Halyomorpha halys (Hemiptera: Pentatomidae) Genetic Diversity in North America and Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Material
2.2. DNA Isolation, Amplification, and Sequencing
2.3. Phylogenetic Analysis
3. Results
3.1. Analysis of Cytochrome Oxidase I Gene
3.2. Analysis of Internal Transcribed Spacer 1
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Josifov, M.V.; Kerzhner, I.M. Heteroptera aus Korea: II. Teil (Aradidae, Berytidae, Lygaeidae, Pyrrhocoridae, Rhopalidae, Alydidae, Coreidae, Urostylidae, Acanthosomatidae, Scutelleridae, Pentatomidae, Cydnidae, Plataspidae. Fragm. Faun. 1978, 23, 137–196. [Google Scholar] [CrossRef]
- Rider, D.A.; Zheng, L.Y.; Kerzhner, I.M. Checklist and nomenclatural notes on the Chinese Pentatomidae (Heteroptera). II. Pentatominae. Zoosyst. Ross. 2002, 11, 135–153. [Google Scholar]
- Lee, D.-H.; Short, B.D.; Joseph, S.V.; Bergh, J.C.; Leskey, T.C.; Adachi, I.; Uchino, K.; Mochizuki, F.; Arakawa, R.; Namura, Y.; et al. Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 2013, 42, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Hoebeke, E.R.; Carter, M.E. Halyomorpha halys (Stal) (Heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in North America. Proc. Entomol. Soc. Wash. 2003, 105, 225–237. [Google Scholar]
- Fogain, R.; Graff, S. First records of the invasive pest, Halyomorpha halys (Hemiptera: Pentatomidae), in Ontario and Quebec. J. Entomol. Soc. Ont. 2011, 142, 45–48. [Google Scholar]
- Cesari, M.; Maistrello, L.; Ganzerli, F.; Dioli, P.; Rebecchi, L.; Guidetti, R. A pest alien invasion in progress: Potential pathways of origin of the brown marmorated stink bug Halyomorpha halys populations in Italy. J. Pest Sci. 2015, 88, 1–7. [Google Scholar] [CrossRef]
- Milonas, P.G.; Partsinevelos, G.K. First report of brown marmorated stink bug Halyomorpha halys Stål (Hemiptera: Pentatomidae) in Greece. EPPO Bull. 2014, 44, 183–186. [Google Scholar] [CrossRef]
- Seat, J. Halyomorpha halys (Stal, 1855) (Heteroptera: Pentatomidae) a new invasive species in Serbia. Acta Entomol. Serb. 2015, 20, 167–171. [Google Scholar]
- Dioli, P.; Leo, P.; Maistrello, L. Prime segnalazioni in Spagna e in Sardegna della specie aliena Halyomorpha halys (Stal, 1855) e note sulla sua distribuzione in Europa (Hemiptera, Pentatomidae). Rev. Gaditana Entomol. 2016, 7, 539–548. [Google Scholar]
- Simov, N. The invasive brown marmorated stink bug Halyomorpha halys (Stal 1855 (Heteroptera: Pentatomidae) already in Bulgaria. Ecol. Montenegrina 2016, 9, 51–53. [Google Scholar]
- Maistrello, L.; Dioli, P.; Bariselli, M.; Mazzoli, G.L.; Giacalone-Forini, I. Citizen science and early detection of invasive species: Phenology of first occurrences of Halyomorpha halys in Southern Europe. Biol. Invasions 2016, 18, 3109–3116. [Google Scholar] [CrossRef]
- Wermelinger, B.; Wyniger, D.; Forster, B. First records of an invasive bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Bull. Soc. Entomol. Suisse 2008, 81, 1–8. [Google Scholar]
- Vétek, G.; Papp, V.; Haltrich, A.; Rédei, D. First record of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae), in Hungary, with description of the genitalia of both sexes. Zootaxa 2014, 3780, 194–200. [Google Scholar] [CrossRef]
- Gariepy, T.D.; Haye, T.; Fraser, H.; Zhang, J. Occurrence, genetic diversity, and potential pathways of entry of Halyomorpha halys in newly invaded areas of Canada and Switzerland. J. Pest Sci. 2014, 87, 17–28. [Google Scholar] [CrossRef]
- Arnold, K. Halyomorpha halys (Stal, 1855), Eine fur die europaische Fauna neu nachgewiesene Wanzenart (Insecta: Heteroptera: Pentatomidae: Cappaeini). Mitteillungen Thuring. Entomol. 2009, 16, 19. [Google Scholar]
- Heckmann, R. Erster nachweis von Halyomorpha halys (Stål, 1855)(Heteroptera: Pentatomidae) für deutschland. Heteropteron 2012, 36, 17–18. [Google Scholar]
- Callot, H.; Brua, C. Halyomorpha halys (Stal, 1855), la Punaise diabolique, nouvelle espece pour la faune de France (Heteroptera Pentatomidae). L’Entomologiste 2013, 69, 69–71. [Google Scholar]
- Hemala, V.; Kment, P. First record of Halyomorpha halys and mass occurrence of Nezara viridula in Slovakia. Plant Prot. Sci. 2017, 53, 1–7. [Google Scholar]
- Macavei, L.; Baetan, R.; Oltean, I.; Florean, T.; Varga, M.; Costi, E.; Maistrello, L. First detection of Halyomorpha halys, a new invasive species with a high potential of damage on agricultural crops in Romania. Lucr. Stiint. Ser. Agron. 2015, 58, 105–108. [Google Scholar]
- Gapon, D.A. First records of the brown marmorated stink bug, Halyomorpha halys (Stal, 1855) (Heteroptera: Pentatomidae) in Russia, Abkhazia, and Georgia. Entomol. Rev. 2016, 96, 1086–1088. [Google Scholar] [CrossRef]
- Faundez, E.I.; Rider, D.A. The brown marmorated stink bug Halyomorpha halys (Stal, 1855) (Heteroptera: Pentatomidae) in Chile. Arq. Entomol. 2017, 17, 305–307. [Google Scholar]
- Kriticos, D.J.; Kean, J.M.; Phillips, C.B.; Senay, S.D.; Acosta, H.; Haye, T. The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity. J. Pest Sci. 2017, 90, 1033–1043. [Google Scholar] [CrossRef]
- Lo, P.L.; Walker, J.T.S.; Rogers, D.J. Risks to pest management in New Zealand’s pipfruit Integrated Fruit Production programme. N. Z. Plant Prot. 2015, 68, 306–312. [Google Scholar]
- Duthie, C.; Michael, T.; Stephenson, B.; Yamoah, E.; McDonald, B. Risk Analysis of Halyomorpha halys (Brown Marmorated Stink Bug) on All Pathways; Biosecurity Risk Analysis Group, Ministry of Agriculture and Forestry of New Zealand: Wellington, New Zealand, 2012.
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, T.D.; Bruin, A.; Haye, T.; Milonas, P.; Vétek, G. Occurrence and genetic diversity of new populations of Halyomorpha halys in Europe. J. Pest Sci. 2015, 88, 451–460. [Google Scholar] [CrossRef]
- Cesari, M.; Maistrello, L.; Piemontese, L.; Bonini, R.; Dioli, P.; Lee, W.; Park, C.-G.; Partsinevels, G.K.; Rebecchi, L.; Guidetti, R. Genetic diversity of the brown marmorated stink bug Halyomorpha halys in the invaded territories of Europe and its patterns of diffusion in Italy. Biol. Invasions 2017, 20, 1073–1092. [Google Scholar] [CrossRef]
- Xu, J.; Fonseca, D.M.; Hamilton, G.C.; Hoelmer, K.A.; Nielsen, A.L. Tracing the origin of US brown marmorated stink bugs, Halyomorpha halys. Biol. Invasions 2014, 16, 153–166. [Google Scholar] [CrossRef]
- Zhu, G.-P.; Ye, Z.; Du, J.; Zhang, D.-L.; Zhen, Y.; Zheng, C.; Zhao, L.; Li, M.; Bu, W.-J. Range wide molecular data and niche modeling revealed the Pleistocene history of a global invader (Halyomorpha halys). Sci. Rep. 2016, 6, 23192. [Google Scholar] [CrossRef]
- Kurata, A.; Fujiwara, A.; Haruyama, N.; Tsuchida, T. Multiplex PCR method for rapid identification of genetic group and symbiont infection status in Bemisia tabaci (Hemiptera: Aleyrodidae). Appl. Entomol. Zool. 2016, 51, 167–172. [Google Scholar] [CrossRef]
- Kumar, L.S.; Shankar, P.; Kulkarni, V.M. Analyses of the internal transcribed rDNA spacers (ITS1 and ITS2) of Indian weevils of Odoiporus longicollis (Olivier) reveal gene flow between locations. Int. J. Trop. Insect Sci. 2018, 38, 313–329. [Google Scholar] [CrossRef]
- Allio, R.; Donega, S.; Galtier, N.; Nabholz, B. Large variation in the ratio of mitochondrial to nuclear mutation rate across animals: Implications for genetic diversity and the use of mitochondrial DNA as a molecular marker. Mol. Biol. Evol. 2017, 34, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Herbertetal_2004. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Valentin, R.E.; Maslo, B.; Lockwood, J.L.; Pote, J.; Fonseca, D.M. Real-time PCR assay to detect brown marmorated stink bug, Halyomorpha halys (Stål), in environmental DNA. Pest Manag. Sci. 2016, 72, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Milonas, P.; Kapantaidaki, D.E.; Cesari, M.; Di Bella, E.; Guidetti, R.; Haye, T.; Maistrello, L.; Moraglio, S.T.; Piemontese, L.; et al. Attraction of Halyomorpha halys (Hemiptera: Pentatomidae) haplotypes in North America and Europe to baited traps. Sci. Rep. 2017, 7, 16941. [Google Scholar] [CrossRef] [PubMed]
- Milligan BG Total DNA Isolation. Molecular Genetic Analysis of Population: A Practical Approach; Hoelzel, A.R., Ed.; Oxford University Press: Oxford, UK; New York, NY, USA; Tokyo, Japan, 1998; pp. 29–64. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Von der Schulenburg, J.H.G.; Hancock, J.M.; Pagnamenta, A.; Sloggett, J.J.; Majerus, M.E.N.; Hurst, G.D.D. Extreme Length and Length Variation in the First Ribosomal Internal Transcribed Spacer of Ladybird Beetles (Coleoptera: Coccinellidae). Mol. Biol. Evol. 2001, 18, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Remigio, E.A.; Blair, D. Relationships among problematic North American stagnicoline snails (Pulmonata: Lymnaeidae) reinvestigated using nuclear ribosomal DNA internal transcribed spacer sequences. Can. J. Zool. 2008, 75, 1540–1545. [Google Scholar] [CrossRef]
- Geneious | Bioinformatics Software for Molecular Sequence Data Analysis. Available online: https://www.geneious.com/ (accessed on 4 April 2019).
- Lee, W.; Kang, J.; Jung, C.; Hoelmer, K.; Lee, S.H.; Lee, S. Complete mitochondrial genome of brown marmorated stink bug Halyomorpha halys (Hemiptera: Pentatomidae), and phylogenetic relationships of hemipteran suborders. Mol. Cells 2009, 28, 155–165. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 36, 1547–1549. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Valentin, R.E.; Nielsen, A.L.; Wiman, N.G.; Lee, D.-H.; Fonseca, D.M. Global invasion network of the brown marmorated stink bug, Halyomorpha halys. Sci. Rep. 2017, 7, 9866. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Guidetti, R.; Cesari, M.; Gariepy, T.D.; Park, Y.-L.; Park, C.-G. Genetic Diversity of Halyomorpha halys (Hemiptera, Pentatomidae) in Korea and Comparison with COI Sequence Datasets from East Asia, Europe, and North America. Florida Entomol. 2018, 101, 49–54. [Google Scholar] [CrossRef]
- Kerdelhué, C.; Boivin, T.; Burban, C. Contrasted invasion processes imprint the genetic structure of an invasive scale insect across southern Europe. Heredity 2014, 113, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Murata, K.; Matsuura, A. Rapid evolution of an introduced insect Ophraella communa LeSage in new environments: Temporal changes and geographical differences in photoperiodic response. Entomol. Sci. 2015, 18, 104–112. [Google Scholar] [CrossRef]
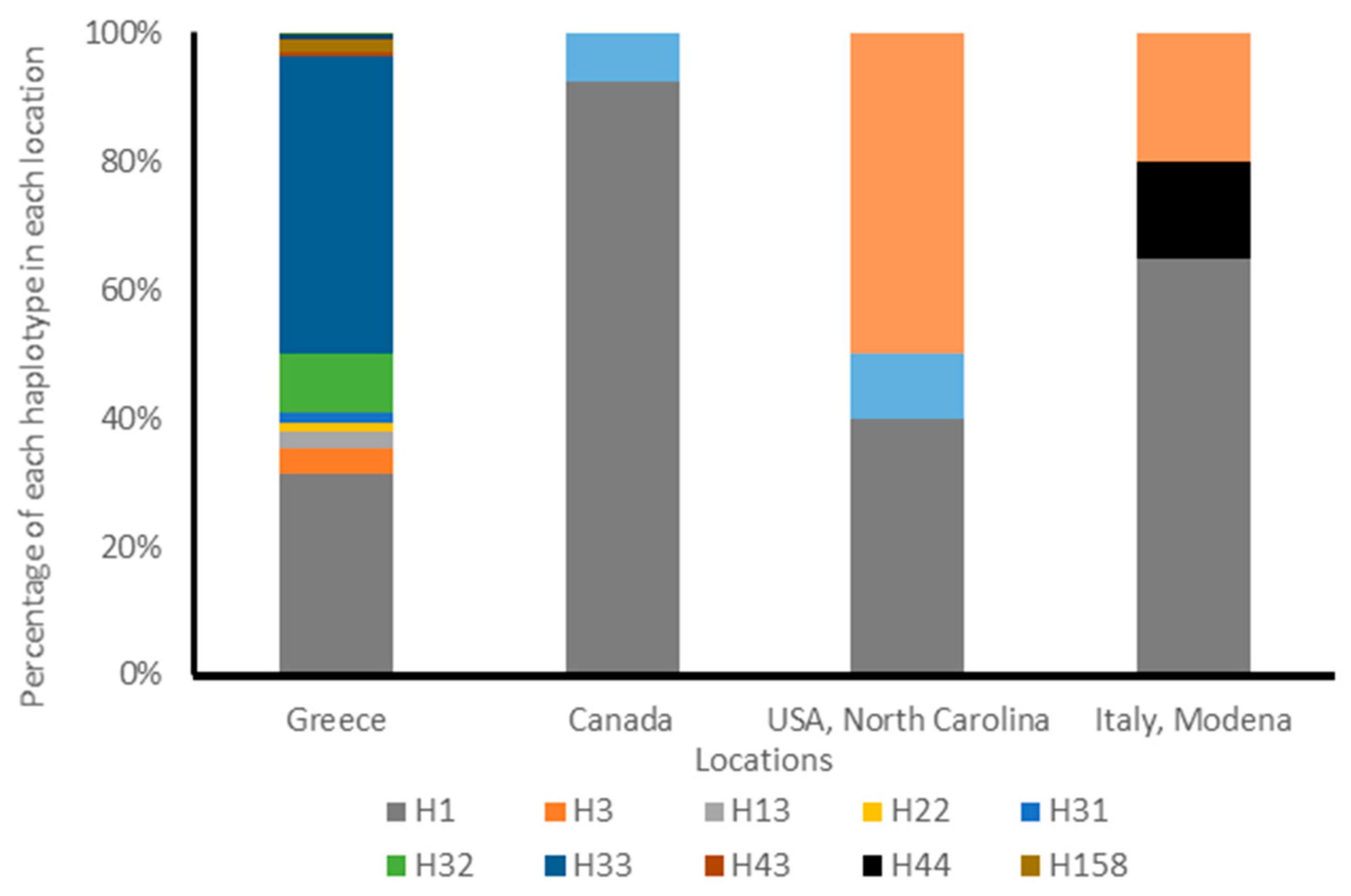
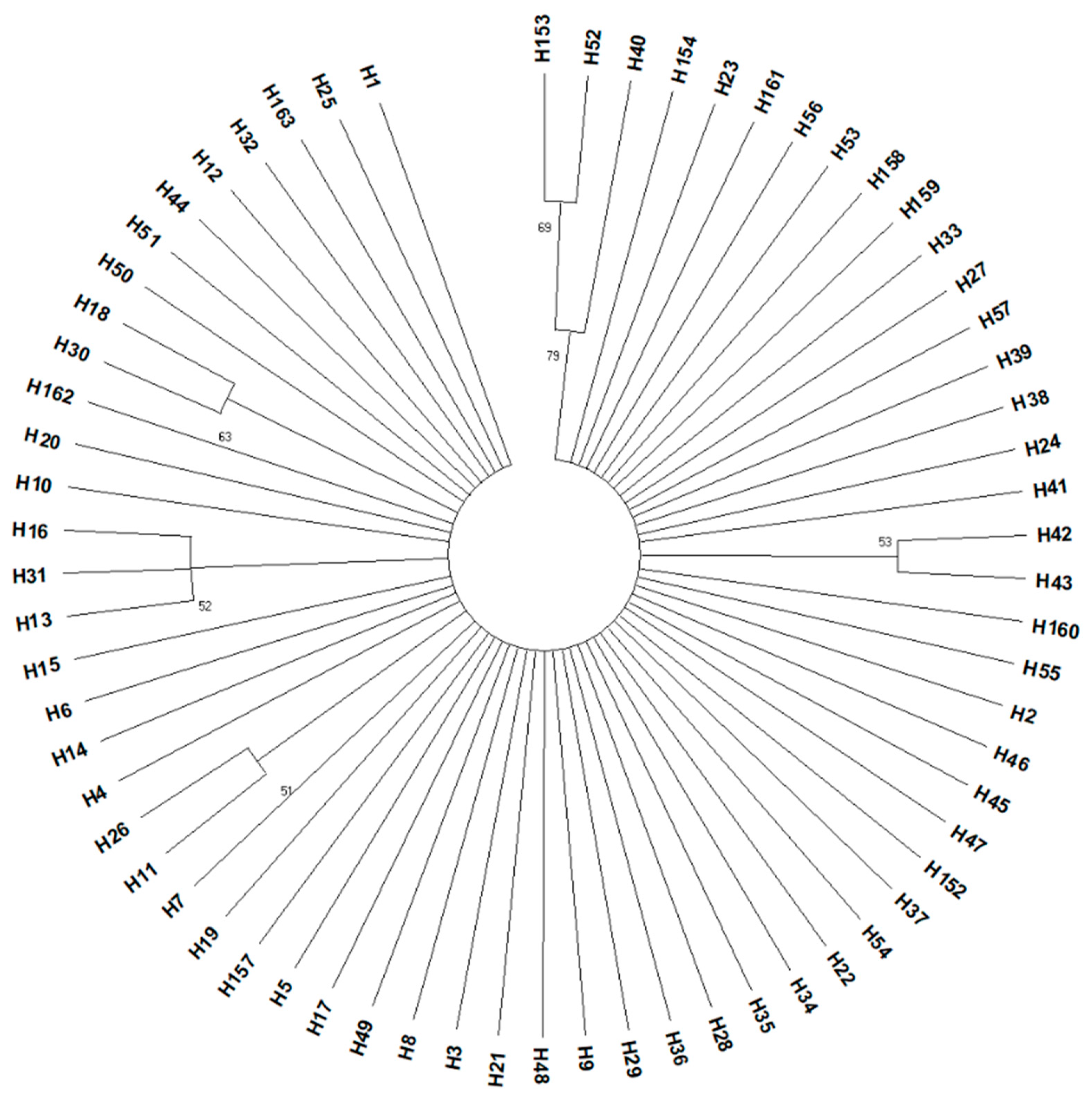
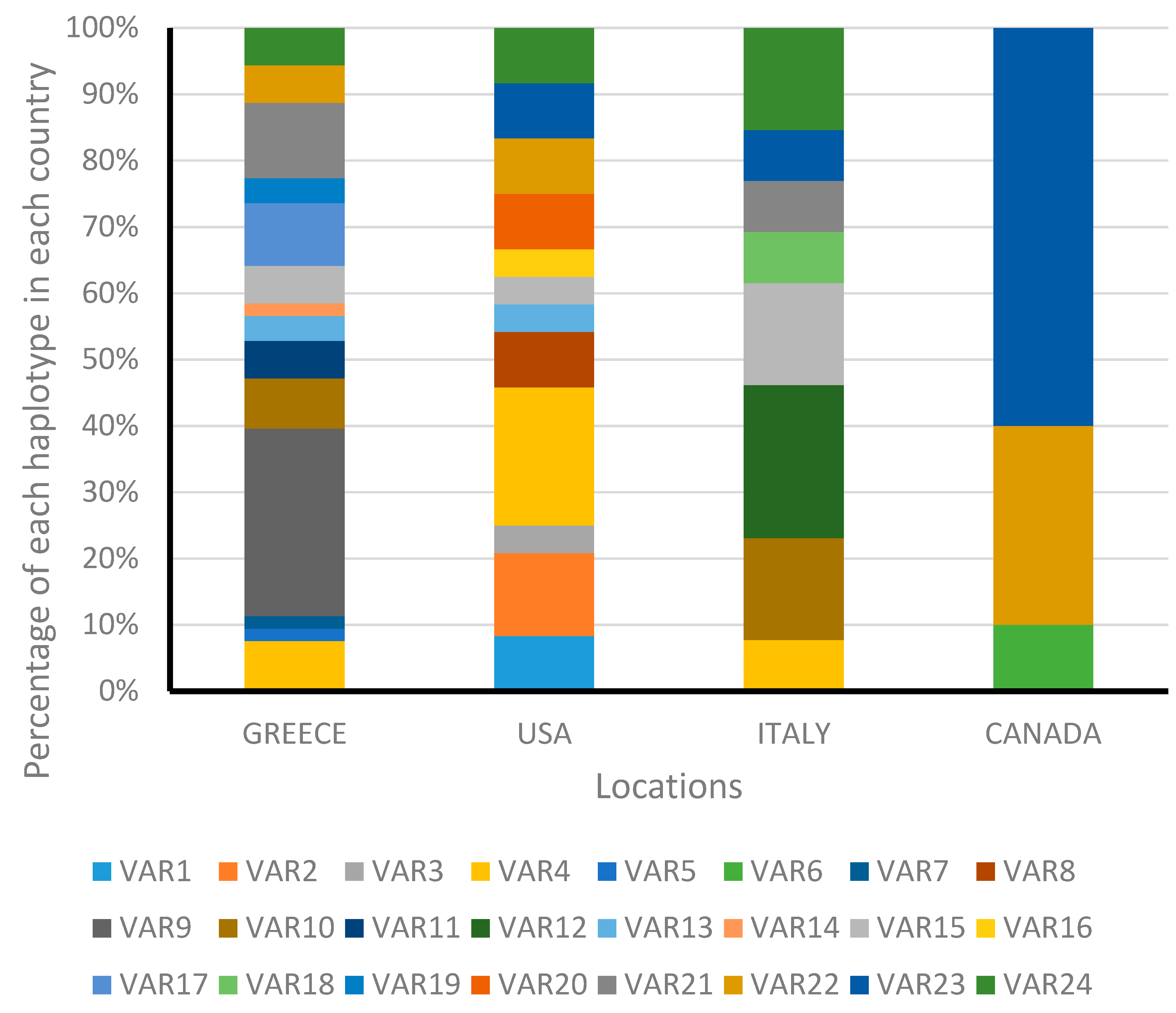
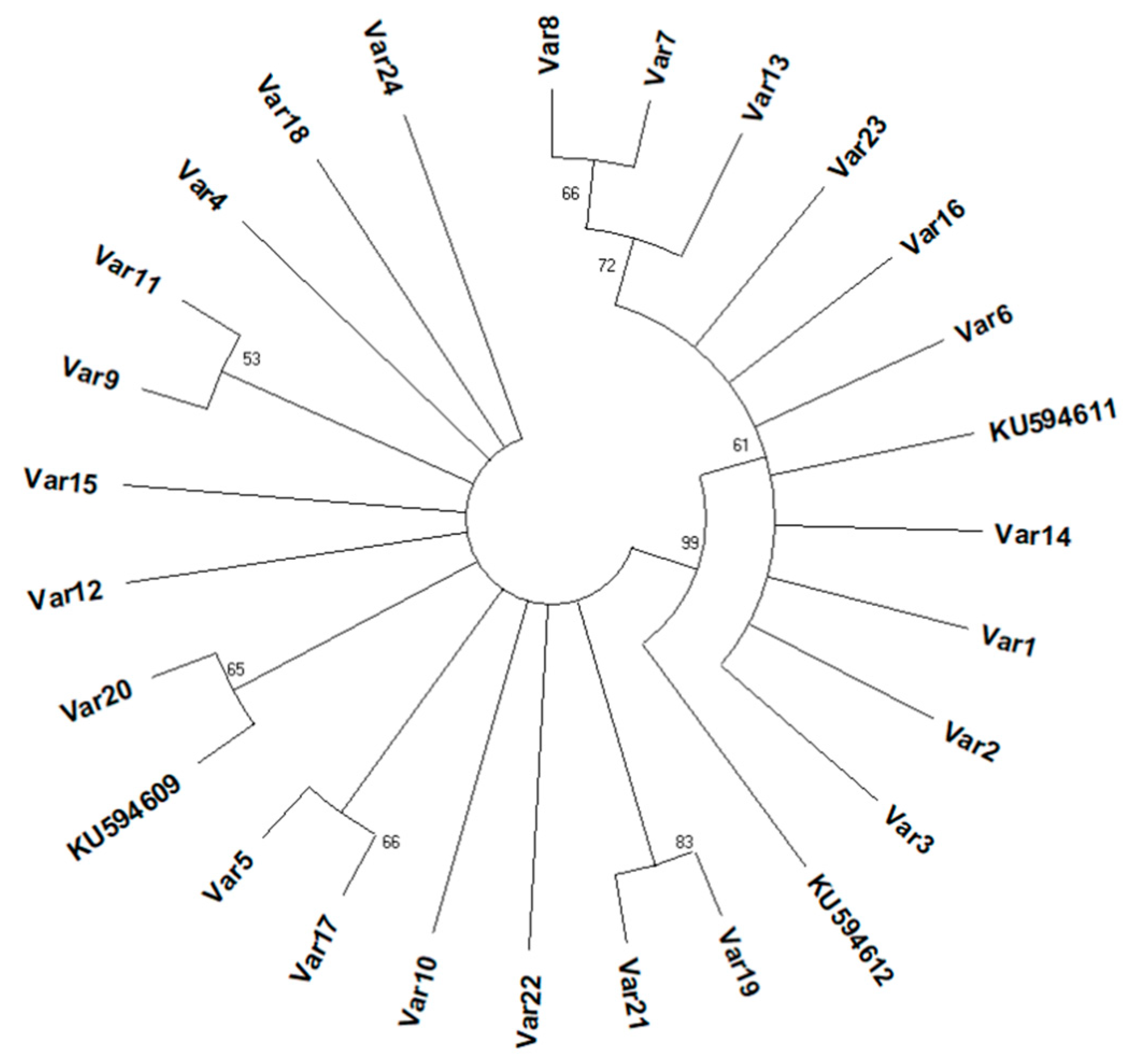
| Country | Region | COI | ITS1 | ||
|---|---|---|---|---|---|
| Morrison et al. 2017 [35] | This Study | Morrison et al. 2017 [35] | This Study | ||
| Greece | Athens | 195 | 50 | ||
| Peloponnese | 2 | 2 | |||
| Chania | 1 | 1 | |||
| Italy | Modena | 20 | 11 | ||
| Perugia | 3 | 2 | |||
| USA | Smithsburg, Maryland | 60 | 19 | ||
| North Carolina | 20 | 5 | |||
| Canada | London, Ontario | 13 | 10 | ||
| Region | N | n | h | H | Hd ± SD | Pi ± SD | S | k | SVS | PIS | Tajima’s D | p-Value | Fu’s Fs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canada | 10 | 10 | 3 | 1 | 0.600 ± 0.131 | 0.011± 0.003 | 12 | 4.800 | 3 | 9 | 0.594 | p > 0.10 | 4.717 |
| Greece | 53 | 53 | 14 | 7 | 0.830 ± 0.034 | 0.022 ± 0.001 | 82 | 7.896 | 2 | 80 | −2.083 | p < 0.05 | 3.967 |
| USA | 24 | 24 | 12 | 6 | 0.728 ± 0.062 | 0.023 ± 0.012 | 64 | 7.909 | 52 | 12 | −2.138 | p < 0.05 | 5.890 |
| Italy | 13 | 13 | 8 | 2 | 0.603 ± 0.131 | 0.005 ± 0.002 | 10 | 1.897 | 8 | 2 | −1.643 | p > 0.05 | 0.934 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapantaidaki, D.E.; Evangelou, V.I.; Morrison, W.R.; Leskey, T.C.; Brodeur, J.; Milonas, P. Halyomorpha halys (Hemiptera: Pentatomidae) Genetic Diversity in North America and Europe. Insects 2019, 10, 174. https://doi.org/10.3390/insects10060174
Kapantaidaki DE, Evangelou VI, Morrison WR, Leskey TC, Brodeur J, Milonas P. Halyomorpha halys (Hemiptera: Pentatomidae) Genetic Diversity in North America and Europe. Insects. 2019; 10(6):174. https://doi.org/10.3390/insects10060174
Chicago/Turabian StyleKapantaidaki, Despoina Ev., Vassiliki I. Evangelou, William R. Morrison, Tracy C. Leskey, Jacques Brodeur, and Panagiotis Milonas. 2019. "Halyomorpha halys (Hemiptera: Pentatomidae) Genetic Diversity in North America and Europe" Insects 10, no. 6: 174. https://doi.org/10.3390/insects10060174
APA StyleKapantaidaki, D. E., Evangelou, V. I., Morrison, W. R., Leskey, T. C., Brodeur, J., & Milonas, P. (2019). Halyomorpha halys (Hemiptera: Pentatomidae) Genetic Diversity in North America and Europe. Insects, 10(6), 174. https://doi.org/10.3390/insects10060174






