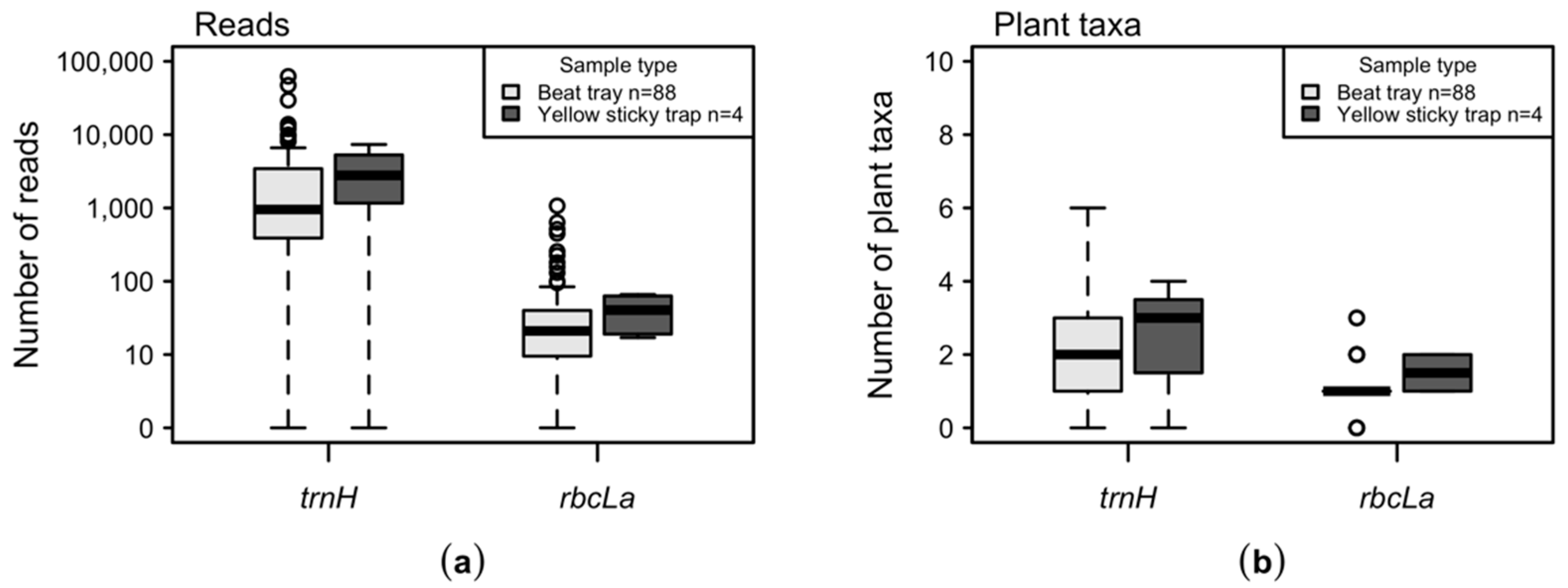
2.2. Molecular Analysis and Deep Sequencing
2.3. Data Analysis
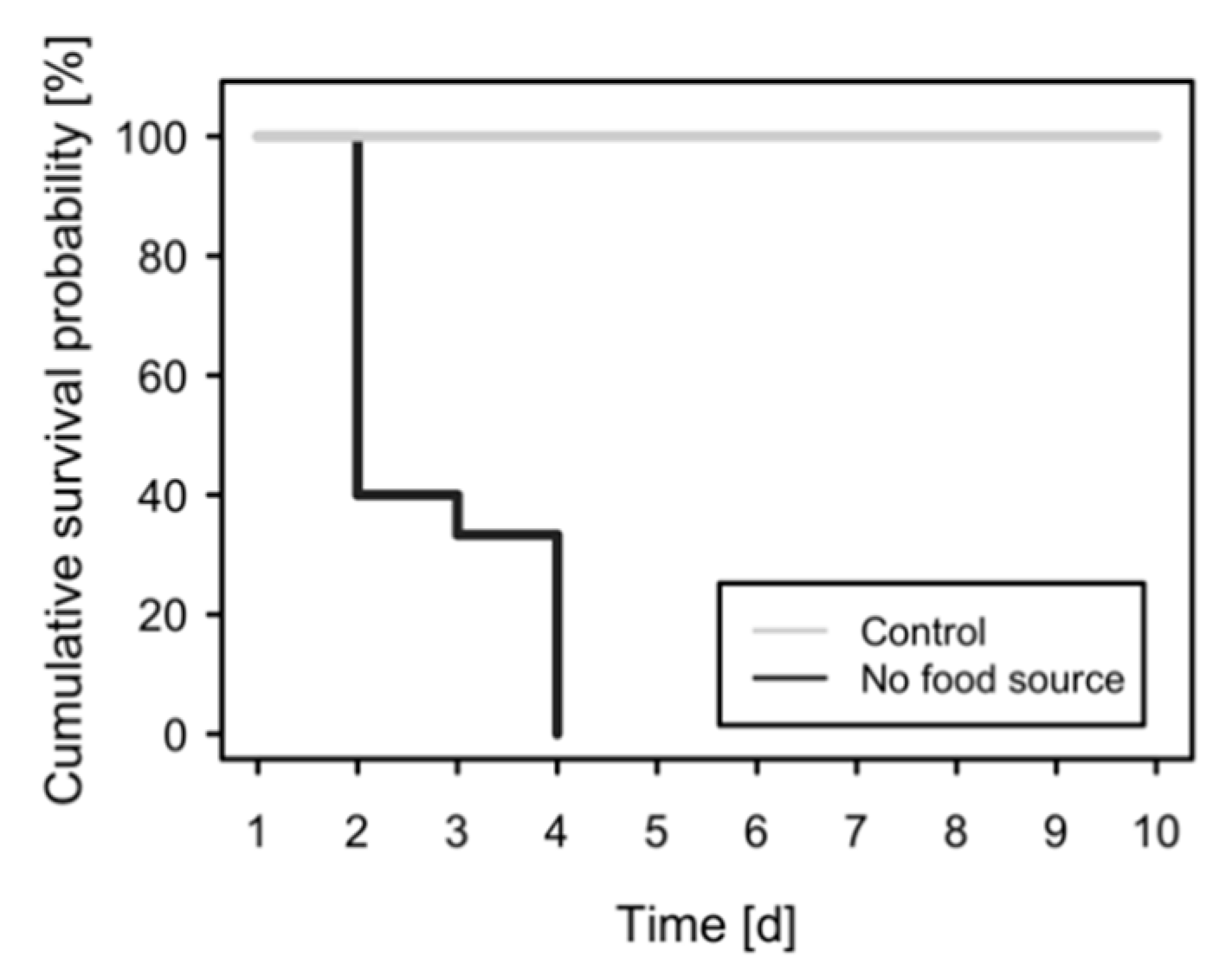
2.4. Longevity under Starvation
2.5. Pollen Staining
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Locus | Status | Min | Mean | Max | Sd | Total |
|---|---|---|---|---|---|---|
| trnH | raw | 22 | 4464 | 71,841 | 9935 | 424,109 |
| trnH | clean | 20 | 4141 | 69,315 | 9577 | 393,442 |
| rbcLa | raw | 7 | 813 | 2754 | 167 | 78,026 |
| rbcLa | clean | 2 | 144 | 1143 | 167 | 13,780 |
| Sample Name | n | nTypes | Sex |
|---|---|---|---|
| C. picta 1 | 1 | 1 | female |
| C. picta 2 | 4 | 4 | female |
| C. picta 3 | 3 | 3 | female |
| C. picta 4 | 3 | 1 | female |
| C. picta 5 | 2 | 2 | female |

References
- Seemüller, E.; Schneider, B. ‘Candidatus Phytoplasma mali’, ‘Candidatus Phytoplasma pyri’ and ‘Candidatus Phytoplasma prunorum’, the causal agents of apple proliferation, pear decline and European stone fruit yellows, respectively. Int. J. Syst. Evol. Microbiol. 2004, 54, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Seemüller, E. Apple proliferation. In Compendium of apple and pear diseases; Jones, A.L., Aldwinkel, H.S., Eds.; APS Press: St. Paul, MN, USA, 1990; pp. 67–68. [Google Scholar]
- Seemüller, E.; Carraro, L.; Jarausch, W.; Schneider, B. Chapter 14: Apple Proliferation Phytoplasma. In Virus and virus-like diseases of pome and stone fruits; Hadidi, A., Barba, M., Candresse, T., Jelkmann, W., Eds.; APS Press: St. Paul, MN, USA, 2011; pp. 67–73. [Google Scholar]
- Strauss, E. Microbiology. Phytoplasma research begins to bloom. Science 2009, 325, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Janik, K.; Barthel, D.; Oppedisano, T.; Anfora, G. Apple Proliferation. A Joint Review; Fondazione Edmund Mach, San Michele all’Adige (TN)/Laimburg Research Centre: Ora, Italy, 2020; ISBN 9788878430549. [Google Scholar]
- Krczal, G.; Krczal, H.; Kunze, L. Fieberiella florii (Stål), a vector of apple proliferation agent. Acta Hortic. 1988, 235, 99–106. [Google Scholar] [CrossRef]
- Frisinghelli, C.; Delaiti, L.; Grando, M.S.; Forti, D.; Vindimian, M.E. Cacopsylla costalis (Flor 1861), as a vector of apple proliferation in Trentino. J. Phytopathol. 2000, 148, 425–431. [Google Scholar] [CrossRef]
- Jarausch, B.; Schwind, N.; Jarausch, W.; Krczal, G.; Dickler, E.; Seemüller, E. First Report of Cacopsylla picta as a Vector of Apple Proliferation Phytoplasma in Germany. Plant Dis. 2003, 87, 101. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.; Alma, A. Transmission of apple proliferation phytoplasma by Cacopsylla melanoneura (Homoptera: Psyllidae). J. Econ. Entomol. 2004, 97, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.; Alma, A. Fieberiella florii (Homoptera: Auchenorrhyncha) as a Vector of ‘Candidatus Phytoplasma mali’. Plant Dis. 2006, 90, 284–290. [Google Scholar] [CrossRef]
- Oppedisano, T.; Panassiti, B.; Pedrazzoli, F.; Mittelberger, C.; Bianchedi, P.L.; Angeli, G.; de Cristofaro, A.; Janik, K.; Anfora, G.; Ioriatti, C. Importance of psyllids’ life stage in the epidemiology of apple proliferation phytoplasma. J Pest Sci 2019, 57, 135. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Beanland, L. Insect vectors of phytoplasmas. Annu. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef]
- Mittelberger, C.; Obkircher, L.; Oettl, S.; Oppedisano, T.; Pedrazzoli, F.; Panassiti, B.; Kerschbamer, C.; Anfora, G.; Janik, K. The insect vector Cacopsylla picta vertically transmits the bacterium ‘Candidatus Phytoplasma mali’ to its progeny. Plant Pathol. 2017, 66, 1015–1021. [Google Scholar] [CrossRef]
- Jarausch, B.; Fuchs, A.; Schwind, N.; Krczal, G.; Jarausch, W. Cacopsylla picta as most important vector for ‘Candidatus Phytoplasma mali’ in Germany and neighbouring regions. Bull. Insectology 2007, 60, 189–190. [Google Scholar]
- Jarausch, B.; Burckhardt, D.; Lauterer, P.; Jarausch, W. Psyllids (Hemiptera, Psylloidea) captured in commercial apple and stone fruit orchards in southwest Germany, eastern France and northwest Switzerland. Mitt. Schweiz. Entomol. Ges. 2009, 82, 205–215. [Google Scholar]
- Jarausch, B.; Tedeschi, R.; Sauvion, N.; Gross, J.; Jarausch, W. Psyllid Vectors. In Phytoplasmas: Plant Pathogenic Bacteria—II; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer Singapore: Singapore, 2019; pp. 53–78. [Google Scholar]
- Reuter, O.M. Charakteristik und Entwicklungsgeschichte der Hemipteren-Fauna (Heteroptera, Auchenorrhynchia und Psyllidae) der palaearktischen Coniferen. Acta Soc. Sci. Fenn. 1909, 36, 1–129. [Google Scholar]
- Ossiannilsson, F. The Psylloidea (Homoptera) of Fennoscandia and Denmark; Brill: Leiden, The Netherlands, 1992; ISBN 90-04-09610-8. [Google Scholar]
- Mattedi, L.; Forno, F.; Cainelli, C.; Grando, M.S.; Jarausch, W. Research on Candidatus Phytoplasma mali transmission by insect vectors in Trentino. Acta Hortic. 2008, 369–374. [Google Scholar] [CrossRef]
- Fischnaller, S.; Parth, M.; Messner, M.; Stocker, R.; Kerschbamer, C.; Reyes-Dominguez, Y.; Janik, K. Occurrence of different Cacopsylla species in apple orchards in South Tyrol (Italy) and detection of apple proliferation phytoplasma in Cacopsylla melanoneura and Cacopsylla picta (Hemiptera: Psylloidea). Cicadina 2017, 17, 37–51. [Google Scholar]
- Čermák, V.; Lauterer, P. Overwintering of psyllids in South Moravia (Czech Republic) with respect to the vectors of the apple proliferation cluster phytoplasmas. Bull. Insectol. 2008, 61, 147–148. [Google Scholar]
- Burckhardt, D.; Ouvrard, D.; Queiroz, D.; Percy, D. Psyllid Host-Plants (Hemiptera: Psylloidea): Resolving a Semantic Problem. Fla. Entomol. 2014, 97, 242–246. [Google Scholar] [CrossRef]
- Thébaud, G.; Yvon, M.; Alary, R.; Sauvion, N.; Labonne, G. Efficient transmission of ‘Candidatus phytoplasma prunorum’ Is delayed by eight months due to a long latency in its host-alternating vector. Phytopathology 2009, 99, 265–273. [Google Scholar] [CrossRef]
- Forno, F.; Mattedi, L.; Vindimian, M.E.; Branz, A.; Forti, D.; Schgraffer, M. Tre anni di osservazioni sulle psille del melo. Terra Trentina 2002, 3, 25–29. [Google Scholar]
- Zimmermann, M.H.; Ziegler, H. Transport in plants I: Phloem transport. Encyclopedia of Plant Physiology; Springer: New York, NY, USA, 1975; Volume 1. [Google Scholar]
- Van Bel, A.J.E. The phloem, a miracle of ingenuity. Plant Cell Environ. 2003, 26, 125–149. [Google Scholar] [CrossRef]
- Civolani, S.; Leis, M.; Grandi, G.; Garzo, E.; Pasqualini, E.; Musacchi, S.; Chicca, M.; Castaldelli, G.; Rossi, R.; Tjallingii, W.F. Stylet penetration of Cacopsylla pyri; an electrical penetration graph (EPG) study. J. Insect Physiol. 2011, 57, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.D.; Walker, G.P.; Trumble, J.T. Feeding disruption of potato psyllid, Bactericera cockerelli, by imidacloprid as measured by electrical penetration graphs. Entomologia Experimentalis et Applicata 2012, 142, 247–257. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, C.; Li, Z.; Xu, L.; Dai, W. Fine structure and sensory apparatus of the mouthparts of the pear psyllid, Cacopsylla chinensis (Yang et Li) (Hemiptera: Psyllidae). Arthropod Struct. Dev. 2013, 42, 495–506. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Ammar, E.-D.; Hall, D.G.; Lapointe, S.L.; Ghanim, M. Sclerenchymatous ring as a barrier to phloem feeding by Asian citrus psyllid: Evidence from electrical penetration graph and visualization of stylet pathways. PLoS ONE 2017, 12, e0173520. [Google Scholar] [CrossRef] [PubMed]
- Kehr, J. Phloem sap proteins: Their identities and potential roles in the interaction between plants and phloem-feeding insects. J. Exp. Bot. 2006, 57, 767–774. [Google Scholar] [CrossRef]
- Blackman, L.M.; Overall, R.L. Immunolocalisation of the cytoskeleton to plasmodesmata of Chara corallina. Plant J. 1998, 14, 733–741. [Google Scholar] [CrossRef]
- Sjolund, R.D. The Phloem Sieve Element: A River Runs through It. Plant Cell 1997, 1137–1146. [Google Scholar] [CrossRef]
- Miles, P.W. Aphid saliva. Biol. Rev. 1999, 74, 41–85. [Google Scholar] [CrossRef]
- Zabotin, A.I.; Barysheva, T.S.; Trofimova, O.I.; Lozovaya, V.V.; Widholm, J. Regulation of Callose Metabolism in Higher Plant Cells in vitro. Russ. J. Plant Physiol. 2002, 49, 792–798. [Google Scholar] [CrossRef]
- Richter, S.; Müssig, J.; Gierlinger, N. Functional plant cell wall design revealed by the Raman imaging approach. Planta 2011, 233, 763–772. [Google Scholar] [CrossRef]
- Ullman, D.E.; McLean, D.L. Anterior alimentary canal of the pear Psylla, Psylla pyricola foerster (Homoptera, Psyllidae). J. Morphol. 1986, 189, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.R.; Krysan, J.L. Host Acceptance Behavior of Pear Psylla (Homoptera: Psyllidae) Affected by Plant Species, Host Deprivation, Habituation, and Eggload. Ann. Entomol. Soc. Am. 1991, 84, 612–627. [Google Scholar] [CrossRef]
- García-Robledo, C.; Erickson, D.L.; Staines, C.L.; Erwin, T.L.; Kress, W.J. Tropical plant-herbivore networks: Reconstructing species interactions using DNA barcodes. PLoS ONE 2013, 8, e52967. [Google Scholar] [CrossRef] [PubMed]
- Díaz Villanueva, V.; Albariño, R. Algal ingestion and digestion by two ephemeropteran larvae from a Patagonian Andean stream. In Research Update on Ephemeroptera & Plecoptera; Gaino, E., Ed.; Università di Perugia: Perugia, Italy, 2003; pp. 468–475. [Google Scholar]
- Hugo, R.L.E.; Merritt, D.J.; Wild, C.H. Gut Content Analysis to Distinguish Between Seed Feeding and Mycophagy of a Biphyllid Beetle Larva Found on Acacia melanoxylon. Biocontrol Sci. Technol. 2010, 13, 355–360. [Google Scholar] [CrossRef]
- Kajtoch, Ł. A DNA metabarcoding study of a polyphagous beetle dietary diversity: The utility of barcodes and sequencing techniques. Folia Biol. 2014, 62, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.R.; Horton, D.R.; Unruh, T.R.; Garczynski, S.F. Gut Content Analysis of a Phloem-Feeding Insect, Bactericera cockerelli (Hemiptera: Triozidae). Environ. Entomol. 2016, 45, 938–944. [Google Scholar] [CrossRef]
- Cooper, W.R.; Horton, D.R.; Wildung, M.R.; Jensen, A.S.; Thinakaran, J.; Rendon, D.; Nottingham, L.B.; Beers, E.H.; Wohleb, C.H.; Hall, D.G.; et al. Host and Non-host ‘Whistle Stops’ for Psyllids: Molecular Gut Content Analysis by High-Throughput Sequencing Reveals Landscape-Level Movements of Psylloidea (Hemiptera). Environ. Entomol. 2019, 48, 603–613. [Google Scholar] [CrossRef]
- De Schepper, V.; de Swaef, T.; Bauweraerts, I.; Steppe, K. Phloem transport: A review of mechanisms and controls. J. Exp. Bot. 2013, 64, 4839–4850. [Google Scholar] [CrossRef]
- Paramonova, N.V.; Krasavina, M.S.; Sokolova, S.V. Ultrastructure of Chloroplasts in Phloem Companion Cells and Mesophyll Cells as Related to the Stimulation of Sink Activity by Cytokinins. Russ. J. Plant Physiol. 2002, 49, 187–195. [Google Scholar] [CrossRef]
- Oettl, S.; Schlink, K. Molecular Identification of Two Vector Species, Cacopsylla melanoneura and Cacopsylla picta (Hemiptera: Psyllidae), of Apple Proliferation Disease and Further Common Psyllids of Northern Italy. J. Econ. Entomol. 2015, 108, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.A.; Simpson, B.B. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Syst. Bot. 2003, 28, 723–737. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L. A two-locus global DNA barcode for land plants: The coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS ONE 2007, 2, e508. [Google Scholar] [CrossRef] [PubMed]
- Kress, W.J.; Erickson, D.L.; Jones, F.A.; Swenson, N.G.; Perez, R.; Sanjur, O.; Bermingham, E. Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc. Natl. Acad. Sci. USA 2009, 106, 18621–18626. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Wilhalm, T.; Niklfeld, H.; Gutermann, W. Katalog der Gefäßpflanzen Südtirols; Folio Verlag: Wien, Austria, 2006. [Google Scholar]
- Autonome Provinz Bozen—Südtirol. Bäume und Sträucher. Available online: http://www.provinz.bz.it/land-forstwirtschaft/wald-holz-almen/forstgaerten/zur-verfuegung-stehende-pflanzen.asp (accessed on 7 October 2020).
- Ullman, D.E.; McLean, D.L. Feeding Behavior of the Winter-Form Pear Psylla, Psylla pyricola (Homoptera: Psyllidae), on Reproductive and Transitory Host Plants. Environ. Entomol. 1988, 17, 675–678. [Google Scholar] [CrossRef]
- Seemüller, E. Apple proliferation: Etiology, epidemiology and detection. In Atti Giornate Fitopatologiche 2002; Giornate Fitopatologiche, Trento, Baselga di Piné, Italy, 7.-11.04.2002; Brunelli, A., Canova, A., Eds.; CLUEB: Bologna, Italy, 2002; pp. 3–6. [Google Scholar]
- Marcone, C.; Ragozzino, A.; Seemüller, E. Association of phytoplasmas with the decline of European hazel in southern Italy. Plant Pathol. 1996, 45, 857–863. [Google Scholar] [CrossRef]
- Lee, I.M.; Bertaccini, A.; Vibio, M.; Gundersen, D.E. Detection of multiple phytoplasmas in perennial fruit trees with decline symptoms in Italy. Phytopathology 1995, 85, 728–735. [Google Scholar] [CrossRef]
- Horton, D.R.; Burts, E.C.; Unruh, T.R.; Krysan, J.L.; Coop, L.B.; Croft, B.A. Intraorchard changes in distribution of winterform pear psylla (Homoptera: Psyllidae) associated with leaf fall in pear. Ann. Entomol. Soc. Am. 1993, 86, 599–608. [Google Scholar] [CrossRef]
- Lauterer, P. Results of the investigation on Hemiptera in Moravia, made by the Moravian museum (Psylloidea 2). Acta Mus. Morav. Sci. Biol. 1999, 84, 71–151. [Google Scholar]
- Mayer, C.J.; Gross, J. Different host plant odours influence migration behaviour of Cacopsylla melanoneura (Forster), an insect vector of the apple proliferation phytoplasma. IOBC/WPRS Bull. 2007, 30, 177–184. [Google Scholar]
- Gallinger, J.; Gross, J. Unraveling the Host Plant Alternation of Cacopsylla pruni—Adults but Not Nymphs Can Survive on Conifers Due to Phloem/Xylem Composition. Front. Plant Sci. 2018, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.J.; Jarausch, B.; Jarausch, W.; Jelkmann, W.; Vilcinskas, A.; Gross, J. Cacopsylla melanoneura has no relevance as vector of apple proliferation in Germany. Phytopathology 2009, 99, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Briem, F.; Zeisler, C.; Guenay, Y.; Staudacher, K.; Vogt, H.; Traugott, M. Identifying plant DNA in the sponging–feeding insect pest Drosophila suzukii. J Pest Sci 2018, 91, 985–994. [Google Scholar] [CrossRef]
- Backus, E.A.; Serrano, M.S.; Ranger, C.M. Mechanisms of hopperburn: An overview of insect taxonomy, behavior, and physiology. Annu. Rev. Entomol. 2005, 50, 125–151. [Google Scholar] [CrossRef]
- Li, W.; Wyckhuys, K.A.G.; Wu, K. Does feeding behavior of a zoophytophagous mirid differ between host plant and insect prey items? Arthropod-Plant Interact. 2016, 10, 79–86. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, W.-F.; Yang, F.; Xu, B.; Yang, Y.-Z. The specific host plant DNA detection suggests a potential migration of Apolygus lucorum from cotton to mungbean fields. PLoS ONE 2017, 12, e0177789. [Google Scholar] [CrossRef]
- Cooper, W.R.; Horton, D.R. Molecular Gut Content Analysis to Pinpoint Where Psylla Overwinter. 2017. Available online: https://treefruitresearch.org/report/molecular-gut-content-analysis-to-pinpoint-where-psylla-overwinter/ (accessed on 20 November 2020).
- Davis, M.A. Why are most insects short fliers? Evol. Theory 1980, 5, 103–111. [Google Scholar]
- Horton, D.R.; Lewis, T.M. Tethered flight activity of pear psylla, Cacopsylla pyricola: Seasonal, host, and morphotypic effects. Entomol. Exp. Appl. 1996, 78, 39–49. [Google Scholar] [CrossRef]
- Burrows, M. Jumping mechanisms in jumping plant lice (Hemiptera, Sternorrhyncha, Psyllidae). J. Exp. Biol. 2012, 215, 3612–3621. [Google Scholar] [CrossRef]
- Barthel, D.; Kerschbamer, C.; Panassiti, B.; Malenovský, I.; Janik, K. Effect of Daytime and Tree Canopy Height on Sampling of Cacopsylla melanoneura, a ‘Candidatus Phytoplasma mali’ Vector. Plants 2020, 9, 1168. [Google Scholar] [CrossRef] [PubMed]
- Willerslev, E.; Hansen, A.J.; Binladen, J.; Brand, T.B.; Gilbert, M.T.P.; Shapiro, B.; Bunce, M.; Wiuf, C.; Gilichinsky, D.A.; Cooper, A. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science 2003, 300, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, W.; Miyagishima, S.-Y.; Jarvis, P. Chloroplast biogenesis: Control of plastid development, protein import, division and inheritance. Arabidopsis Book 2008, 6, e0110. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, R.; Tang, L.Y.; Zhang, L.; Yamada, H.; Twell, D.; Sakamoto, W. A conserved, Mg²+-dependent exonuclease degrades organelle DNA during Arabidopsis pollen development. Plant Cell 2011, 23, 1608–1624. [Google Scholar] [CrossRef] [PubMed]

| Sample Location | Region | Coordinates | Sea Level (m s.l.m.) | n | Specimen Code | Type | Date | Status |
|---|---|---|---|---|---|---|---|---|
| Bosentino | TR | 46°0′ N, 11°13′ E | 688 | 32 (31) | Bosentino 1–31 | Beat | 4 May 2016 | Remi |
| Eyrs | ST | 46°63′ N, 10°65′ E | 900 | 4 | Eyrs 1 | Trap | 21 April 2015 | Remi |
| Eyrs 2 | Trap | 29 April 2015 | Remi | |||||
| Eyrs 3 | Beat | 21 April 2015 | Remi | |||||
| Eyrs 4 | Beat | 3 May 2016 | Remi | |||||
| Kortsch | ST | 46°63′ N, 10°75′ E | 800 | 1 | Kortsch 1 | Beat | 29 April 2015 | Remi |
| Latsch | ST | 46°37′ N, 10°52′ E | 639 | 2 | Latsch 1 | Beat | 29 April 2015 | Remi |
| Latsch 2 | Beat | 11 April 2016 | Remi | |||||
| Mais | ST | 46°64′ N, 11°19′ E | 700 | 14 (13) | Mais 1 | Beat | 30 March 2015 | Remi |
| Mais 2–3 | Beat | 17 April 2015 | Remi | |||||
| Mais 4–9 | Beat | 10 April 2012 | Remi | |||||
| Mais 10 | Beat | 2 April 2013 | Remi | |||||
| Mais 11 | Beat | 26 April 2013 | Remi | |||||
| Mais 12 | Beat | 9 May 2013 | Remi | |||||
| Mais 13 | Beat | 22 May 2013 | Remi | |||||
| Mais | ST | 46°64′ N, 11°19′ E | 700 | 24 (23) | Mais 14–21, 26 | Beat | 26 June 2013 | Emi |
| Mais 22–24 | Beat | 20 June 2012 | Emi | |||||
| Mais 25 | Beat | 18 June 2013 | Emi | |||||
| Mais 27–32 | Beat | 9 July 2013 | Emi | |||||
| Mais 33–36 | Beat | 4 July 2013 | Emi | |||||
| Mais Moarhof | ST | 46°66′ N, 11°19′ E | 600 | 2 | Mais Moarhof 1 | Beat | 7 May 2013 | Remi |
| Mais Moarhof 2 | Beat | 3 May 2013 | Remi | |||||
| Niederlana | ST | 46°37′ N, 11°9′ E | 310 | 1 | Niederlana 1 | Beat | 7 May 2015 | Remi |
| Partschins | ST | 46°41′ N, 11°4′ E | 626 | 2 | Partschins 1 | Beat | 10 April 2012 | Remi |
| Partschins 2 | Beat | 16 April 2014 | Remi | |||||
| Rabland | ST | 46°67′ N, 11°06′ E | 525 | 1 | Rabland 1 | Beat | 9 May 2013 | Remi |
| Tarsch | ST | 46°36′ N, 10°53′ E | 800 | 1 | Tarsch 1 | Beat | 29 April 2015 | Remi |
| Tartsch | ST | 46°68′ N, 10°56′ E | 1029 | 1 | Tartsch 1 | Beat | 22 April 2016 | Remi |
| Tenna/ Bosentino | TR | 46°1′ N, 11°16′ E/46°0′ N, 11°13′ E | 569/688 | 3 | Tenna/ Bosentino 1–3 | Beat | 20 April 2015 | Remi |
| Dorf Tirol | ST | 46°41′ N, 11°9′ E | 594 | 2 | Dorf Tirol 1 | Beat | 16 April 2013 | Remi |
| Dorf Tirol 2 | Beat | 23 April 2013 | Remi | |||||
| Tscherms | ST | 46°38′ N, 11°9′ E | 292 | 5 | Tscherms 1 | Beat | 27 March 2012 | Remi |
| Tscherms 2–5 | Beat | 03 April 2012 | Remi |
| Order | Family | Genus | n | (%) | n Reads | Mean ± SD | Locus |
|---|---|---|---|---|---|---|---|
| Rosales | Rosaceae | - | 69 | 75 | 4506 | 65 ± 18 | rbcLa |
| Rosales | Rosaceae | Malus | 80 | 87 | 307,347 | 3842 ± 1022 | trnH |
| Rosales | Ulmaceae | Ulmus | 4 | 4 | 1801 | 450 ± 419 | trnH |
| Rosales | Cannabaceae | Humulus | 1 | 1 | 159 | 159 | trnH |
| Rosales | Urticaceae | Urtica | 1 | 1 | 1794 | 1794 | trnH |
| Coniferales | Pinaceae | - | 17 | 18 | 884 | 52 ± 17 | rbcLa |
| Coniferales | Pinaceae | Picea | 6 | 7 | 727 | 121 ± 61 | trnH |
| Coniferales | Pinaceae | Pinus | 6 | 7 | 375 | 62 ± 28 | trnH |
| Coniferales | Pinaceae | Cedrus | 5 | 5 | 6189 | 1238 ± 807 | trnH |
| Coniferales | Cupressaceae | Juniperus | 1 | 1 | 111 | 111 | trnH |
| Fagales | Betulaceae | - | 12 | 13 | 134 | 11 ± 2 | rbcLa |
| Fagales | Betulaceae | Betula | 9 | 10 | 706 | 78 ± 23 | trnH |
| Fagales | Fagaceae | - | 9 | 10 | 172 | 19 ± 5 | rbcLa |
| Fagales | Fagaceae | Quercus | 6 | 7 | 156 | 26 ± 11 | trnH |
| Fagales | Fagaceae | Fagus | 1 | 1 | 274 | 274 | trnH |
| Fagales | Juglandaceae | Juglans | 10 | 11 | 12,472 | 1247 ± 864 | trnH |
| Lamiales | Oleaceae | Fraxinus | 19 | 21 | 1983 | 104 ± 36 | trnH |
| Caryophyllales | Polygonaceae | Rumex | 15 | 16 | 5946 | 396 ± 242 | trnH |
| Malpighiales | Salicaceae | Salix | 10 | 11 | 4759 | 476 ± 273 | trnH |
| Malpighiales | Salicaceae | Populus | 4 | 4 | 767 | 192 ± 96 | trnH |
| Cucurbitales | Cucurbitaceae | Cucurbita | 5 | 5 | 965 | 193 ± 88 | trnH |
| Cucurbitales | Cucurbitaceae | Cucumis | 3 | 3 | 415 | 138 ± 115 | trnH |
| Asterales | Asteraceae | Taraxacum | 4 | 4 | 325 | 81 ± 20 | trnH |
| Asterales | Asteraceae | Lactuca | 2 | 2 | 117 | 58 ± 19 | trnH |
| Apiales | Apiaceae | Daucus | 2 | 2 | 1986 | 993 ± 507 | trnH |
| Grimmiales | Grimmiaceae | Schistidium | 1 | 1 | 725 | 725 | trnH |
| Vitales | Vitaceae | Vitis | 1 | 1 | 153 | 153 | trnH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barthel, D.; Schuler, H.; Galli, J.; Borruso, L.; Geier, J.; Heer, K.; Burckhardt, D.; Janik, K. Identification of Plant DNA in Adults of the Phytoplasma Vector Cacopsylla picta Helps Understanding Its Feeding Behavior. Insects 2020, 11, 835. https://doi.org/10.3390/insects11120835
Barthel D, Schuler H, Galli J, Borruso L, Geier J, Heer K, Burckhardt D, Janik K. Identification of Plant DNA in Adults of the Phytoplasma Vector Cacopsylla picta Helps Understanding Its Feeding Behavior. Insects. 2020; 11(12):835. https://doi.org/10.3390/insects11120835
Chicago/Turabian StyleBarthel, Dana, Hannes Schuler, Jonas Galli, Luigimaria Borruso, Jacob Geier, Katrin Heer, Daniel Burckhardt, and Katrin Janik. 2020. "Identification of Plant DNA in Adults of the Phytoplasma Vector Cacopsylla picta Helps Understanding Its Feeding Behavior" Insects 11, no. 12: 835. https://doi.org/10.3390/insects11120835
APA StyleBarthel, D., Schuler, H., Galli, J., Borruso, L., Geier, J., Heer, K., Burckhardt, D., & Janik, K. (2020). Identification of Plant DNA in Adults of the Phytoplasma Vector Cacopsylla picta Helps Understanding Its Feeding Behavior. Insects, 11(12), 835. https://doi.org/10.3390/insects11120835








