New Insights into Cockroach Control: Using Functional Diversity of Blattella germanica Symbionts
Simple Summary
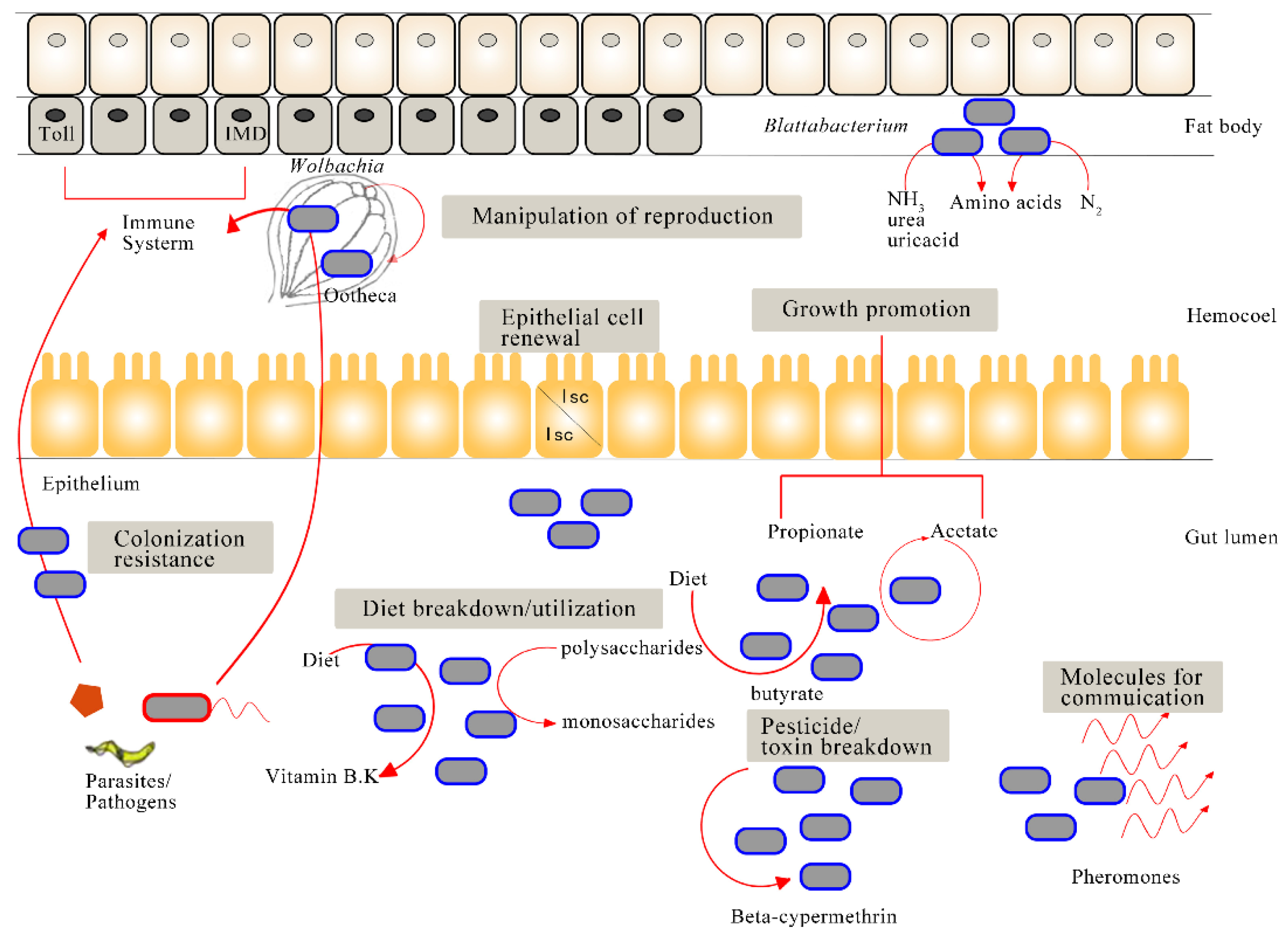
Abstract
1. Introduction
| Bacteria | Category | Distribution | Function | Reference |
|---|---|---|---|---|
| Blattabacterium | Flavobacteriales | A special cell of fat body | Participate in nitrogen assimilation, uric acid degradation, and nutrient provisioning | [19] |
| Wolbachia | Proteobacteria | Reproductive tissues, digestive tract, thorax, abdomen, salivary gland, etc. | Reproductive regulations (e.g., cytoplasmic incompatibility) | [30] |
| Salmonella spp. | Proteobacteria | Gut | Increase the host drug resistant | [10] |
| Bacteroides | Bacteroidetes | Gut | Carbohydrate metabolism and transport; assist the host defense; entomopathogenic fungi | [31] |
| Lachnospira | Firmicutes | Gut | Hydrolyze polysaccharide; assist digestion; synthesize acetate, propionate, and butyrate | [32] |
| Pseudomonas | Proteobacteria | Gut | Secrete versatile secondary metabolites; provide protection from parasites and pathogens | [33] |
| Bacillus | Bacteriophyta | Gut | Inhibit microbial growth by secreting antifungal compounds and antibiotic-like compounds | [34] |
| Weissella | Firmicutes | Gut | Produce many antimicrobial agents such as adhesion inhibitors, organic acids, and bacteriocins | [35] |
| Rickettsia | Proteobacteria | Digestive organs, salivary glands, reproductive organs, etc. | Participate in reproductive regulation; increase host resistance | [36] |
| Acetobacteraceae | Proteobacteria | Gut | Participate in carbohydrate fermentation and lactate metabolism | [37] |
| Providencia | Proteobacteria | Gut | Assist the host defense natural predators | [10] |
| Fusobacterium | Fusobacteria | Gut | Ferment both glucose and amino acids | [32] |
| Enterococcus sp. | Firmicutes | Gut | Anti-phytopathogenic fungal activity | [38] |
2. Nutrition and Development: Reducing Survivability
3. Reproductive Regulation: Potential Population Suppress Strategy Using Wolbachia
4. Resistance to Pesticides: Symbiont-Mediated Potential Direction of Resistance Management
5. Immune and Defense: Improving the Effect of Biological Control Using Pathogenic Microorganisms
6. Behavior: Interference with Aggregation and Trap Killing
7. Paratransgenesis
8. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Bell, W.J.; Roth, L.M.; Nalepa, C.A.; Wilson, E.O. Cockroaches: Ecology, Behavior, and Natural History; Johns Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Cochran, D.G. Blattodea: (cockroaches). In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: New York, NY, USA, 2009; pp. 108–112. [Google Scholar]
- Beccaloni, G.W. Cockroach Species File Online. Version 5.0/5.0. 2014. Available online: http://Cockroach.SpeciesFile.org (accessed on 1 September 2020).
- Nazari, M.; Motlagh, B.A.; Nasirian, H. Toxicity of cypermethrin and chlorpyrifos against German cockroach [Blattella germanica (Blattaria: Blattellidae)] strains from Hamadan, Iran. Pak. J. Biol. Sci. 2016, 19, 259–264. [Google Scholar] [PubMed]
- Nasirian, H. Infestation of cockroaches (Insecta: Blattaria) in the human dwelling environments: A systematic review and meta-analysis. Acta Trop. 2017, 167, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Lihoreau, M.; Costa, J.; Rivault, C. The social biology of domiciliary cockroaches: Colony structure, kin recognition and collective decisions. Insectes Soc. 2012, 59, 445–452. [Google Scholar] [CrossRef]
- Moges, F.; Eshetie, S.; Endris, M.; Huruy, K.; Muluye, D.; Feleke, T.; G/Silassie, F.; Ayalew, G.; Nagappan, R. Cockroaches as a source of high bacterial pathogens with multidrug resistant strains in Gondar Town, Ethiopia. Biomed. Res. Int. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Naqqash, M.N.; Saeed, Q.; Saeed, S.; Jaleel, W.; Zaka, S.M.; Faheem, M.; Bakhtawar, M.; Rehman, S. A cross sectional survey of community awareness about typhoid and its major vector cockroach in Southern Punjab, Pakistan. Middle-East J. Sci. Res. 2014, 21, 602–608. [Google Scholar]
- Wannigama, D.L.; Dwivedi, R.; Zahraei-Ramazani, A. Prevalence and antibiotic resistance of gram-negative pathogenic bacteria species isolated from Periplaneta americana and Blattella germanica in Varanasi, India. J. Arthropod Borne Dis. 2013, 8, 10–20. [Google Scholar]
- Akinjogunla, O.J.; Odeyemi, A.T.; Udoinyang, E.T. Cockroaches (Periplaneta americana and Blattella germanica): Reservoirs of multi drug resistant (MDR) bacteria in Uyo, Akwa Ibom State. Sci. J. Biol. Sci. 2012, 1, 269–279. [Google Scholar]
- Vazirianzadeh, B.; Mehdinejad, M.; Dehghani, R. Identification of bacteria which possible transmitted by Polyphaga aegyptica (Blattodea: Blattidae) in the region of Ahvaz, Southwest Iran. Jundishapur J. Microbiol. 2009, 2, 36–40. [Google Scholar]
- Douglas, A.E. Multiorganismal Insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Eichler, S.; Schaub, G. Development of symbionts in triatomine bugs and the effects of infections with trypanosomatids. Exp. Parasitol. 2002, 100, 17–27. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects-diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- McMeniman, C.J.; Lanem, R.V.; Cass, B.N.; Fong, A.W.C.; Sidhu, M.; Wang, Y.F.; O’Neill, S.L. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Chouaia, B.; Rossi, P.; Epis, S.; Mosca, M.; Ricci, I.; Damiani, C.; Ulissi, U.; Crotti, E.; Daffonchio, D.; Bandi, C.; et al. Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiol. 2012, 12 (Suppl. S1), S2. [Google Scholar] [CrossRef] [PubMed]
- Ricci, I.; Valzano, M.; Ulissi, U.; Epis, S.; Cappelli, A.; Favia, G. Symbiotic control of mosquito borne disease. Pathog. Glob. Health 2012, 106, 380–385. [Google Scholar] [CrossRef]
- Eappen, A.G.; Smith, R.C.; Jacobs-Lorena, M. Enterobacter-activated mosquito immune responses to Plasmodium involve activation of SRPN6 in Anopheles stephensi. PLoS ONE 2013, 8, e62937. [Google Scholar] [CrossRef]
- Sabree, Z.L.; Kambhampati, S.; Moran, N.A. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc. Natl. Acad. Sci. USA 2009, 106, 19521–19526. [Google Scholar] [CrossRef]
- Wang, S.; Qu, S. Insect Symbionts and Their Potential Application in Pest and Vector-borne Disease Control. Bull. Chin. Acad. Sci. 2017, 32, 863–872. [Google Scholar]
- Chavshin, A.R.; Oshaghi, M.A.; Vatandoost, H.; Yakhchali, B.; Zarenejad, F.; Terenius, O. Malpighian tubules are important determinants of Pseudomonas transstadial transmission and longtime persistence in Anopheles stephensi. Parasites Vectors 2015, 8, 36. [Google Scholar] [CrossRef]
- Wang, S.; Dos-Santos, A.L.A.; Huang, W.; Liu, K.C.; Oshaghi, M.A.; Wei, G. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 2017, 357, 1399–1402. [Google Scholar] [CrossRef]
- Abdul Rahman, N.; Parks, D.H.; Willner, D.L.; Engelbrektson, A.L.; Goffredi, S.K.; Warnecke, F.; Scheffrahn, R.H.; Hugenholtz, P. A molecular survey of Australian and North American termite genera indicates that vertical inheritance is the primary force shaping termite gut microbiomes. Microbiome 2015, 3, 5. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, B.D.; Scott, J.G. Insecticide Resistance Mechanisms in the German Cockroach, Blattella germanica (L.). Mol. Mech. Insectic. Resist. 1992, 218–230. [Google Scholar] [CrossRef]
- Catalgol, B.K.; Ozden, S.; Alpertunga, B. Effects of trichlorfon on malondialdehyde and antioxidant system in human erythrocytes. Toxicol. In Vitro 2007, 21, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Ashauer, R.; Boxall, A.; Brown, C. Uptake and Elimination of Chlorpyrifos and Pentachlorophenol into the Freshwater Amphipod Gammarus pulex. Arch. Environ. Contam. Toxicol. 2006, 51, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, A.K.; Pachauri, D.C.; Jindal, T. Maximum Residue Limit and Risk Assessment of Beta-Cyfluthrin and Imidacloprid on Tomato (Lycopersicon esculentum Mill). Bull. Environ. Contam. Toxicol. 2003, 70, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y. Insecticide resistance and its underlying mechanisms in the German cockroach, Blattella germanica (Linn.) (Dictyoptera: Blattellidae). J. Biosci 1997, 8, 156–172. [Google Scholar]
- Vaishampayan, P.A.; Dhotre, D.P.; Gupta, R.P.; Lalwani, P.; Ghate, H.; Patole, M.S.; Shouche, Y.S. Molecular evidence and phylogenetic affiliations of Wolbachia in cockroaches. Mol. Phylogenet. Evol. 2007, 44, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, P.; Pérez-Cobas, A.E.; van de Pol, C.; Baixeras, J.; Moya, A.; Latorre, A. Succession of the gut microbiota in the cockroach Blattella germanica. Int. Microbiol. 2014, 17, 99–109. [Google Scholar]
- Zhang, F.; Huang, Y.H.; Liu, S.Z.; Zhang, L.; Li, B.T.; Zhao, X.X.; Fu, Y.; Liu, J.J.; Zhang, X.X. Pseudomonas reactans, a bacterial strain isolated from the intestinal flora of Blattella germanica with anti-Beauveria bassiana activity. Environ. Entomol. 2013, 42, 453–459. [Google Scholar] [CrossRef]
- Martirani, L.; Varcamonti, M.; Naclerio, G.; Felice, M.D. Purification and partial characterization of bacillocin 490, a novel bacteriocin produced by a thermophilic strain of Bacillus licheniformis. Microb. Cell Fact. 2002, 1, 1–5. [Google Scholar] [CrossRef]
- Stiles, M.E.; Holzapfel, W.H. Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 1997, 36, 1–29. [Google Scholar] [CrossRef]
- Perlman, S.J.; Hunter, M.S.; Zchori-Fein, E. The emerging diversity of Rickettsia. Proc. R. Soc. B 2006, 273, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Thaochan, N.; Drew, R.A.I.; Hughes, J.M.; Vijaysegaran, S.; Chinajariyawong, A. Alimentary tract bacteria isolated and identified with API-20E and molecular cloning techniques from Australian tropical fruit flies, Bactrocera cacuminata and B. tryoni. J. Insect Sci. 2010, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.N.; Seo, M.J.; Youn, Y.N.; Yu, Y.M. Antifungal activity against plant pathogenic fungi on insect Enterobacteriaceae. Korean J. Pestic. Sci. 2015, 19, 71–79. [Google Scholar] [CrossRef]
- Douglas, A.E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009, 23, 38–47. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wang, X.J.; Zhang, F.; Huo, X.B.; Fu, R.S.; Liu, J.J.; Sun, W.B.; Kang, D.M.; Jing, X. The identification of a bacterial strain BGI-1 isolated from the intestinal flora of Blattella germanica, and its anti-entomopathogenic fungi activity. J. Econ. Entomol. 2013, 106, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, X.X.; Zhang, X.C.; Zhang, S.; Lu, J.; Xia, Y.M.; Huang, Y.H.; Wang, X.J. The interactions between gut microbiota and entomopathogenic fungi: A potential approach for biological control of Blattella germanica (L.). Pest Manag. Sci. 2018, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Wada-Katsumata, A.; Zurek, L.; Nalyanya, G.; Roelofs, W.L.; Schal, C. Gut bacteria mediate aggregation in the German cockroach. Proc. Natl. Acad. Sci. USA 2015, 112, 15678–15683. [Google Scholar] [CrossRef]
- Liu, H. Study on the Symbiotic Bacteria Population Change and Insecticide Resistance of Blattella germannica. Master’s Thesis, Shandong Normal University, Jinan, China, 2013. [Google Scholar]
- Li, L.W.; Zhang, X.C.; Xia, J.; Zhang, S.; Zhao, D.Q.; Zhang, F. Effect of removing symbionts on the biological fitness of Blattella germanica. J. Shandong Norm. Univ. Nat. Sci. 2016, 31, 143–147. [Google Scholar]
- Zhang, F.; Yang, R. Life history and functional capacity of the microbiome are altered in beta-cypermethrin-resistant cockroaches. Int. J. Parasitol. 2019, 49, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Lou, M.F.; Shen, W.; Fu, R.S.; Wang, D.H. A maternal low-fiber diet predisposes offspring to improved metabolic phenotypes in adulthood in an herbivorous rodent. Physiol. Biochem. Zool. 2017, 90, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?-A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef]
- Jin, X.B.; Chu, F.J.; Zhu, J.Y. Molecular identification and analysis of German cockroach symbiotic bacteria Wolbachia. Chin. J. Vector Biol. Control 2008, 19, 121–122. [Google Scholar]
- Bourtzis, K.; Miller, T. Insect Symbiosis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Dobson, S.L.; Fox, C.W.; Jiggins, F.M. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc. Biol. Sci. 2002, 269, 437–445. [Google Scholar] [CrossRef]
- Sinkins, S.P. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 723–729. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Hoffman, A.A.; Werren, J.H. Influential passengers: Inherited microorganisms and arthropod reproduction. Q. Rev. Biol. 1997, 141–143. [Google Scholar]
- Berasategui, A.; Shukla, S.; Salem, H.; Kaltenpoth, M. Potential applications of insect symbionts in biotechnology. Appl. Microbiol. Biotechnol. 2016, 100, 1567–1577. [Google Scholar] [CrossRef]
- Van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, K.; Higgs, S.; O Neill, S.L. Impact of Wolbachia on infection with Chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.D.D.; Jiggins, F.M. Male-killing bacteria in insects: Mechanisms, incidence, and implications. Emerg. Infect. Dis. 2000, 6, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Hurst, G.D.D.; Hornett, E.A.; Josling, J.A.; Engelstädter, J. High incidence of the maternally inherited bacterium Cardinium in spiders. Mol. Ecol. 2008, 17, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.F.; McKemey, A.R.; Nimmo, D.; Curtis, Z.; Black, I.; Morgan, S.A.; Oviedo, M.N.; Lacroix, R.; Naish, N.; Morrison, N.I.; et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 2012, 30, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A. Entomology: Incompatible mosquitoes. Nature 2005, 436, 189. [Google Scholar] [CrossRef] [PubMed]
- Zabalou, S.; Riegler, M.; Theodorakopoulou, M.; Stauffer, C.; Savakis, C.; Bourtzis, K. Wolbachia induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. USA 2004, 101, 15042–15045. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Xi, Z.Y.; Dean, J.L.; Khoo, C.; Dobson, S.L. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem. Mol. Biol. 2005, 35, 903–910. [Google Scholar] [CrossRef]
- Xi, Z.; Khoo, C.C.H.; Dobson, S.L. Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc. R. Soc. B 2006, 273, 1317–1322. [Google Scholar] [CrossRef]
- Blagrove, M.S.C.; Arias-Goeta, C.; Failloux, A.B.; Sinkins, S.P. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl. Acad. Sci. USA 2012, 109, 255–260. [Google Scholar] [CrossRef]
- Hancock, P.A.; Sinkins, S.P.; Godfray, H.C.J. Strategies for introducing Wolbachia to reduce transmission of mosquito-borne diseases. PLoS Negl. Trop. Dis. 2011, 5, e1024. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.Q.; Robinson, A.S. The first releases of transgenic mosquitoes: An argument for the sterile insect technique. Trends Parasitol. 2003, 19, 349–355. [Google Scholar] [CrossRef]
- Tanaka, M.; Daimon, T. First molecular genetic evidence for automictic parthenogenesis in cockroaches. Insect Sci. 2019, 26, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Xian, X. Effects of mating on oviposition, and possibility of parthenogenesis of three domestic cockroach species, the American cockroach, Periplaneta americana; the Smoky brown cockroach, Periplaneta fuliginosa; and the German cockroach, Blattella germanica. Med. Entomol. Zool. 1998, 49, 27–32. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.J.; Huang, Y.H.; Zhao, Z.G.; Zhang, S.S.; Gong, X.S.; Xie, L.; Kang, D.M.; Jing, X. Differential expression of hemolymph proteins between susceptible and insecticide-resistant Blattella germanica (Blattodea: Blattellidae). Environ. Entomol. 2014, 43, 1117–1123. [Google Scholar] [CrossRef]
- Rahimian, A.A.; Hanafi-Bojd, A.A.; Vatandoost, H.; Zaim, M. A Review on the Insecticide Resistance of Three Species of Cockroaches (Blattodea: Blattidae) in Iran. J. Econ. Entomol. 2019, 112, 1–10. [Google Scholar] [CrossRef]
- Rahayu, R.; Madona, W.R.; Bestari, W.; Jannatan, R. Resistance monitoring of some commercial insecticides to German cockroach (Blattella germanica (L.) in Indonesia. J. Entomol. Zool. Stud. 2016, 4, 709–712. [Google Scholar]
- Liang, D.; McGill, J.; Pietri, J.E. Unidirectional cross-resistance in German cockroach (Blattodea: Blattellidae) populations under exposure to insecticidal baits. J. Econ. Entomol. 2017, 110, 1713–1718. [Google Scholar] [CrossRef]
- Wu, X.; Appel, A.G. Insecticide resistance of several field-collected German cockroach (Dictyoptera: Blattellidae) strains. J. Econ. Entomol. 2017, 110, 1203–1209. [Google Scholar] [CrossRef]
- Ruan, C.L.; Mi, Z.; Zhu, Y. Research progress on mechanism of insect resistance to insecticides. Sci. Ser. 2012, 38, 322–328. [Google Scholar]
- Gressel, J. Microbiome facilitated pest resistance: Potential problem and uses. Pest Manag. Sci. 2018, 74, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef]
- Dowd, P.F.; Shen, S.K. The contribution of symbiontic yeast to toxin resistance of the Cigatette beetle (Lasioderma serricome). Entomol. Exp. Appl. 1990, 56, 241–248. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.M.; Zhang, Y.F.; He, Y.P.; Chen, L.Z. Comparison for activities of detoxifying enzymes between in resistant-strains and susceptible-imidacloprid endosymbiotic strains of rice brown planthopper, Nilaparvata lugens. Acta Agric. Univ. Zhejiangensis 2010, 22, 653–659. [Google Scholar]
- Xia, X.; Sun, B.; Gurr, M.G.; Vasseur, L.; Xue, M.; You, M. Gut Microbiota Mediate Insecticide Resistance in the Diamondback Moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef]
- Adams, A.S.; Aylward, F.O.; Adams, S.M.; Erbilgin, N.; Aukema, B.H.; Currie, C.R.; Suen, G.; Raffa, K.F. Mountain pine beetles colonizing historical and naive host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Appl. Environ. Microbiol. 2013, 79, 3468–3475. [Google Scholar] [CrossRef]
- You, M.; Yue, Z.; He, W.; Yang, X.; Yang, G.; Xie, M.; Zhan, D.; Baxter, S.W.; Vasseur, L.; Gurr, G.M.; et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013, 45, 220–225. [Google Scholar] [CrossRef]
- Pietri, J.E.; Tiffany, C.; Liang, D. Disruption of the microbiota affects physiological and evolutionary aspects of insecticide resistance in the German cockroach, an important urban pest. PLoS ONE 2018, 13, e0207985. [Google Scholar] [CrossRef]
- Yuki, M.; Kuwahara, H.; Shintani, M.; Izawa, K.; Sato, T.; Starns, D.; Hongoh, Y.; Ohkuma, M. Dominant ectosymbiotic bacteria of cellulolytic protists in the termite gut also have the potential to digest lignocellulose. Environ. Microbiol. 2015, 17, 4942–4953. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Llorens, C.; Comas, J.; Guerrero, R. Gut bacterial community of the xylophagous cockroaches Cryptocercus punctulatus and Parasphaeria boleiriana. PLoS ONE 2016, 11, e0152400. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.J.; Vennard, C.T.; Buckling, A.; Charnley, A.K. Diversity of locust gut bacteria protects against pathogen invasion. Ecol. Lett. 2005, 8, 1291–1298. [Google Scholar] [CrossRef]
- Dong, Y.M.; Manfredini, F.; Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef]
- Wei, G.; Lai, Y.; Wang, G.; Chen, H.; Li, F.; Wang, S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA 2017, 114, 5994–5999. [Google Scholar] [CrossRef]
- Evans, J.D.; Lopez, D.L. Bacterial probiotics induce an immune response in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 2004, 97, 752–756. [Google Scholar] [CrossRef]
- Shokal, U.; Yadav, S.; Atri, J.; Accetta, J.; Kenney, E.; Banks, K.; Katakam, A.; Jaenike, J.; Eleftherianos, I. Effects of co-occurring Wolbachia and Spiroplasma endosymbionts on the Drosophila immune response against insect pathogenic and non-pathogenic bacteria. BMC Microbiol. 2016, 16, 16. [Google Scholar] [CrossRef]
- Kellner, R.L.L. Molecular identification of an endosymbiotic bacterium associated with pederin biosynthesis in Paederus sabaeus (Coleoptera: Staphylinidae). Insect Biochem. Mol. Biol. 2002, 32, 389–395. [Google Scholar] [CrossRef]
- Kellner, R.L.L. What is the basis of pederin polymorphism in Paederus riparius rove beetles? The endosymbiotic hypothesis. Entomol. Exp. Appl. 1999, 93, 41–49. [Google Scholar] [CrossRef]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Carrillo, L.; Audisio, M.C. Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Res. Microbiol. 2009, 160, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, D.C.; Audisio, M.C. Inhibitory activity of surfactin, produced by different Bacillus subtilis subsp. subtilis strains, against Listeria monocytogenes sensitive and bacteriocin-resistant strains. Microbiol. Res. 2013, 168, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.C.; Poulsen, M.; Currie, C.R.; Clardy, J. Dentigerumycin: A bacterial mediator of an anti-fungus symbiosis. Nat. Chem. Biol. 2009, 5, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Koga, R.; Horikawa, M.; Tsunoda, T.; Maoka, T.; Matsumoto, S.; Simon, J.; Fukatsu, T.; Fukatsu, T. Symbiotic bacterium modifies aphid body color. Science 2010, 330, 1102–1104. [Google Scholar] [CrossRef]
- Oliver, K.M.; Moran, N.A.; Hunter, M.S. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl. Acad. Sci. USA 2005, 102, 12795–12800. [Google Scholar] [CrossRef]
- Broderick, N.A.; Raffa, K.F.; Handelsman, J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Natl. Acad. Sci. USA 2006, 103, 15196–15199. [Google Scholar] [CrossRef]
- Broderick, N.A.; Robinson, C.J.; McMahon, M.D.; Holt, J.; Handelsman, J.; Raffa, K.F. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol. 2009, 7, 11. [Google Scholar] [CrossRef]
- Sharon, G.; Segal, D.; Ringo, J.M.; Hefetz, A.; Zilber-Rosenberg, L.; Rosenberg, E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2010, 107, 20051–20056. [Google Scholar] [CrossRef]
- Li, Y.J.; Su, W.Z.; Hu, K.K.; Li, P.C.; Liu, W.; Yao, H. Lactobacillus plantarum promotes the growth and development of Drosophila melanogaster. Acta Entomol. Sin. 2017, 60, 544–552. [Google Scholar]
- Dillon, R.J.; Vennard, C.T.; Charnley, A.K. Pheromones-exploitation of gut bacteria in the locust. Nature 2000, 403, 851. [Google Scholar] [CrossRef]
- Shi, W.; Guo, Y.; Xu, C.; Tan, S.; Miao, J.; Feng, Y.; Zhao, H.; St Leger, R.J.; Fang, W. Unveiling the mechanism by which microsporidian parasites prevent locust swarm behavior. Proc. Natl. Acad. Sci. USA 2014, 111, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, M.; Ueda, S.; Ishii, S. Aggregation effects on the growth of the German cockroach, Blattella germanica (L.) (Blattaria: Blattellidae). Appl. Entomol. Zool. 1970, 5, 159–171. [Google Scholar] [CrossRef]
- Krause, J.; Ruxton, G.D. Living in Groups; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Uzsák, A.; Schal, C. Social interaction facilitates reproduction in male German cockroaches, Blattella germanica. Anim. Behav. 2013, 85, 1501–1509. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Li, Z.L.; Zheng, W.W. The application of Hansenula anomala in attractant preparation of citrus flies. In CN102754665A; Kaiyuan Intellectual Property Agency Co., LTD: Wuhan, China, 2012. [Google Scholar]
- Hurwitz, I.; Fieck, A.; Read, A.; Hillesland, H.; Klein, N.; Kang, A.; Durvasula, R. Paratransgenic control of vector borne diseases. Int. J. Biol. Sci. 2011, 7, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ghosh, A.K.; Bongio, N.; Stebbings, K.A.; Lampe, D.J.; Jacobslorena, M. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl. Acad. Sci. USA 2012, 109, 12734–12739. [Google Scholar] [CrossRef]
- Wang, S.; Jacobs-Lorena, M. Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol. 2013, 31, 185–193. [Google Scholar] [CrossRef]
- Durvasula, R.V.; Gumbs, A.; Panackal, A.; Kruglov, O.; Taneja, J.; Kang, A.S.; Cordon-Rosales, C.; Richards, F.F.; Whitham, R.G.; Beard, C.B. Expression of a functional antibody fragment in the gut of Rhodnius prolixus via transgenic bacterial symbiont Rhodococcus rhodnii. Med. Vet. Entomol. 1999, 13, 115–119. [Google Scholar] [CrossRef]
- Kuzina, L.V.; Miller, E.D.; Ge, B.; Miller, T.A. Transformation of Enterobacter gergoviae isolated from pink bollworm (Lepidoptera: Gelechiidae) gut with Bacillus thuringiensis toxin. Curr. Microbiol. 2002, 44, 1–4. [Google Scholar] [CrossRef]
- Keller, R.; Pedroso, M.Z.; Ritchmann, R.; Silva, R.M. Occurrence of virulence-associated properties in Enterobacter cloacae. Infect. Immun. 1998, 66, 645–649. [Google Scholar] [CrossRef]
- Akbari, S.; Oshaghi, M.A.; Hashemi-Aghdam, S.S.; Hajikhani, S.; Oshaghi, G.; Shirazi, M.H. Aerobic Bacterial Community of American Cockroach Periplaneta americana, a Step toward Finding Suitable Paratransgenesis Candidates. J. Arthropod Borne Dis. 2014, 9, 35–48. [Google Scholar]
- Watanabe, K.; Abe, K.; Sato, M. Biological control of an insect pest by gutcolonizing Enterobacter cloacae transformed with ice nucleation gene. J. Appl. Microbiol. 2000, 88, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.F.; Simpson, S.J.; Douglas, A.E. The Insects: Structure and Function, 5th ed.; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Kopanic, R.J.; Holbrook, G.L.; Sevala, V.; Schal, C. An adaptive benefit of facultative coprophagy in the German cockroach Blattella germanica. Ecol. Entomol. 2001, 26, 154–162. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Wang, X.; Zhang, F. New Insights into Cockroach Control: Using Functional Diversity of Blattella germanica Symbionts. Insects 2020, 11, 696. https://doi.org/10.3390/insects11100696
Pan X, Wang X, Zhang F. New Insights into Cockroach Control: Using Functional Diversity of Blattella germanica Symbionts. Insects. 2020; 11(10):696. https://doi.org/10.3390/insects11100696
Chicago/Turabian StylePan, Xiaoyuan, Xuejun Wang, and Fan Zhang. 2020. "New Insights into Cockroach Control: Using Functional Diversity of Blattella germanica Symbionts" Insects 11, no. 10: 696. https://doi.org/10.3390/insects11100696
APA StylePan, X., Wang, X., & Zhang, F. (2020). New Insights into Cockroach Control: Using Functional Diversity of Blattella germanica Symbionts. Insects, 11(10), 696. https://doi.org/10.3390/insects11100696





