Can Generalist Predators Control Bemisia tabaci?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Experimental Evidence for Generalist Predator Importance in Whitefly Biocontrol
3.1. Direct Predation of Bemisia tabaci by Generalist Predators
3.2. Placing Generalist Predators in a Community-Ecology Context
3.2.1. Complementarity among Generalist Predators of Bemisia tabaci
3.2.2. Intraguild Predation among Generalist Predators of Bemisia tabaci
3.3. Non-Trophic Impacts of Generalist Predators on Bemisia tabaci
4. Within Field Studies of Generalist Predators of Bemisia tabaci
4.1. Life-Table Analysis of Generalist Predator Effects on Bemisia tabaci
4.2. Molecular Gut Content Analysis to Determine Predators of Bemisia tabaci
5. Conserving Generalist Predators to Strengthen Biocontrol
6. Current Synthesis of Predator Roles in the US Whitefly Biocontrol
6.1. Open Field Agroecosystems
6.2. Controlled Environment Agroecosystems
6.3. Impact of Environmental Stress
7. Future Research Opportunities
7.1. Expanding the Geographical Range and Scale
7.2. Integrating Generalist Predators into Economic Thresholds
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naranjo, S.E.; Ellsworth, P.C. Special Issue: Challenges and opportunities for pest management of Bemisia tabaci in the new century. Crop Prot. 2001, 20, 707. [Google Scholar] [CrossRef]
- Oliveira, M.R.V.; Henneberry, T.E.; Anderson, P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef] [Green Version]
- Castle, S.J.; Prabhaker, N. Monitoring changes in Bemisia tabaci (Hemiptera: Aleyrodidae) susceptibility to neonicotinoid insecticides in Arizona and California. J. Econ. Entomol. 2013, 106, 1404–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellsworth, P.C.; Martinez-Carrillo, J.L. IPM for Bemisia tabaci: A case study from North America. Crop Prot. 2001, 20, 853–869. [Google Scholar] [CrossRef]
- Simmons, A.M.; Jackson, D.M. Evaluation of foliar-applied insecticides on abundance of parasitoids of Bemisia argentifolii (Homoptera: Aleyrodidae) in vegetables. J. Entomol. Sci. 2000, 35, 1–8. [Google Scholar] [CrossRef]
- Simmons, A.M.; Abd-Rabou, S. Inundative field releases and evaluation of three predators for Bemisia tabaci (Hemiptera: Aleyrodidae) management in three vegetable crops. Insect Sci. 2011, 18, 195–202. [Google Scholar] [CrossRef]
- Simmons, A.M.; Abd-Rabou, S. Incidence of parasitism of Bemisia tabaci (Homoptera: Aleyrodidae) in three vegetable crops after application of biorational insecticides. J. Entomol. Sci. 2005, 40, 474–477. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Khasdan, V.; Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. Physiol. 2005, 58, 216–225. [Google Scholar] [CrossRef]
- Gould, J.; Hoelmer, K.; Goolsby, J. Classical Biological Control of Bemisia tabaci in the United States—A Review of Interagency Research and Implementation; Springer Science + Business Media B.V.: New York, NY, USA, 2008; Volume 4. [Google Scholar]
- Naranjo, S.E. Conservation and evaluation of natural enemies in IPM systems for Bemisia tabaci. Crop Prot. 2001, 20, 835–852. [Google Scholar] [CrossRef] [Green Version]
- Gerling, D.; Alomar, Ò.; Arnò, J. Biological control of Bemisia tabaci using predators and parasitoids. Crop Prot. 2001, 20, 779–799. [Google Scholar] [CrossRef]
- Liu, T.-X.; Stansly, P.A.; Gerling, D. Whitefly parasitoids: Distribution, life history, bionomics, and utilization. Annu. Rev. Entomol. 2015, 60, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Letourneau, D.K.; Jedlicka, J.A.; Bothwell, S.G.; Moreno, C.R. Effects of natural enemy biodiversity on the suppression of arthropod herbivores in terrestrial ecosystems. In Annual Review of Ecology Evolution and Systematics; Annual Reviews: Palo Alto, CA, USA, 2009; Volume 40, pp. 573–592. [Google Scholar]
- Rosenfeld, J.S. Functional redundancy in ecology and conservation. Oikos 2002, 98, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Gurr, G.M.; Wratten, S.D.; Snyder, W.E. Biodiversity and Insect Pests: Key Issues for Sustainable Management; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Hagler, J.R.; Blackmer, F. Identifying inter- and intra-guild feeding activity of an arthropod predator assemblage. Ecol. Entomol. 2013, 38, 258–271. [Google Scholar] [CrossRef]
- Hagler, J.R.; Naranjo, S.E. Use of a gut content ELISA to detect whitefly predator feeding activity after field exposure to different insecticide treatments. Biocontrol Sci. Technnol. 2005, 15, 321–339. [Google Scholar] [CrossRef]
- Vandervoet, T.F.; Ellsworth, P.C.; Carrière, Y.; Naranjo, S.E. Quantifying conservation biological control for management of Bemisia tabaci (Hemiptera: Aleyrodidae) in cotton. J. Econ. Entomol. 2018, 111, 1056–1068. [Google Scholar] [CrossRef]
- Naranjo, S.E. Retrospective analysis of a classical biological control programme. J. Appl. Ecol. 2018, 55, 2439–2450. [Google Scholar] [CrossRef] [Green Version]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [Green Version]
- King, R.A.; Read, D.S.; Traugott, M.; Symondson, W.O.C. Molecular analysis of predation: A review of best practice for DNA-based approaches. Mol. Ecol. 2008, 17, 947–963. [Google Scholar] [CrossRef] [PubMed]
- González-Chang, M.; Wratten, S.D.; Lefort, M.-C.; Boyer, S. Food webs and biological control: A review of molecular tools used to reveal trophic interactions in agricultural systems. Food Webs 2016, 9, 4–11. [Google Scholar] [CrossRef]
- Symondson, W.O. The molecular revolution: Using polymerase chain reaction based methods to explore the role of predators in terrestrial food webs. In Biodiersity and Insect Pests: Key Issues for Sustainable Management; Gurr, G.M., Wratten, S., Snyder, W.E., Read, M.Y., Eds.; Wiley-Blackwell: West Sussex, UK, 2012; pp. 166–184. [Google Scholar]
- Ellis, D.; McAvoy, R.; Ayyash, L.A.; Flanagan, M.; Ciomperlik, M. Evaluation of Serangium parcesetosum (Coleoptera: Coccinellidae) for biological control of silverleaf whitefly, Bemisia argentifolii (Homoptera: Aleyrodidae), on poinsettia. Fla. Entomol. 2001, 84, 215–221. [Google Scholar] [CrossRef]
- Asiimwe, P.; Ellsworth, P.C.; Naranjo, S.E. Natural enemy impacts on Bemisia tabaci (MEAM1) dominate plant quality effects in the cotton system. Ecol. Entomol. 2016, 41, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Asiimwe, P.; Naranjo, S.E.; Ellsworth, P.C. Relative influence of plant quality and natural enemies on the seasonal dynamics of Bemisia tabaci (Hemiptera: Aleyrodidae) in cotton. J. Econ. Entomol. 2013, 106, 1260–1273. [Google Scholar] [CrossRef] [Green Version]
- Razze, J.M.; Liburd, O.E.; McSorley, R. Preference of Bemisia tabaci biotype B on zucchini squash and buckwheat and the effect of Delphastus catalinae on whitefly populations. Pest Manag. Sci. 2015, 72, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Razze, J.M.; Liburd, O.E.; Nuessly, G.S.; Samuel-Foo, M. Evaluation of bioinsecticides for management of Bemisia tabaci (Hemiptera: Aleyrodidae) and the effect on the whitefly predator Delphastus catalinae (Coleoptera: Coccinellidae) in organic squash. J. Econ. Entomol. 2016, 109, 1766–1771. [Google Scholar] [CrossRef]
- Döker, İ.; Hernandez, Y.V.; Mannion, C.; Carrillo, D. First report of Amblyseius tamatavensis (Acari: Phytoseiidae) in the United States of America. Int. J. Acarol. 2018, 44, 101–104. [Google Scholar] [CrossRef]
- Vafaie, E.K.; Pemberton, H.B.; Gu, M.; Kerns, D.; Eubanks, M.D.; Heinz, K.M. A comparison of repetitive releases of single or multiple natural enemy species on the suppression of Bemisia tabaci infesting poinsettias. Biol. Control. 2020, 151, 104407. [Google Scholar] [CrossRef]
- Smith, H.A.; Krey, K.L. Three release rates of Dicyphus hesperus (Hemiptera: Miridae) for management of Bemisia tabaci (Hemiptera: Aleyrodidae) on greenhouse tomato. Insects 2019, 10, 213. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.X.; Stansly, P.A. Functional response and plant preference of Nephaspis Oculatus (Coleoptera: Coccinellidae), preying on Bemisia Argentifolii (Homoptera: Aleyrodidm) in the laboratory. Insect Sci. 2002, 9, 1–9. [Google Scholar]
- Legaspi, J.C.; Simmons, A.M.; Legaspi, B.C. Prey preference by Delphastus catalinae (Coleoptera: Coccinellidae) on Bemisia argentifolii (Homoptera: Aleyrodidae): Effects of plant species and prey stages. Fla. Entomol. 2006, 89, 218–222. [Google Scholar] [CrossRef]
- Kumar, V.; Mehra, L.; McKenzie, C.L.; Osborne, L.S. Functional response and prey stage preference of Delphastus catalinae and D. pallidus (Coleoptera: Coccinellidae) on Bemisia tabaci (Hemiptera: Aleyrodidae). Biocontrol Sci. Technnol. 2020, 30, 584–591. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C. Mortality dynamics and population regulation in Bemisia tabaci. Entomol. Exp. Appl. 2005, 116, 93–108. [Google Scholar] [CrossRef]
- Hagler, J. Foraging behavior, host stage selection and gut content analysis of field collected Drapetis nr. divergens: A predatory fly of Bemisia argentifolii. Southw. Entomol. 2002, 27, 241–250. [Google Scholar]
- Hagler, J.R.; Jackson, C.G.; Isaacs, R.; Machtley, S.A. Foraging behavior and prey interactions by a guild of predators on various lifestages of Bemisia tabaci. J. Insect Sci. 2004, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Hernandez, Y.V.; Kumar, V.; Francis, A.; Skelley, P.; Rohrig, E.; McKenzie, C.; Osborne, L.; Mannion, C. Pallidus beetle, Delphastus pallidus LeConte (Insecta: Coleoptera: Coccinellidae), a native predatory beetle of whitefly species in Florida. Fla. Dep. Agric. Consum. Serv. Div. Plant Ind. 2017, 435. [Google Scholar]
- Zang, L.-S.; Liu, T.-X. Intraguild interactions between an oligophagous predator, Delphastus catalinae (Coleoptera: Coccinellidae), and a parasitoid, Encarsia sophia (Hymenoptera: Aphelinidae), of Bemisia tabaci (Homoptera: Aleyrodidae). Biol. Control 2007, 41, 142–150. [Google Scholar] [CrossRef]
- Naranjo, S.E. Intraguild predation on Eretmocerus sp. nr. emiratus, a parasitoid of Bemisia tabaci, by three generalist predators with implications for estimating the level and impact of parasitism. Biocontrol Sci. Technnol. 2007, 17, 605–622. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Akey, D.H. Conservation of natural enemies in cotton: Comparative selectivity of acetamiprid in the management of Bemisia tabaci. Pest Manag. Sci. Former. Pestic. Sci. 2005, 61, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, S.E.; Ellsworth, P.C. The contribution of conservation biological control to integrated control of Bemisia tabaci in cotton. Biol. Control 2009, 51, 458–470. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Chu, C.C.; Henneberry, T.J. Conservation of predatory arthropods in cotton: Role of action thresholds for Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 2002, 95, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, S.E.; Hagler, J.R.; Ellsworth, P.C. Improved conservation of natural enemies with selective management systems for Bemisia tabaci (Homoptera: Aleyrodidae) in cotton. Biocontrol Sci. Technnol. 2003, 13, 571–587. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Hagler, J.R. Conservation of natural enemies in cotton: Role of insect growth regulators in management of Bemisia tabaci. Biol. Control 2004, 30, 52–72. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-H.; Nyrop, J.P.; Sanderson, J.P. Non-consumptive effects of the predatory beetle Delphastus catalinae (Coleoptera: Coccinellidae) on habitat use patterns of adult whitefly Bemisia argentifolii (Hemiptera: Aleyrodidae). Appl. Entomol. Zool. 2014, 49, 599–606. [Google Scholar] [CrossRef]
- Lee, D.-H.; Nyrop, J.P.; Sanderson, J.P. Avoidance of natural enemies by adult whiteflies, Bemisia argentifolii, and effects on host plant choice. Biol. Control 2011, 58, 302–309. [Google Scholar] [CrossRef]
- Xiao, Y.; Avery, P.; Chen, J.; McKenzie, C.; Osborne, L. Ornamental pepper as banker plants for establishment of Amblyseius swirskii (Acari: Phytoseiidae) for biological control of multiple pests in greenhouse vegetable production. Biol. Control 2012, 63, 279–286. [Google Scholar] [CrossRef]
- Rincon, D.F.; Cañas, L.A.; Hoy, C.W. Intra-plant spatial interaction between Delphastus catalinae (Coleoptera: Coccinellidae) and Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) and its effect on predation rates. Biol. Control 2016, 95, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Hagler, J.; Naranjo, S. A multiple ELISA system for simultaneously monitoring intercrop movement and feeding activity of mass-released insect predators. Int J. Pest Manag. 2004, 50, 199–207. [Google Scholar] [CrossRef]
- Simmons, A.M.; Legaspi, J.C. Survival and predation of Delphastus catalinae (Coleoptera: Coccinellidae), a predator of whiteflies (Homoptera: Aleyrodidae), after exposure to a range of constant temperatures. Environ. Entomol. 2004, 33, 839–843. [Google Scholar] [CrossRef]
- Simmons, A.M.; Legaspi, J.C. Ability of Delphastus catalinae (Coleoptera: Coccinellidae), a predator of whiteflies (Homoptera: Aleyrodidae), to survive mild winters. J. Entomol. Sci. 2007, 42, 163–173. [Google Scholar] [CrossRef]
- Simmons, A.M.; Legaspi, J.C.; Legaspi, B.C. Responses of Delphastus catalinae (Coleoptera: Coccinellidae), a predator of whiteflies (Hemiptera: Aleyrodidae), to relative humidity: Oviposition, hatch, and immature survival. Ann. Entomol. Soc. Am. 2008, 101, 378–383. [Google Scholar] [CrossRef]
- Legaspi, J.C.; Legaspi, B.C., Jr.; Simmons, A.M.; Soumare, M. Life table analysis for immatures and female adults of the predatory beetle, Delphastus catalinae, feeding on whiteflies under three constant temperatures. J. Insect Sci. 2008, 8. [Google Scholar] [CrossRef] [Green Version]
- Simmons, A.M. Capture of Bemisia tabaci (Homoptera: Aleyrodidae) and Delphastus catalinae (Coleoptera: Coccinellidae) on three colors of sticky traps. J. Entomol. Sci. 2003, 38, 481–484. [Google Scholar] [CrossRef]
- Simmons, A.M.; Chu, C.-C.; Henneberry, T.J. Yellow sticky cards equipped with light-emitting diodes: A natural enemies compatible management tool for whiteflies (Homoptera: Aleyrodidae) and other greenhouse vegetable pests. J. Entomol. Sci. 2004, 39, 298–300. [Google Scholar] [CrossRef]
- Chu, C.C.; Chen, T.; Simmons, A.; Alexander, P.; Henneberry, T. Development of Light-Emitting Diode (LED) Equipped Insect Traps for Monitoring Pest Insects in Greenhouses and Fields, 2001–2004; Caribbean Food Crops Society: St. Croix, U.S. Virgin Islands, 2004; pp. 106–113. [Google Scholar]
- Bowers, C.L.; Toews, M.D.; Schmidt, J.M. Beyond soil health: The trophic effects of cover crops shape predator communities. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Finke, D.L.; Denno, R.F. Predator diversity dampens trophic cascades. Nature 2004, 429, 407–410. [Google Scholar] [CrossRef]
- Ives, A.R.; Cardinale, B.J.; Snyder, W.E. A synthesis of subdisciplines: Predator–prey interactions, and biodiversity and ecosystem functioning. Ecol. Lett. 2005, 8, 102–116. [Google Scholar] [CrossRef]
- Latham, D.R.; Mills, N.J. Quantifying insect predation: A comparison of three methods for estimating daily per capita consumption of two aphidophagous predators. Environ. Entomol. 2009, 38, 1117–1125. [Google Scholar] [CrossRef] [Green Version]
- Kheirodin, A.; Costamagna, A.C.; Cárcamo, H.A. Laboratory and field tests of predation on the cereal leaf beetle, Oulema melanopus (Coleoptera: Chrysomelidae). Biocontrol Sci. Technnol. 2019, 29, 451–465. [Google Scholar] [CrossRef]
- Sheppard, S.; Harwood, J. Advances in molecular ecology: Tracking trophic links through predator–prey food-webs. Funct. Ecol. 2005, 19, 751–762. [Google Scholar] [CrossRef]
- Simmons, A.M.; Weber, D.C.; Payton, M.E.; Hu, J.S.; Greenstone, M.H. Do Heteropterans have longer molecular prey detectability half-lives than other predators? A test With Geocoris punctipes (Heteroptera: Geocoridae) and Orius insidiosus (Heteroptera: Anthocoridae). J. Entomol. Sci. 2015, 50, 99–105, 107. [Google Scholar] [CrossRef]
- Jansen, J.P. Pest Select Database: A New Tool to Use Selective Pesticide for IPM. Available online: https://www.iobc-wprs.org/ip_ipm/archive/IPM_Future_Jansen_20130320.pdf (accessed on 26 October 2020).
- BioBest-Group. Side Effect Manual. Available online: https://www.biobestgroup.com/en/side-effect-manual (accessed on 26 October 2020).
- IOBC. Pesticide Side Effect Databse. Available online: https://www.iobc-wprs.org/ip_ipm/IOBC_Pesticide_Side_Effect_Database.html (accessed on 26 October 2020).
- Koppert-Biological-Systems. Side Effects Database. Available online: https://sideeffects.koppert.com (accessed on 26 October 2020).
- Snyder, W.E. Give predators a complement: Conserving natural enemy biodiversity to improve biocontrol. Biol. Control 2019, 135, 73–82. [Google Scholar] [CrossRef]
- Greenop, A.; Woodcock, B.A.; Wilby, A.; Cook, S.M.; Pywell, R.F. Functional diversity positively affects prey suppression by invertebrate predators: A meta-analysis. Ecology 2018, 99, 1771–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Peter, B.; Bengtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.F.; et al. Landscape moderation of biodiversity patterns and processes—Eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Kheirodin, A.; Cárcamo, H.A.; Costamagna, A.C. Contrasting effects of host crops and crop diversity on the abundance and parasitism of a specialist herbivore in agricultural landscapes. Landsc. Ecol. 2020, 35, 1073–1087. [Google Scholar] [CrossRef]
- Greenstone, M.H.; Szendrei, Z.; Payton, M.E.; Rowley, D.L.; Coudron, T.C.; Weber, D.C. Choosing natural enemies for conservation biological control: Use of the prey detectability half-life to rank key predators of Colorado potato beetle. Entomol. Exp. Appl. 2010, 136, 97–107. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Harwood, J.D.; Rypstra, A.L. Foraging activity of a dominant epigeal predator: Molecular evidence for the effect of prey density on consumption. Oikos 2012, 121, 1715–1724. [Google Scholar] [CrossRef]
- Kheirodin, A.; Sharanowski, B.J.; Cárcamo, H.A.; Costamagna, A.C. Consumption of cereal leaf beetle, Oulema melanopus, by generalist predators in wheat fields detected by molecular analysis. Entomol. Exp. Appl. 2020, 168, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Szendrei, Z.; Bryant, A.; Rowley, D.; Furlong, M.J.; Schmidt, J.M.; Greenstone, M.H. Linking habitat complexity with predation of pests through molecular gut-content analyses. Biocontrol Sci. Technnol. 2014, 24, 1425–1438. [Google Scholar] [CrossRef]
- Hallett, R.H.; Bahlai, C.A.; Xue, Y.; Schaafsma, A.W. Incorporating natural enemy units into a dynamic action threshold for the soybean aphid, Aphis glycines (Homoptera: Aphididae). Pest Manag. Sci. 2014, 70, 879–888. [Google Scholar] [CrossRef]
- Zhang, W.; Swinton, S.M. Incorporating natural enemies in an economic threshold for dynamically optimal pest management. Ecol. Model. 2009, 220, 1315–1324. [Google Scholar] [CrossRef]
- Bowers, C.; Toews, M.; Liu, Y.; Schmidt, J.M. Cover crops improve early season natural enemy recruitment and pest management in cotton production. Biol. Control 2020, 141, 104149. [Google Scholar] [CrossRef]
- Almeida, G.; Pratissoli, D.; Zanuncio, J.; Vicentini, V.; Holtz, A.; Serrão, J. Calcium silicate and organic mineral fertilizer increase the resistance of tomato plants to Frankliniella schultzei. Phytoparasitica 2009, 37, 225–230. [Google Scholar] [CrossRef]
- AAFC. Cereal Aphid Manager Mobile App. Available online: https://www.agr.gc.ca/eng/scientific-collaboration-and-research-in-agriculture/agricultural-research-results/cereal-aphid-manager-mobile-app/?id=1521633279786 (accessed on 26 October 2020).
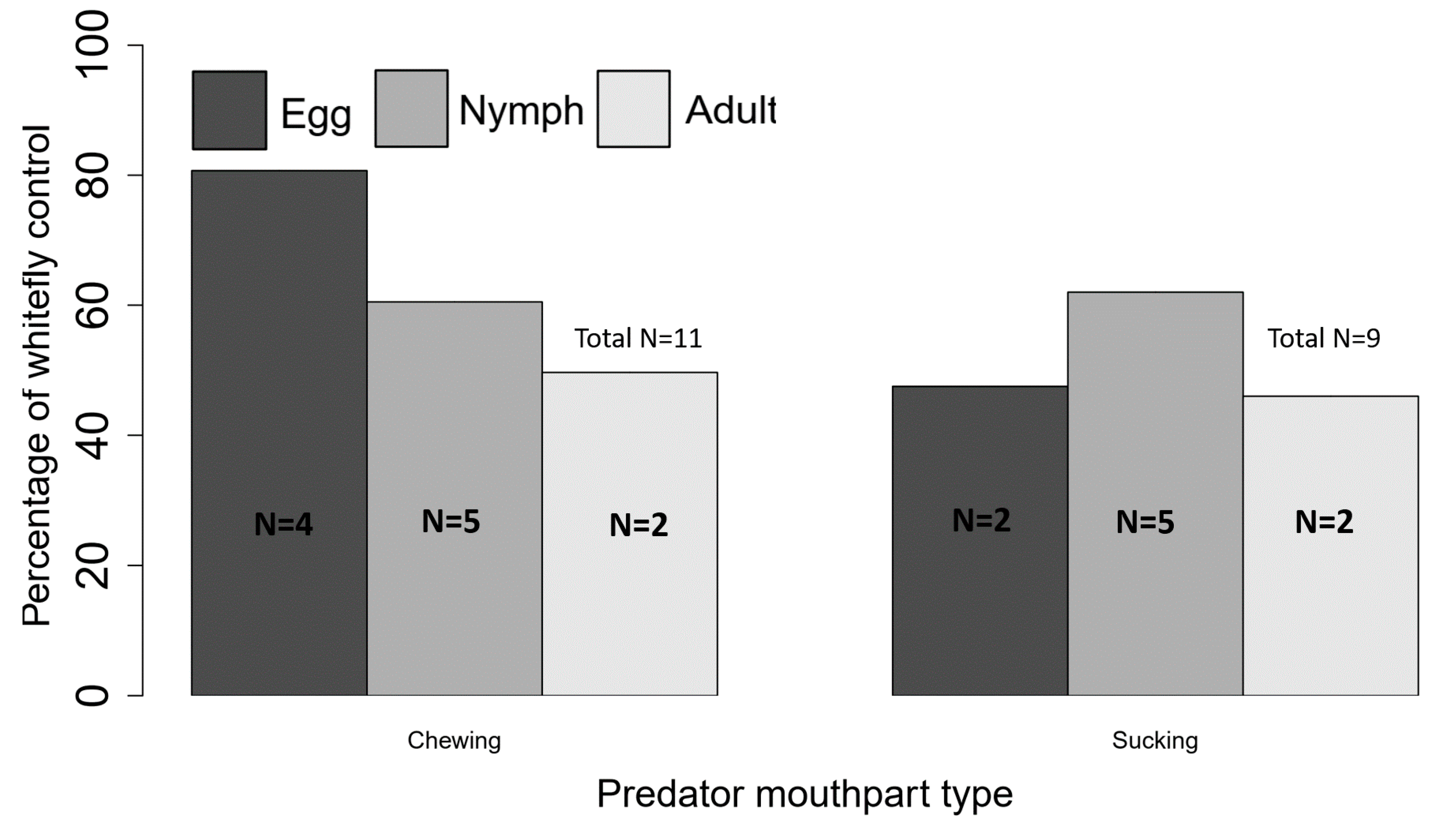
| Concept Studied | Lab# | Greenhouse# | Field# | Total# | References |
|---|---|---|---|---|---|
| Direct effect of predators on whitefly population growth and control | 2 | 5 | 6 | 13 | [19,20,25,26,27,28,29,30,31,32,33,34,35,36] |
| Predator prey life stage preference and host plant preference | 5 | 0 | 0 | 5 | [34,35,37,38,39] |
| Predator gut content analysis | 1 | 0 | 5 | 6 | [17,18,37,40,41] |
| Intraguild predation among predators and parasitoids | 2 | 0 | 0 | 2 | [40,41] |
| Insecticide effects on B. tabaci predation | 0 | 1 | 8 | 9 | [18,19,27,29,42,43,44,45,46] |
| Indirect effects of predators on B. tabaci dispersal | 0 | 2 | 0 | 2 | [47,48] |
| Thresholds and predator-prey ratios testing | 0 | 0 | 2 | 2 | [19,44] |
| Banker plant effect on B. tabaci biocontrol | 0 | 2 | 0 | 2 | [28,49] |
| Predator movement pattern from release site | 0 | 1 | 1 | 2 | [50,51] |
| Environmental stress effect on predator | 3 | 0 | 1 | 4 | [52,53,54,55] |
| Compatibility with whitefly sticky traps | 1 | 2 | 0 | 3 | [56,57,58] |
| Order: Family | Species | Crops | Spatial Scale | Prey Stage Preference | Preferred Crop | Control and/or Predation Rates | References | ||
| Coleoptera: | Delphastus catalinae | Cotton, collard, tomato, hibiscus, and squash | Laboratory, greenhouse and field | Eggs | Cotton | 98.6% in petri-dish, 75% population growth reduction in the greenhouse | [23,25,30,35,52] | ||
| Coccinellidae | Hippodamia convergens | Cotton and cantaloupe | Laboratory and field | Eggs, parasitized nymphs | Not studied | 45.5% nymphal mortality petri-dish, 50% of individuals positive for B. tabaci DNA | [17,38,43] | ||
| Delphastus pallidus | fiscus hedge, cotton | Laboratory | Eggs & early nymphs | Not studied | 68.0% and 55.1% eggs and nymph mortality on leaf disc, respectively | [35,39] | |||
| Serangium parcesetosum | Poinsettia | Greenhouse | Not studied | Not studied | Up to 60% B. tabaci mortality with four individuals per plant | [28] | |||
| Nephaspis oculatus | Collards, soybean and tomato | Laboratory | Not studied | Collards | 72.55% average egg predation on leaf discs at range of egg densities in 24 h | [34] | |||
| Melyridae | Collops vittatus | Cotton | Laboratory and field | Eggs & adults | Not studied | 86% positive for B. tabaci DNA in cotton fields.13.1 eggs consumed per hour. Reduce B. tabaci immatures | [17,19,38] | ||
| Hemiptera: | Geocoris punctipes | Cotton | Laboratory and field | Adult and parasitized nymphs | Not studied | 36% nymphal predation in petri dish, predation on 4th instar nymph considered B. tabaci key mortality factor in cotton fields | [29,36,38,43,46] | ||
| Geocoridae | Geocoris pallens | Cotton | Field | Adult | Not studied | Considered one of the key predators of B. tabaci with predator-prey ratios of 0.75 G. pallens per 100 sweeps to one large B. tabaci nymph | [17,19,29,36] | ||
| Anthocoridae | Orius tristicolor | Cotton | Laboratory and field | Adult and nymphal stages | Not studied | In the laboratory, adults consumed up to 2.1 B. tabaci adults per hour. In fields, considered key predators of B. tabaci | [18,19,29,38] | ||
| Order: Family | Species | Crops | Spatial Scale | Prey stage Preference | Preferred Crop | Control and/or Predation Rates | References | ||
| Miridae | Spanagonicus albofasciatus | Cotton | Field | Not studied | Not studied | A range of 30–50% tested positive for B. tabaci eggs or adult females’ antigen | [18] | ||
| Lygus hesperus | Cotton | Laboratory and field | nymphs | Not studied | Adults consumed up to 2.4 B. tabaci nymphs per hour. Also, it is a cotton pest. Its density was correlated with predation on B. tabaci life stages in cotton fields | [19,36,38] | |||
| Pseudatomocelis seriatus | Cotton | Field | Not studied | Not studied | A range of 30–50% P. seriatus individuals fed on B. tabaci eggs or adult females | [18] | |||
| Dicyphus hesperus | Tomato | Greenhouse | Not studied | Not studied | Up to 70% and 64% B. tabaci egg and nymph population control over six weeks | [33] | |||
| Rhinacloa forticornis | Cotton | Field | Not studied | Not studied | Not significant or minimal levels of control in cotton fields | [19,24,36] | |||
| Reduviidae | Zelus renardii | Cotton | Field | Not studied | Not studied | On average, 49.3 % of individuals Z. renardii had B. tabaci DNA in their gut | [17] | ||
| Sinea confuse | Cotton | Field | Not studied. | Not studied | On average, 15.3% of individuals had B. tabaci DNA in their gut | [17] | |||
| Nabidae | Nabis alternatus | Cotton | Field | Not studied. | Not studied | On average, 32.4% of individuals had B. tabaci DNA in their gut | [17] | ||
| Diptera: Empididae | Drapetis nr. divergens | Cotton | Field | Adults and eggs | Not studied | The predator-B. tabaci ratio of 8 or 44 D. nr divergens per 100 sweeps to one adult or one large B. tabaci nymph. 32.4% of individuals positives for B. tabaci DNA | [18,19,37] | ||
| Neuroptera: Chrysopidae | Chrysoperla carnea | Cotton | Field | Not studied | Not studied | 49% positive for B. tabaci DNA. Significant negative effect on B. tabaci immature stages in cotton fields | [17,19,36] | ||
| Acari: Phytoseiidae | Amblyseius swirskii | Green bean and pepper | Greenhouse | Not studied | Pepper | Significant suppression of the B. tabaci population on green bean plants by A. swirskii in the greenhouse | [32,50,51] | ||
| Amblyseius tamataven | black nightshade | Laboratory and field | Not studied | Not studied | Can complete development on B. tabaci, suggesting suitability | [31] | |||
| Order: Family | Species | Crops | Spatial Scale | Prey stage Preference | Preferred Crop | Control and/or Predation Rates | References | ||
| Araneae Thomisidae | Misumenops celer | Cotton | Field | Not studied | Not studied | 31% and 32.5% individuals tested positive for B. tabaci antigen and DNA, respectively. Significant reduction in B. tabaci density in cotton fields with high M. celer density | [17,18,19,24] | ||
| Dictynidae | Dictyna reticulata | Cotton | Field | Not studied | Not studied | On average, 39.5% of individuals had B. tabaci DNA in their gut | [17] | ||
| Clubionidae | Clubiona spp. | Cotton | Field | Not studied | Not studied | 31.6% positive for B. tabaci DNA | [17] | ||
| Salticidae | No species identity | Cotton | Field | Not studied | Not studied | 8% positive for B. tabaci DNA | [17,59] | ||
| Lycosidae | Hogna spp. | Cotton | Field | Not studied | Not studied | 22.2% positive for B. tabaci DNA | [17] | ||
| Araneidae | No species identity | Cotton | Field | Not studied | Not studied | 25% positive for B. tabaci DNA | [17] | ||
| Miturgidae | Cheiracanthium inclusum | Cotton | Field | Not studied | Not studied | 71.4% positive for B. tabaci DNA | [17] | ||
| Corinnidae | Trachelas spp. | Cotton | Field | Not studied | Not studied | An average of 33.3% of individuals was positive for B. tabaci DNA. But the species had a low density | [17] | ||
| Gnaphosidae | No species identity | Cotton | Field | Not studied | Not studied | An average of 50% of individuals was positive for B. tabaci DNA. But the species had a low density | [17] | ||
| Predator 1 | Description of Predator Effects | References |
|---|---|---|
Delphastus catalinae |
| [23,25,30,35,52] |
Collops vittatus |
| [17,19,36,38] |
Orius tristicolor |
| [18,19,29,36,38] |
Geocoris sp. |
| [19,29,36,38,43,46] |
Dapetis nr. divergens |
| [18,19,37] |
Misumenops celer |
| [17,18,19,27,59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheirodin, A.; Simmons, A.M.; Legaspi, J.C.; Grabarczyk, E.E.; Toews, M.D.; Roberts, P.M.; Chong, J.-H.; Snyder, W.E.; Schmidt, J.M. Can Generalist Predators Control Bemisia tabaci? Insects 2020, 11, 823. https://doi.org/10.3390/insects11110823
Kheirodin A, Simmons AM, Legaspi JC, Grabarczyk EE, Toews MD, Roberts PM, Chong J-H, Snyder WE, Schmidt JM. Can Generalist Predators Control Bemisia tabaci? Insects. 2020; 11(11):823. https://doi.org/10.3390/insects11110823
Chicago/Turabian StyleKheirodin, Arash, Alvin M. Simmons, Jesusa C. Legaspi, Erin E. Grabarczyk, Michael D. Toews, Phillip M. Roberts, Juang-Horng Chong, William E. Snyder, and Jason M. Schmidt. 2020. "Can Generalist Predators Control Bemisia tabaci?" Insects 11, no. 11: 823. https://doi.org/10.3390/insects11110823





