First Description of the Adult Male of the Gall-Like Scale Insect Allokermes galliformis (Riley) (Hemiptera: Coccomorpha: Kermesidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Slide Mounting
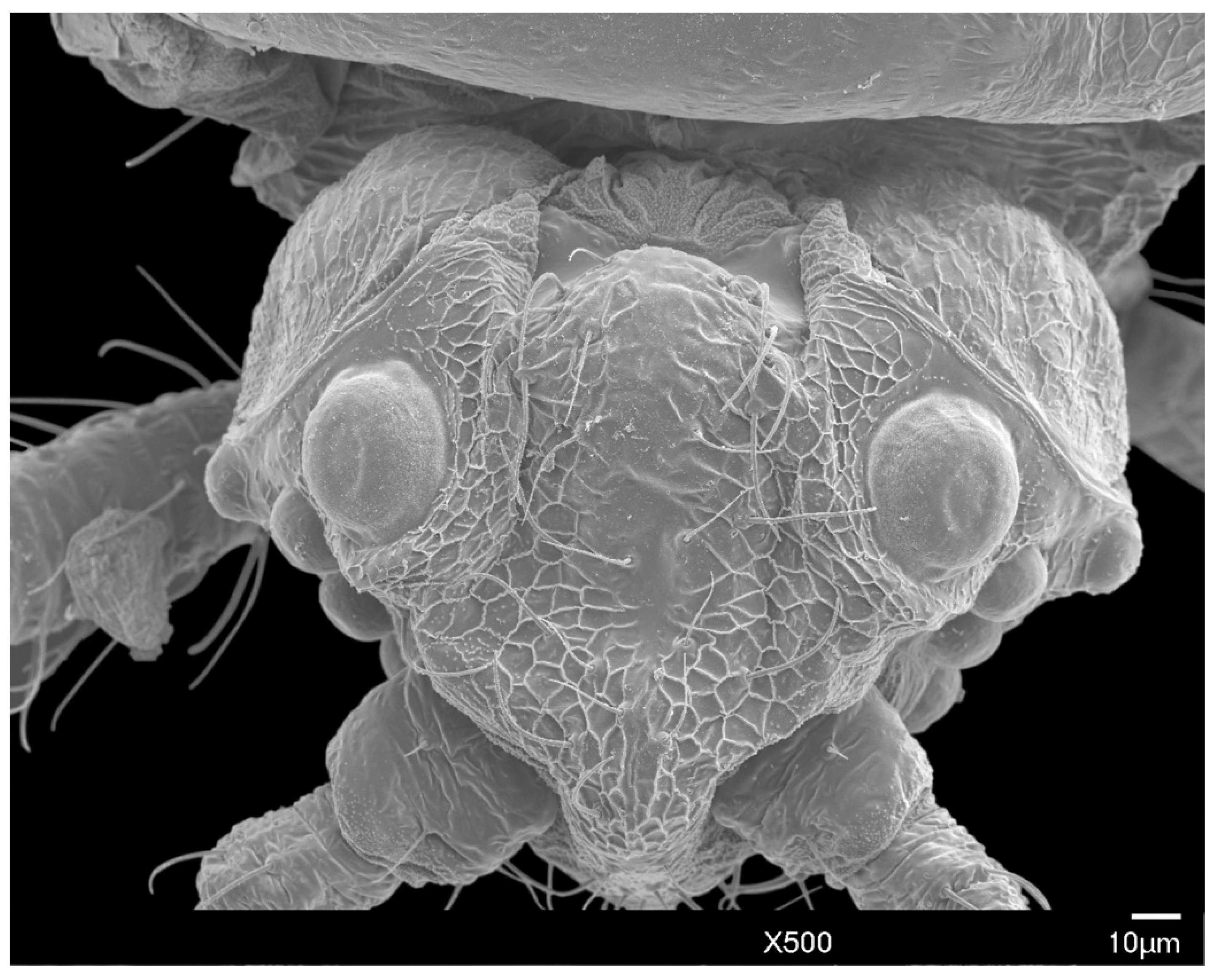
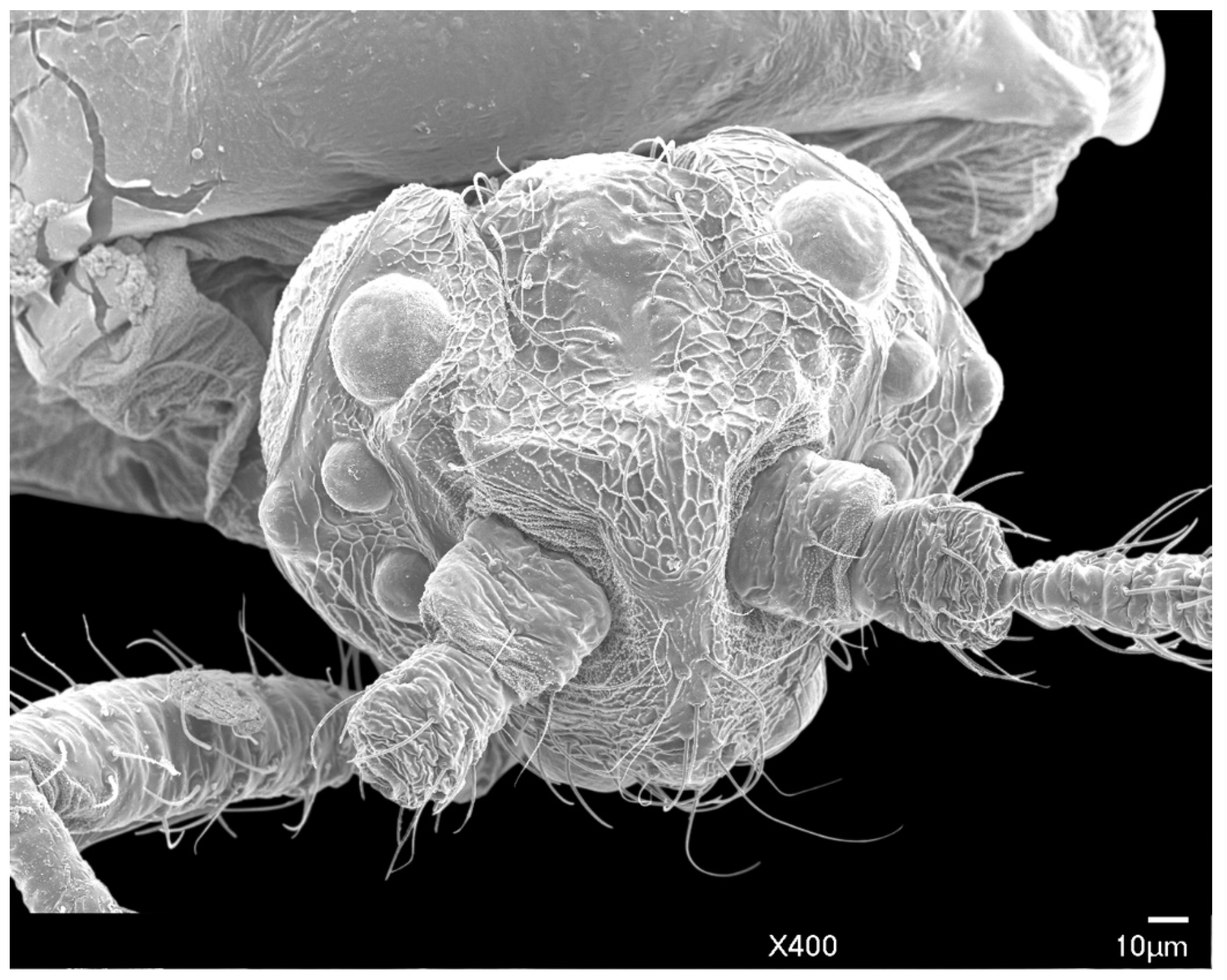
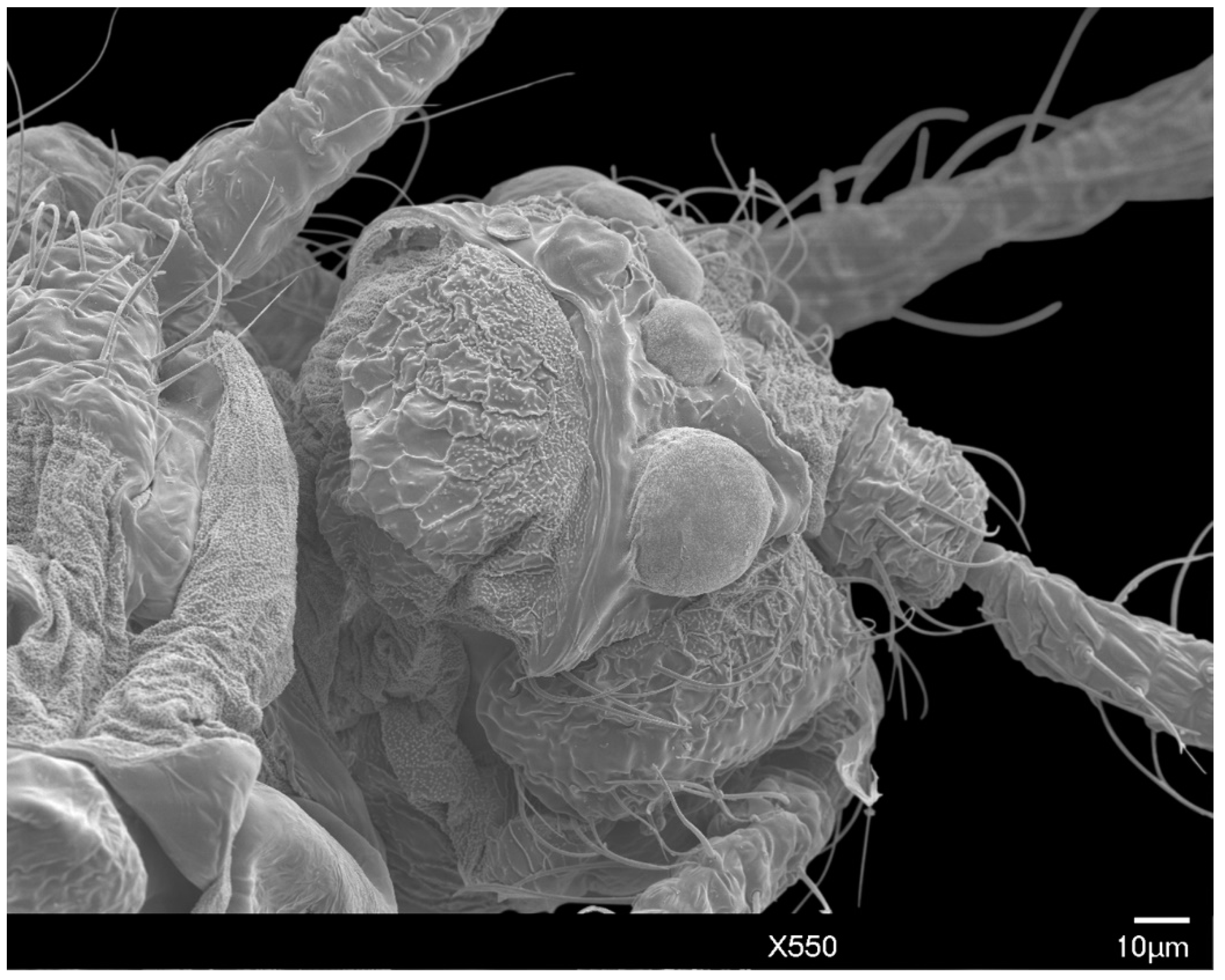
2.3. Scanning Electron Microscopy
3. Results
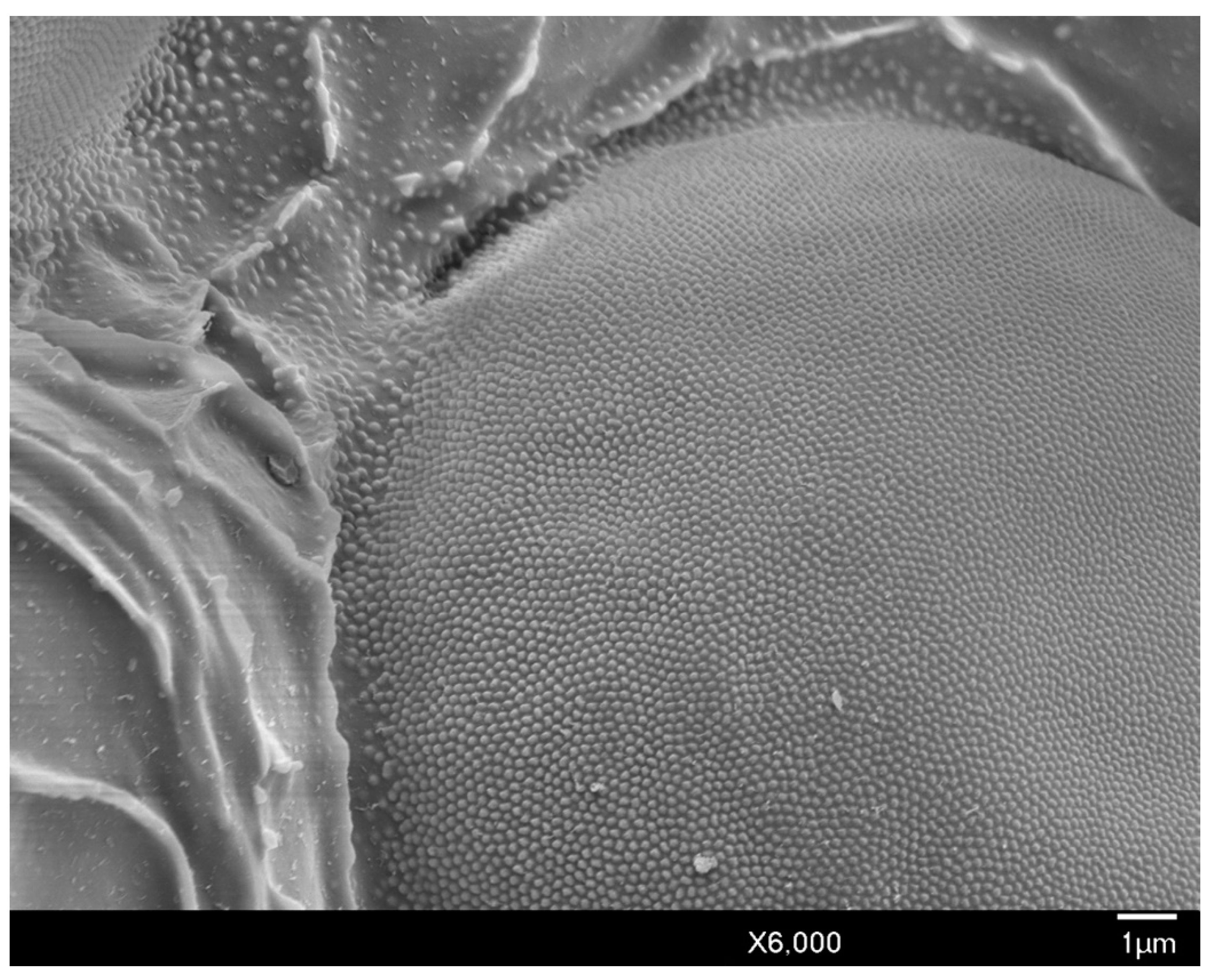
3.1. Description of the Adult Male of Allokermes galliformis (Riley, 1881)
3.1.1. Head
3.1.2. Body Setae and Pores
3.1.3. Thorax
3.1.4. Abdomen
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kosztarab, M. Scale Insects of Northeastern North America: Identification, Biology, and Distribution; Virginia Museum of Natural History: Martinsville, VA, USA, 1996; 650p. [Google Scholar]
- Spodek, M.; Ben-Dov, Y. A taxonomic revision of the Kermesidae (Hemiptera: Coccoidea) in Israel, with a description of a new species. Zootaxa 2014, 3781, 1–99. [Google Scholar] [CrossRef] [PubMed]
- Gullan, P.J.; Cook, L.G. Phylogeny and higher classification of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Zootaxa 2007, 1668, 413–425. [Google Scholar]
- García Morales, M.; Denno, B.D.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N.B. ScaleNet: A literature-based model of scale insect biology and systematics. Database 2016. Available online: http://scalenet.info (accessed on 8 July 2018). [CrossRef]
- Gullan, P.J.; Kosztarab, M. Adaptations in scale insects. Ann. Rev. Entomol. 1997, 42, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Sirisena, U.G.A.I.; Watson, G.W.; Hemachandra, K.S.; Wijayagunasekara, H.N.P. A modified technique for the preparation of specimens of Sternorrhyncha for taxonomic studies. Trop. Agric. Res. 2013, 24, 139–149. [Google Scholar]
- Hodgson, C.J.; Hardy, N.B. The phylogeny of the superfamily Coccoidea (Hemiptera: Sternorrhyncha) based on the morphology of extant and extinct macropterous males. Syst. Entomol. 2013, 38, 794–804. [Google Scholar] [CrossRef]
- Riley, C.V. A new species of oak coccid mistaken for a gall. Am. Nat. 1881, 15, 482. [Google Scholar]
- Baer, R.G.; Kosztarab, M. A morphological and systematic study of the first and second instars of the family Kermesidae in the Nearctic region (Homoptera: Coccidea). Va. Polytech. Inst. State Univ. Bull. 1985, 85, 119–261. [Google Scholar]
- Bullington, S.; Kosztarab, M. Revision of the family Kermesidae (Homoptera) in the Nearctic Region based on adult and third instar females. Va. Polytech. Inst. State Univ. Bull. 1985, 85–11, 1–118. [Google Scholar]
- Sitz, R.A.; Cranshaw, W.S. Life history of Allokermes galliformis (Hemiptera: Kermesidae) in Colorado. Ann. Entomol. Soc. Am. 2018, 111, 265–270. [Google Scholar] [CrossRef]
- Sitz, R.A.; Zerillo, M.M.; Snelling, J.; Caballero, J.I.; Alexander, K.; Nash, K.; Tisserat, N.A.; Cranshaw, W.S.; Stewart, J.E. Drippy blight, a disease of red oaks in Colorado produced from the combined effect of the scale insect Allokermes galliformis and the bacterium Lonsdalea quercina subsp. quercina. Arboric. Urban For. 2018, 44, 146–153. [Google Scholar]
- Sitz, R.A.; Aquino, V.; Tisserat, N.A.; Cranshaw, W.S.; Stewart, J.E. Insects visiting drippy blight diseased red oak trees are contaminated with the pathogenic bacterium Lonsdalea quercina. Plant Dis. 2019. accepted. [Google Scholar] [CrossRef] [PubMed]
- Cockerell, T.D.A. XXXVII—New North-American insects. J. Nat. Hist. 1898, 2, 321–331. [Google Scholar] [CrossRef][Green Version]
- Hamon, A.B.; Lambdin, P.L.; Kosztarab, M. Life history and morphology of Kermes kingii in Virginia. Va. Polytech. Inst. State Univ. Bull. 1976, 111, 1–31. [Google Scholar]
- Afini, S.A.; Kosztarab, M. Morphological and systematic studies on the adult males of some species of Lecanodiaspis (Homoptera: Coccoidea: Lecanodiaspididae). Va. Polytech. Inst. State Univ. Bull. 1969, 36, 1–23. [Google Scholar]
- Afini, S.A.M. Systematic status of the family Conchaspididae, based on the males of Conchaspis lata Hempel. (Homoptera: Coccoidea). Va. Polytech. Inst. State Univ. Bull. 1969, 36, 25–37. [Google Scholar]
- Sternlicht, M. Kermes bytinskii n. spec. (Coccoidea, Kermesidae) in Israel and observations on its life history. Isr. J. Entomol. 1969, 4, 251–270. [Google Scholar]
- Buschbeck, E.K.; Hauser, M. The visual system of male scale insects. Naturwissenschaften 2009, 96, 365–374. [Google Scholar] [CrossRef]
- Balachowsky, A. Sur les Kermes Boitard (Hom: Coccoidea) des Chenes du Bassin Oriental de la Méditerranée. Revue de Pathologie Végétale et d’Entomologie Agricole de France 1953, 32, 181–189. (In French) [Google Scholar]
- Bodenheimr, F.S. Zur Kenntnis de paläartkischen Kermes-arten (Rhy. Cocc.). Konowia 1931, 10, 241–247. [Google Scholar]
- Cook, L.G.; Gullan, P.J.; Trueman, H.E. A preliminary phylogeny of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea) based on nuclear small-subunit ribosomal DNA. Mol. Phylogenet. Evol. 2002, 25, 43–52. [Google Scholar] [CrossRef]
- Marcus, J.M. Our love-hate relationship with DNA barcodes, the Y2K problem, and the search for next generation barcodes. AIMS Genet. 2018, 5, 1–23. [Google Scholar] [CrossRef]
- Miller, D.R.; Miller, G.L. Description of a new genus of scale insect with a discussion of relationships among families related to the Kermesidae (Homoptera: Coccoidea). Syst. Entomol. 1993, 18, 237–251. [Google Scholar] [CrossRef]
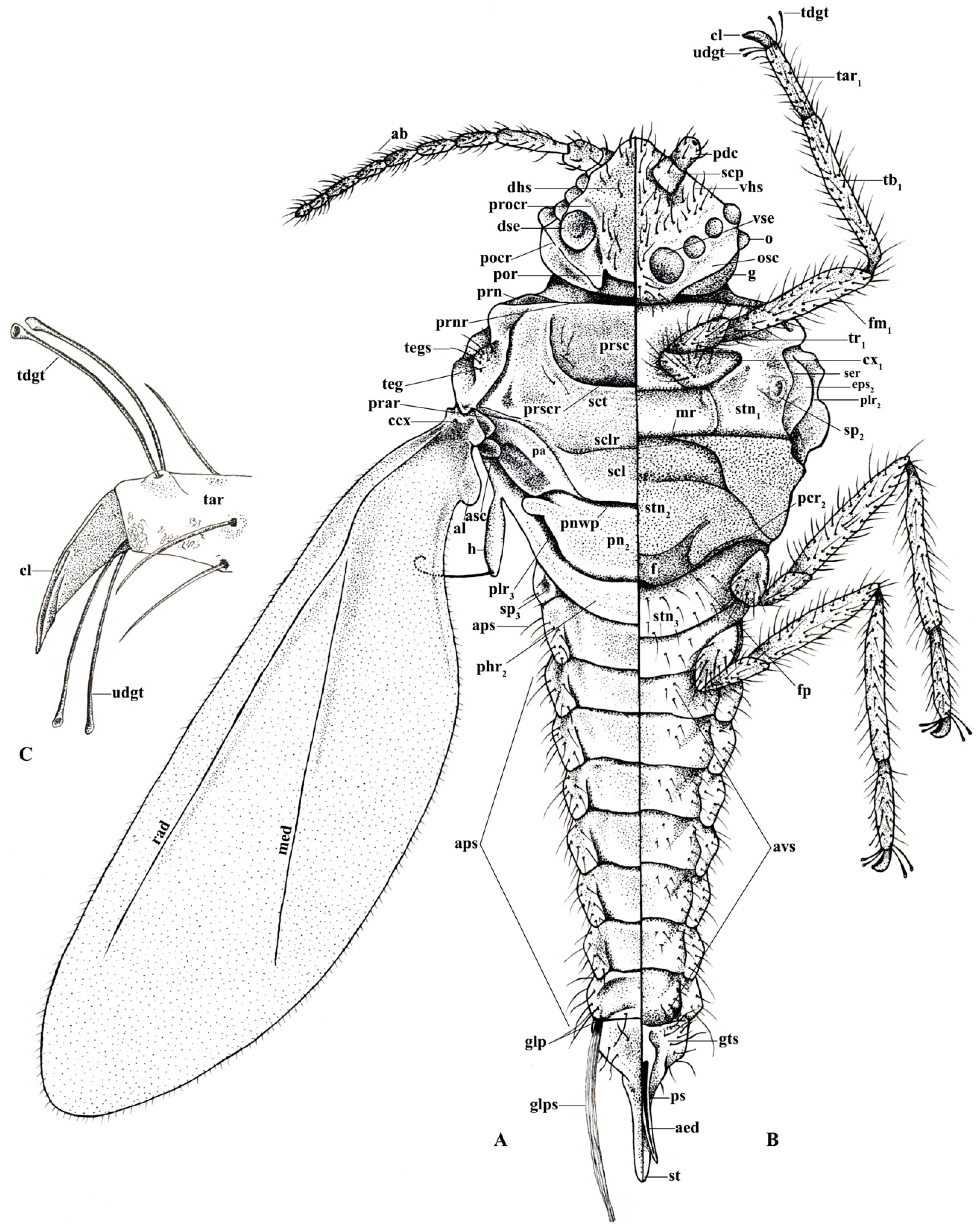






| aed | aedeagus | med | media | ps | penile sheath |
| al | alar lobe | mr | marginal ridge | rad | radius |
| aps | abdominal pleural setae | o | ocellus | scp | scape |
| al | alar lobe | osc | ocular sclerite | scl | scutellum |
| asc | additional sclerite | pa | postalar | sclr | scutellar ridge |
| avs | abdominal ventral setae | pcr2 | precoxal ridge | sct | scutum |
| ccx | costal complex of wing veins | pdc | pedicel | ser | subepisternal ridge |
| cl | claw | phr2 | mesopostphragma | sp | thoracic spiracles (sp2–3) |
| cx | coxa (cx1–3) | plr2 | mesopleural ridge | st | style |
| dhs | dorsal head setae | plr3 | metapleural ridge | stn | sternum (stn1–3) |
| dse | dorsal simple eyes | pn2 | mesopostnotum | tar | tarsus (tar1–3) |
| eps | episternum (eps2–3) | pnwp | posterior notal wing process | tb | tibia (tb1–3) |
| f | furca | pocr | postocular ridge | tdgt | tarsal digitules |
| fm | femur (fm1–3) | por | postoccipital ridge | teg | tegula |
| fp | furcal pit | prar | prealar ridge | tegs | tegular setae |
| g | genae | prn | lateral pronotal sclerite | tr | trochanter (tr1–3) |
| glp | glandular pouch | prnr | pronotal ridge | ugdt | ungual digitules |
| glps | setae of the glandular pouch | procr | preocular ridge | vhs | ventral head setae |
| gts | setae of genital segment | prsc | prescutum | vse | ventral simple eyes |
| h | hamulohaltera | prscr | prescutal ridge |
| Segments | Scape (scp) | Pedicel (pdc) | III | IV | V | VI | VII | VIII | IX | X |
|---|---|---|---|---|---|---|---|---|---|---|
| Length range | 32–55 | 46–53 | 95–107 | 57–82 | 32–67 | 55–65 | 42–69 | 40–55 | 38–53 | 36–42 |
| Length mean | (41) | (49) | (100) | (64) | (53) | (59) | (59) | (47) | (44) | (39) |
| Width range | 38–44 | 32–32 | 19–23 | 19–23 | 17–21 | 19–21 | 19–23 | 19–25 | 11–25 | 19–21 |
| Width mean | (41) | (32) | (21) | (21) | (20) | (20) | (21) | (22) | (22) | (20) |
| Leg | Coxa (cx) | Trochanter (tr) | Femur (fm) | Tibia (tb) | Tarsus (tar) | Claw (cl) | Total |
|---|---|---|---|---|---|---|---|
| Prothoracic length width | 84–88 (85) 44–50 (46) | 46–53 (50) 23–29 (26) | 160–181 (174) 29–38 (33) | 160–181 (170) 17–21 (19) | 74–92 (82) 15–19 (17) | 17–25 (22) | 548–626 (591) |
| Mesothoracic length width | 78–84 (81) 42–46 (43) | 50–53 (51) 23–27 (25) | 155–185 (170) 29–34 (32) | 168–181 (174) 17–21 (20) | 69–86 (81) 16–21 (19) | 19–25 (22) | 540–613 (579) |
| Metathoracic length width | 55–65 (59) 38–44 (41) | 50–57 (53) 23–29 (25) | 126–147 (137) 29–34 (32) | 160–181 (169) 19–25 (22) | 74–82 (80) 17–21 (19) | 21–25 (24) | 485–557 (520) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krutil, K.D.; Hall, A.L.; Cranshaw, W.S.; Kondratieff, B.C.; Sitz, R.A. First Description of the Adult Male of the Gall-Like Scale Insect Allokermes galliformis (Riley) (Hemiptera: Coccomorpha: Kermesidae). Insects 2019, 10, 250. https://doi.org/10.3390/insects10080250
Krutil KD, Hall AL, Cranshaw WS, Kondratieff BC, Sitz RA. First Description of the Adult Male of the Gall-Like Scale Insect Allokermes galliformis (Riley) (Hemiptera: Coccomorpha: Kermesidae). Insects. 2019; 10(8):250. https://doi.org/10.3390/insects10080250
Chicago/Turabian StyleKrutil, Kyle D., Alison L. Hall, Whitney S. Cranshaw, Boris C. Kondratieff, and Rachael A. Sitz. 2019. "First Description of the Adult Male of the Gall-Like Scale Insect Allokermes galliformis (Riley) (Hemiptera: Coccomorpha: Kermesidae)" Insects 10, no. 8: 250. https://doi.org/10.3390/insects10080250
APA StyleKrutil, K. D., Hall, A. L., Cranshaw, W. S., Kondratieff, B. C., & Sitz, R. A. (2019). First Description of the Adult Male of the Gall-Like Scale Insect Allokermes galliformis (Riley) (Hemiptera: Coccomorpha: Kermesidae). Insects, 10(8), 250. https://doi.org/10.3390/insects10080250





