Physalis peruviana L. (Solanaceae) Is Not a Host of Ceratitis capitata (Diptera: Tephritidae): Evidence from Multi-Year Field and Laboratory Studies in Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Sampling in the Field and Adult Medfly Trapping
Probit 9 Determination
2.2. Field-Cage Studies
2.2.1. Study Sites and Experimental Treatments
2.2.2. Experimental Fly and Fruit Handling
2.2.3. Observation Protocol
2.3. Forced Infestation under Artificial Laboratory Conditions
2.4. Volatile Collections in P. peruviana Branches
2.5. Data Analyses
3. Results
3.1. Fruit Sampling in the Field and Adult Fly Trapping
Probit 9 Calculations
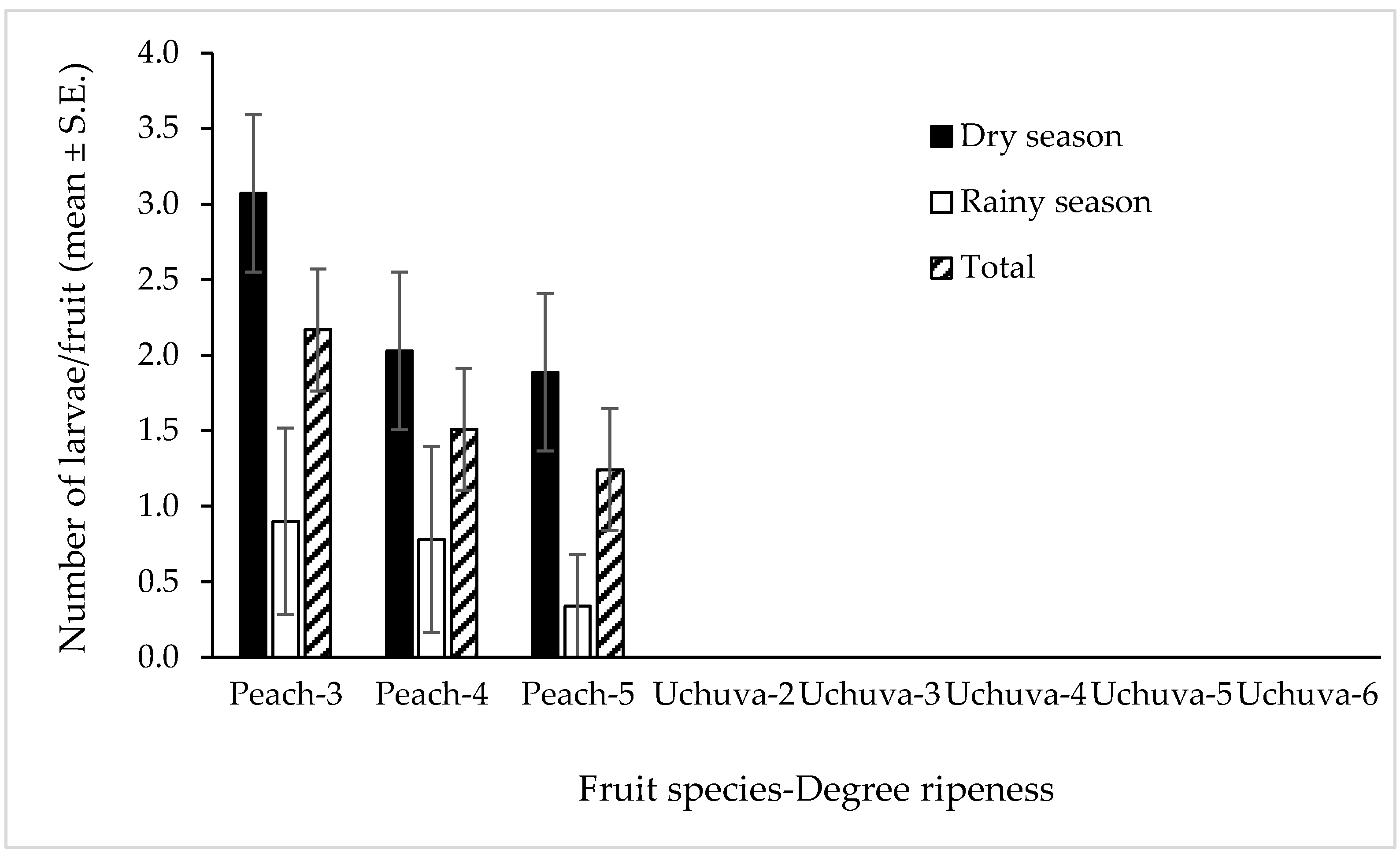
3.2. Forced Infestation Studies under Field-Cage Conditions
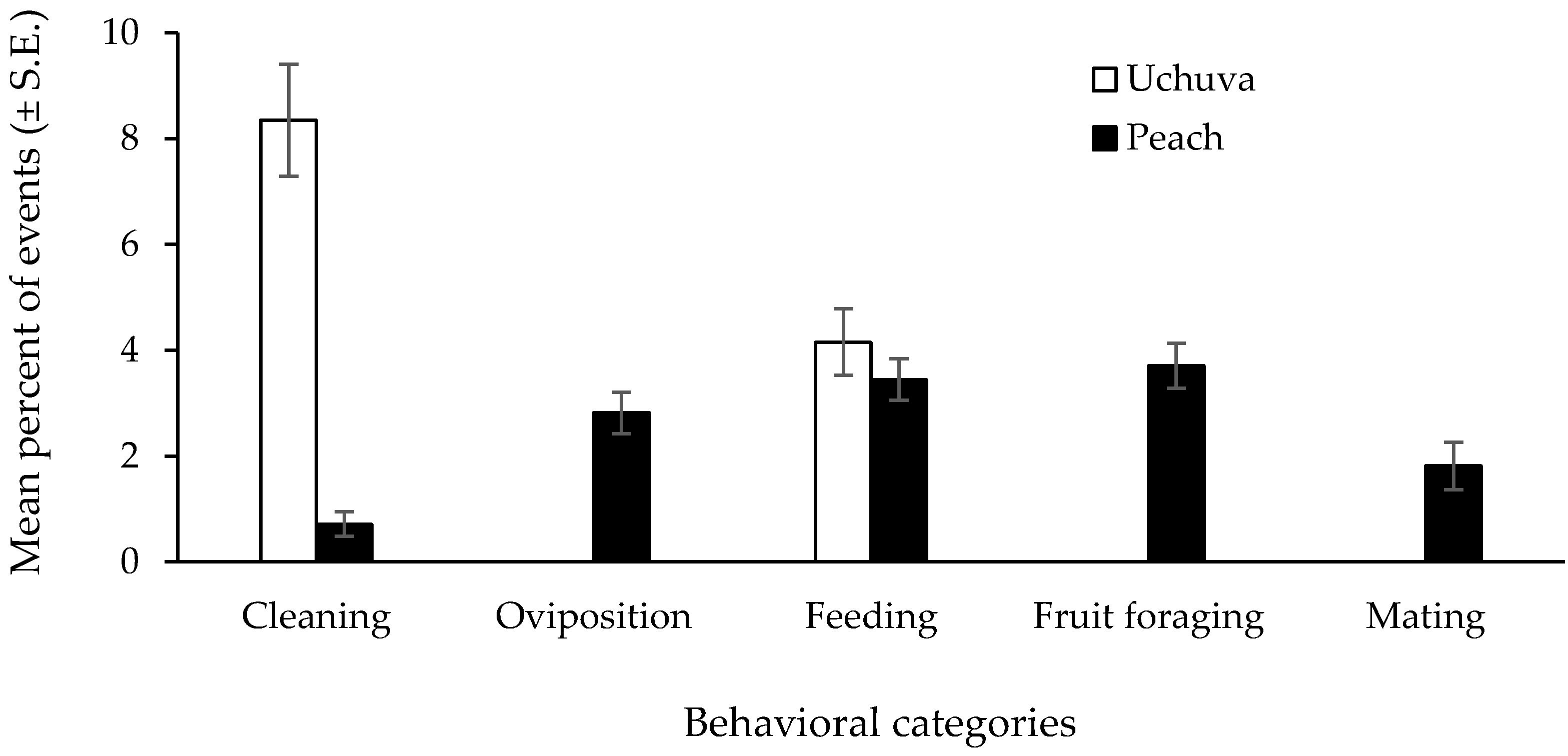
Fly Behavioral Patterns in Field-Cages
3.3. Laboratory Studies
3.4. Volatile Collections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cowley, J.M.; Baker, R.T.; Harte, D.S. Definition and determination of host status for multivoltine fruit fly (Diptera: Tephritidae) species. J. Econ. Entomol. 1992, 85, 312–317. [Google Scholar] [CrossRef]
- Aluja, M.; Díaz-Fleischer, F.; Arredondo, J. Nonhost status of commercial Persea americana ‘Hass’ to Anastrepha ludens, Anastrepha obliqua, Anastrepha serpentina and Anastrepha striata (Diptera: Tephritidae) in Mexico. J. Econ. Entomol. 2004, 97, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, M.K. Guidelines for the Determination and Designation of Host Status of a Commodity for Fruit Flies (Tephritidae); USDA-CPHST: Orlando, FL, USA, 2007. [Google Scholar]
- Aluja, M.; Mangan, R.L. Fruit fly (Diptera: Tephritidae) host status determination: Critical conceptual, methodological, and regulatory considerations. Annu. Rev. Entomol. 2008, 53, 473–502. [Google Scholar] [CrossRef] [PubMed]
- Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO). NIMF 37: Determinación de la condición de una fruta como hospedante de moscas de la fruta (Tephritidae). In Convención Internacional de Protección Fitosanitaria; Secretaría de la Convención Internacional de Protección Fitosanitaria: Rome, Italy, 2019; pp. 1–14. [Google Scholar]
- Legge, A. Notes on the history, cultivation and uses of Physalis peruviana L. J. R. Hortic. Soc. 1974, 99, 310–314. [Google Scholar]
- National Research Council (NRC). Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation; National Research Council—National Academy Press: Washington, DC, USA, 1990. [Google Scholar]
- Puente, L.A.; Pinto-Muñoz, C.A.; Castro, E.S.; Cortes, M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: A review. Food Res. Int. 2011, 44, 1733–1740. [Google Scholar] [CrossRef]
- Brito, D. Producción de Uvilla para Exportación: Agroexportación de Productos no Tradicionales; Fundación Aliñambi: Quito, Ecuador, 2002. [Google Scholar]
- Fischer, G.; Herrera, A.; Almanza, P.J. Cape Gooseberry (Physalis peruviana L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead: Philadelphia, PA, USA, 2011; pp. 374–396. [Google Scholar]
- USDA-ARS. Germplasm Resources Information Network (GRIN). Online Database. Beltsville, Maryland, USA: National Germplasm Resources Laboratory. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearch.aspx (accessed on 29 May 2019).
- CABI. Invasive Species Compendium—Physalis peruviana (Cape Gooseberry). Available online: https://www.cabi.org/isc/datasheet/40713#FBB493AB-61B9-467D-AC06-7FA9B17C8598 (accessed on 30 May 2019).
- Njoroge, G.N.; Bussmann, R.W.; Gemmill, B.; Newton, L.E.; Ngumi, V.W. Utilization of weed species as sources of traditional medicines in central Kenya. Lyonia 2004, 7, 71–87. [Google Scholar]
- Global Invasive Species Database (GISD). Species profile: Physalis Peruviana. Available online: http://www.iucngisd.org/gisd/speciesname/Physalis+peruviana (accessed on 2 June 2019).
- Morton, J. Cape Gooseberry. In Fruits for Warm Climates; Media Incorporated: Greensboro, NC, USA, 1987; p. 27419. [Google Scholar]
- Perea-Dallos, M.; Rodríguez, N.C.; Fischer, G.; Velásquez-Lozano, M.; Gutiérrez, Y. Uchuva Physalis peruviana L. (Solanaceae). In Biotecnología Aplicada al Mejoramiento de los Cultivos de Frutas Tropicales; Perea-Dallos, M., Matallana-Ramirez, L.P., Tirado-Perea, A., Eds.; Universidad Nacional de Colombia: Bogotá, Colombia, 2010; pp. 466–490. [Google Scholar]
- Fischer, G. Effect of Root Zone Temperature and Tropical Altitude on the Growth, Development and Fruit Quality of the Cape Gooseberry (Physalis peruviana L.). Ph.D. Thesis, Humboldt-Universität zu Berlin, Berlin, Germany, 1995. [Google Scholar]
- Mendoza, C.H.; Rodríguez de S, J.H.; Millán, C.P. Physical and chemical characterization of Golden Berry fruit (Physalis peruviana) in the region of Silvia Cauca. Rev. Bio. Agro. 2012, 10, 188–196. [Google Scholar]
- Ariza, R. Manejo de Plagas. In Producción, Poscosecha y Exportación de la Uchuva (Physalis peruviana L.); Flórez, V., Fischer, G., Sora, A., Eds.; Universidad Nacional de Colombia: Santafé de Bogotá, Colombia, 2000; pp. 67–73. [Google Scholar]
- Benavides, M.A.; Mora, H.R. Los insectos-plaga limitantes en el cultivo de la Uchuva y su manejo. In Avances en Cultivo, Poscosecha y Exportación de la Uchuva (Physalis peruviana L.) en Colombia; Fischer, G., Miranda, D., Piedrahíta, W., Romero, J., Eds.; Unibiblos—Universidad Nacional de Colombia: Bogotá, Colombia, 2005; pp. 83–96. [Google Scholar]
- Nakagawa, S.; Farias, G.J.; Urago, T. Newly recognized hosts of the Oriental fruit fly, Melon fly, and Mediterranean fruit fly. J. Econ. Entomol. 1968, 61, 339–340. [Google Scholar] [CrossRef]
- Liquido, N.J.; Cunningham, R.T.; Nakagawa, S. Host plants of Mediterranean fruit fly on the island of Hawaii (1949–1985 survey). J. Econ. Entomol. 1990, 83, 1863–1878. [Google Scholar] [CrossRef]
- Liquido, N.; Harris, E.J.; Dekker, L.A. Ecology of Bactrocera latifrons (Diptera: Tephritidae) populations: Host plants, natural enemies, distribution and abundance. Ann. Entomol. Soc. Am. 1994, 87, 71–84. [Google Scholar] [CrossRef]
- Vargas, R.I.; Piñero, J.C.; Mau, R.F.L.; Jang, E.B.; Klungness, L.M.; Mclnnis, D.O.; Harris, E.B.; McQuate, G.T.; Bautista, R.C.; Wong, L. Area-wide suppression of the Mediterranean fruit fly, Ceratitis capitata, and the Oriental fruit fly, Bactrocera dorsalis, in Kamuela, Hawaii. J. Insect Sci. 2010, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Vilatuña, J.; Valenzuela, P.; Bolaños, J.; Hidalgo, R.; Mariño, A. Hospederos de moscas de la fruta Anastrepha spp. y Ceratitis capitata (Diptera: Tephritidae) en Ecuador. Rev. Científica Ecuat. 2016, 3, 52–57. [Google Scholar] [CrossRef]
- Bado, S.; Mareggiani, G.; Amiano, N.; Burton, G.; Veleiro, A.S. Lethal and sublethal effects of withanolides from Salpichroa origanifolia and analogues on Ceratitis capitata. J. Agric. Food Chem. 2004, 52, 2875–2878. [Google Scholar] [CrossRef] [PubMed]
- Cirigliano, A.; Colamarino, I.; Mareggiani, G.; Bado, S. Biological effects of Physalis peruviana L. (Solanaceae) crude extracts and its major withanolides on Ceratitis capitata Wiedemann (Diptera: Tephritidae). Bol. Sanid. Veg. Plagas 2008, 34, 509–515. [Google Scholar]
- Baumann, T.W.; Meier, C.M. Chemical defence by withanolides during fruit development in Physalis peruviana. Phytochemistry 1993, 33, 317–321. [Google Scholar] [CrossRef]
- Ascher, K.R.S.; Nemny, N.E.; Eliyahu, M.; Kirson, I.; Abraham, A.; Glotter, E. Insect antifeedant properties of withanolides and related steroids from Solanaceae. Experientia 1980, 36, 998–999. [Google Scholar] [CrossRef]
- Glotter, E. Withanolides and related ergostane-type steroids. Nat. Prod. Rep. 1991, 8, 415–440. [Google Scholar] [CrossRef]
- Veleiro, A.; Oberti, J.; Burton, G. Chemistry and bioactivity of withanolides from South American Solanaceae. In Studies in Natural Products Chemistry; Ur-Rahman, A., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2005; Volume 32, Part L; pp. 1019–1052. [Google Scholar]
- Franco, L.; Ocampo, Y.; Gómez, H.; De la Puerta, R.; Espartelo, J.; Ospina, L. Sucrose esters from Physalis peruviana calyces with anti-inflammatory activity. Planta Med. 2014, 80, 1605–1614. [Google Scholar] [CrossRef]
- Jácome, I.; Aluja, M.; Liedo, P.; Nestel, D. The influence of adult diet and age on lipid reserves in the tropical fruit fly Anastrepha serpentina (Diptera: Tephritidae). J. Insect Physiol. 1995, 41, 1079–1086. [Google Scholar] [CrossRef]
- Aluja, M.; Jácome, I.; Birke, A.; Lozada, N.; Quintero, G. Basic patterns of behavior in wild Anastrepha striata (Diptera: Tephritidae) flies under field-cage conditions. Ann. Entomol. Soc. Am. 1993, 86, 776–793. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System); Version 10; tatSoft, Inc.: Tulsa, OK, USA, 2011. [Google Scholar]
- Follett, P.A.; Hennessey, M.N. Confidence limits and sample size for determining nonhost status of fruits and vegetables to tephritid fruit flies as a quarantine measure. J. Econ. Entomol. 2007, 100, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.P.; Nava, D.E.; García, M.S.; Silva, F.F.; Valgas, R.A. Oviposition of fruit flies (Diptera: Tephritidae) and its relationship with the pericarp of citrus fruits. Braz. J. Biol. 2018, 78, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Llano, S.M.; Muñoz-Jiménez, A.M.; Jiménez-Cartagena, C.; Londoño-Londoño, J.; Medina, S. Untargeted metabolomics reveals specific withanolides and fatty acyl glycoside as tentative metabolites to differentiate organic and conventional Physalis peruviana fruit. Food Chem. 2018, 244, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Tsai, J.Y.; Chang, S.P.; Lin, D.L.; Wang, S.S.; Huang, S.N.; Ng, L.T. Supercritical carbon dioxide extract exhibits enhanced antioxidant and anti-inflammatory activities of Physalis peruviana. J. Ethnopharmacol. 2006, 108, 407–413. [Google Scholar] [CrossRef]
- Ramadan-Hassanien, M.F. Physalis peruviana: A rich source of bioactive phytochemicals for functional foods and pharmaceuticals. Food Rev. Int. 2013, 27, 259–273. [Google Scholar] [CrossRef]
- Kumar-Gautam, S.; Dwivedi, D.H.; Kumar, P. Preliminary studies on the bioactive phytochemicals in extract of Cape Gooseberry (Physalis peruviana L.) fruits and their products. J. Pharm. Phytochem. 2015, 3, 93–95. [Google Scholar]
- Olivares-Tenorio, M.L.; Dekker, M.; Verkerk, R.; Van Boekel, M.A. Health-promoting compounds in Cape Gooseberry (Physalis peruviana L.): Review from supply chain perspective. Trends Food Sci. Technol. 2016, 57, 83–92. [Google Scholar] [CrossRef]
- Ames, T.; Malagamba, P. First International conference on sweet potato food and health. Acta Hortic. 2002, 583, 244. [Google Scholar]
- Zoubiri, S.; Baaliouamer, A. Potentiality of plants as source of insecticide principles. J. Saudi Chem. Soc. 2014, 18, 925–938. [Google Scholar] [CrossRef]
- Adjalian, E.; Sessou, P.; Odjo, T.; Figueredo, G.; Kossou, D.; Avlessi, F.; Menut, C.; Sohounhloué, D. Chemical composition and insecticidal and repellent effect of essential oils of two Premna species against Sitotroga cerealella. J. Insects 2015, 2015, 319045. [Google Scholar] [CrossRef]
- Santos da Silva, R.C.S.; Milet-Pinheiro, P.; Bezerra da Silva, P.C.; da Silva, A.G.; da Silva, M.V.; Ferraz Navarro, D.M.A.; da Silva, N.H. (E)-Caryophyllene and α-humulene: Aedes aegypti oviposition deterrents elucidated by gas chromatography-electrophysiological assay of Commiphora leptophloeos leaf oil. PLoS ONE 2015, 10, e0144586. [Google Scholar]
- Dambolena, J.S.; Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Areco, V.A.; Zygadlo, J.A. Terpenes: Natural products for controlling insects of importance to human health—A structure-activity relationship study. Psyche 2016, 2016, 4595823. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, W.; Liang, J.; You, C.; Geng, Z.; Wang, C.; Du, S. Contact and repellent activities of the essential oil from Juniperus formosana against two stored product insects. Molecules 2016, 21, 504. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; Colares, H.C.; Campos, J.M.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Toxic effects of two essential oils and their constituents on the mealworm beetle, Tenebrio molitor. Bull. Entomol. Res. 2018, 108, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Aluja, M.; Lozada, N.; Piñero, J.; Birke, A.; Hernández-Ortíz, V.; Díaz-Fleischer, F. Basic behavior of Rhagoletis turpiniae (Diptera: Tephritidae) with comparative notes on the sexual behavior of Rhagoletis pomonella and Rhagoletis zoqui. Ann. Entomol. Soc. Am. 2001, 94, 268–274. [Google Scholar]
- Rani, K.; Arya, S.S.; Devi, S.; Kaur, V. Plant volatiles and defense. In Volatiles and Food Security; Choudhary, D.K., Sharma, A.K., Agarwal, P., Varma, A., Tuteja, N., Eds.; Springer: Singapore, 2017; pp. 113–134. [Google Scholar]
- Sistema de Información Sanitaria para Importación y Exportación de Productos Agrícolas y Pecuarios-Instituto Colombiano Agropecuario (SISPAP). Certificación Fitosanitaria de Uchuva Fresca. In Sugerencia de Protección Fronteriza, Grupo Nacional de Cuarentena Vegetal-SISPAP 21/12/2018; Instituto Colombiano Agropecuario: Bogotá, Colombia, 2019. [Google Scholar]
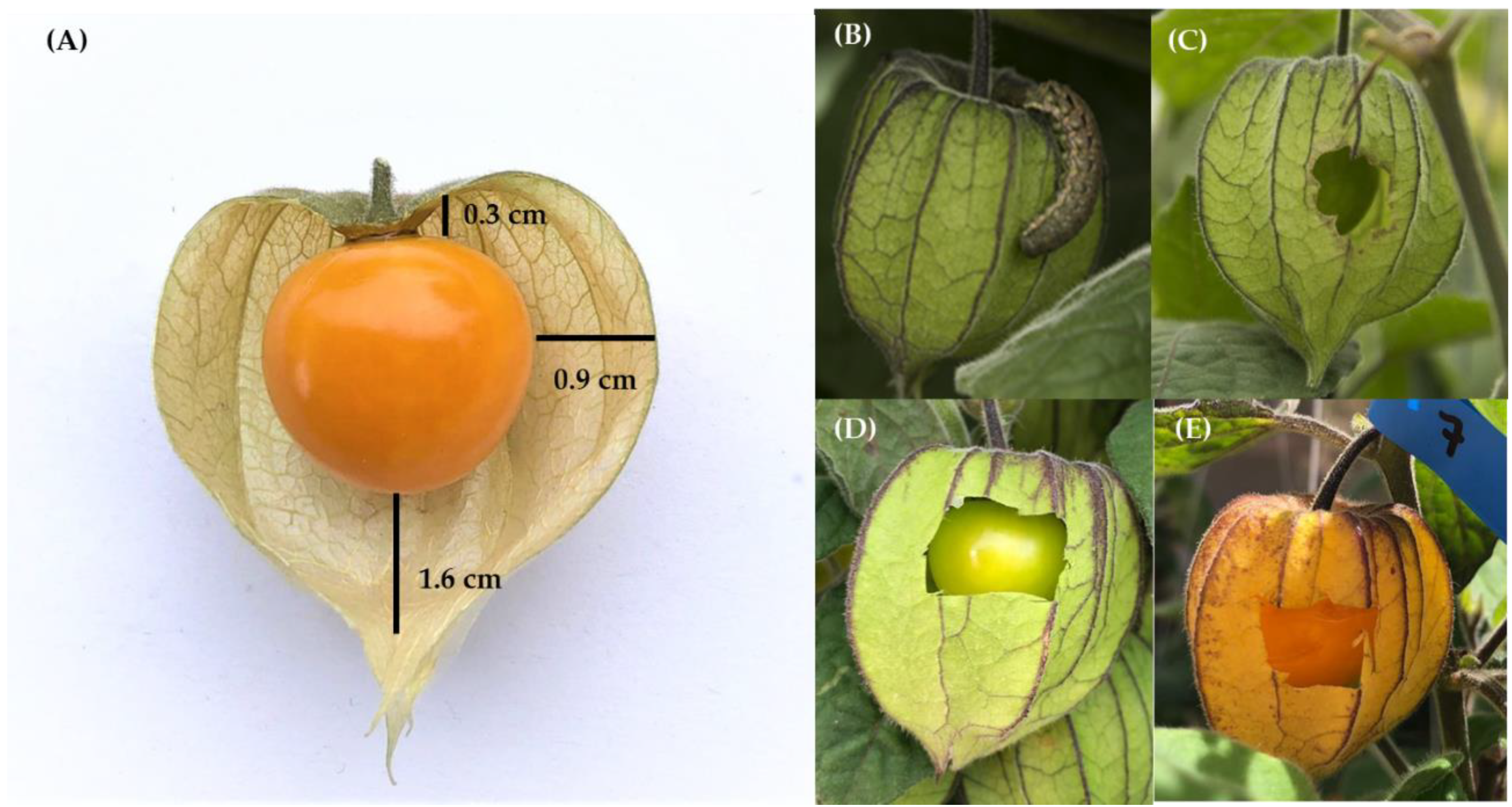

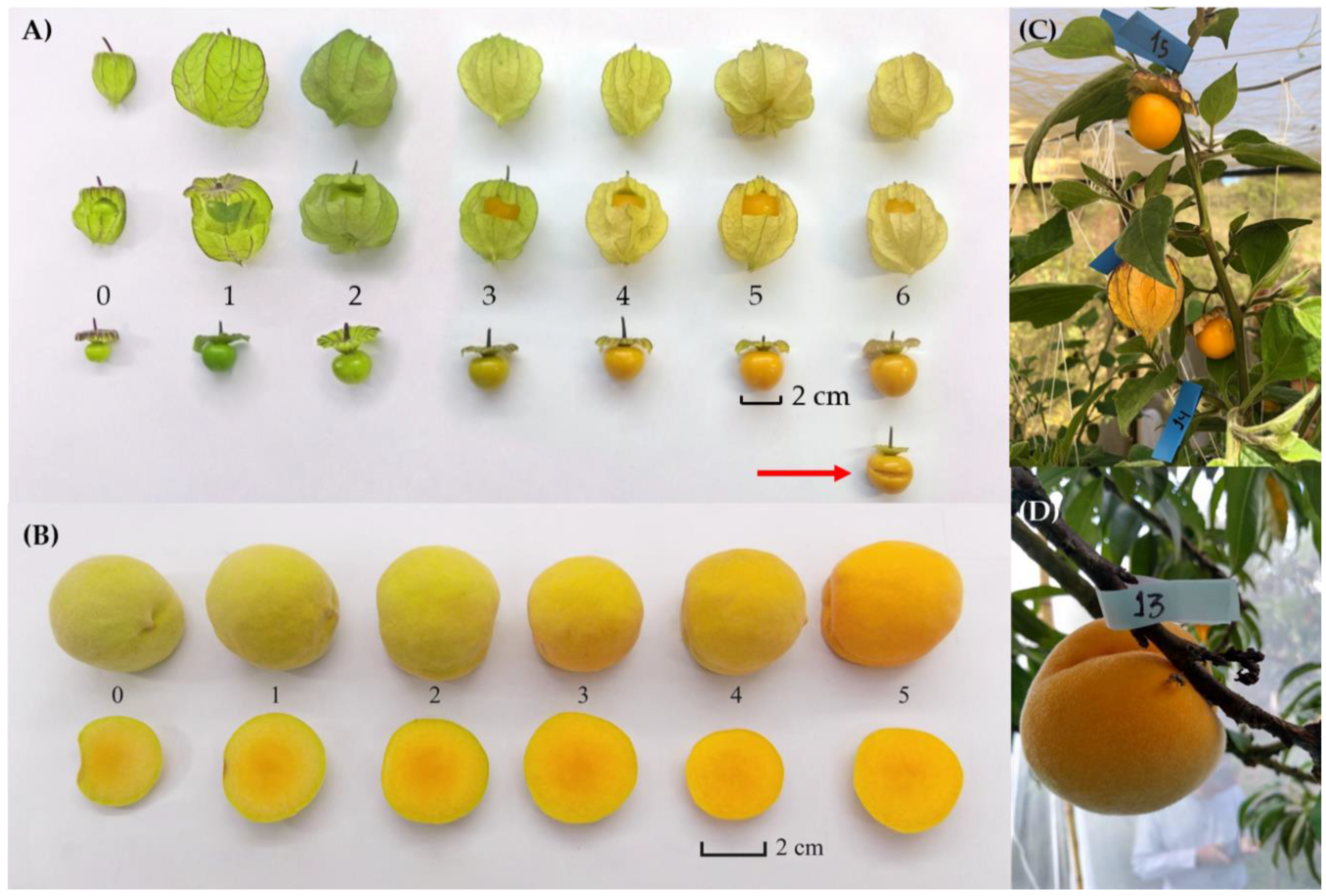

| Year | Fruit | Sampling Part | Kg of Fruit | Total no. Fruit |
|---|---|---|---|---|
| Total 2016 | 184.6 | 33,079 | ||
| Commercial Uchuva plantations | 171.4 | 30,537 | ||
| Plant | 166.2 | 29,052 | ||
| Soil | 5.2 | 1485 | ||
| “Feral” Uchuva plants | Plant | 13.2 | 2542 | |
| Total 2017 | 445.4 | 60,221 | ||
| Commercial Uchuva plantations | 427.0 | 57,317 | ||
| Plant | 425.0 | 57,085 | ||
| Soil | 1.9 | 232 | ||
| “Feral” Uchuva plants | 18.4 | 2904 | ||
| Plant | 15.4 | 2408 | ||
| Soil | 3.0 | 496 | ||
| Total 2018 | 32.6 | 4832 | ||
| Commercial Uchuva plantations | Plant | 31.7 | 4649 | |
| “Feral” Uchuva plants | Plant | 0.9 | 183 | |
| Total | 662.5 | 98,132 |
| Compounds | Intact Branches | Recently Pruned Branches | Branches Pruned 24 h Prior to Volatile Collection | |||
|---|---|---|---|---|---|---|
| RT | Relative Area | RT | Relative Area | RT | Relative Area | |
| Methyl isopentanoate | 3.310 | 16,473,762 | ||||
| ? | 3.622 | 160,694,822 | 3.616 | 95,356,938 | ||
| ? | 3.81 | 26,810,425 | ||||
| Methyl 2,3-dimethylbutanoate | 4.310 | 13,745,891.3 | ||||
| 3-Hexen-1-ol, (Z) * | 4.394 | 610,92,890.7 | ||||
| trans-2-Hexenol | 4.534 | 26,142,420.7 | ||||
| Octylcyclopropane/1-Nonene | 4.879 | 8,460,853 | ||||
| Cyclene | 5.472 | 7,052,744 | 5.467 | 44,547,522 | 5.462 | 61,727,006.7 |
| alpha-Pinene * | 5.650 | 7,101,577 | 5.645 | 14,490,525 | 5.639 | 28,033,637 |
| ? | 5.905 | 32,962,783.5 | 5.900 | 38,290,510 | 5.896 | 12,457,773 |
| beta-Phellandrene | 6.273 | 14,704,868.5 | 6.269 | 19,964,512.5 | ||
| Butyl butanoate | 6.537 | 193,621,106 | 6.532 | 177,265,806 | ||
| sec-Amyl butyrate | 7.549 | 26,157,546 | 7.543 | 34,564,042 | ||
| ? | 8.134 | 36,909,580.5 | ||||
| Methyl octanoate | 8.590 | 8,737,293.5 | ||||
| Ethyl octanoate * | 9.726 | 91,124,017.5 | 9.720 | 47,461,136 | 9.717 | 34,196,081 |
| delta-EIemene | 12.028 | 9,593,930.5 | ||||
| Ylangene | 12.579 | 42,290,590 | 12.576 | 52,852,747.3 | 12.573 | 64,819,090.7 |
| alfa-Copaene * | 12.645 | 22,192,225.7 | 12.640 | 30,216,856.3 | ||
| ? | 12.657 | 18,306,226 | ||||
| beta-Bourbonene | 12.810 | 116,855,780 | 12.815 | 113,935,875 | 12.808 | 142,049,337 |
| beta-Cubebene | 13.285 | 37,045,601.3 | ||||
| Caryophyllene * | 13.323 | 37,115,958.7 | 13.321 | 145,049,228 | 13.318 | 193,915,800 |
| beta-copaene * | 13.433 | 28,194,287.3 | 13.430 | 31,627,144 | 13.427 | 28,854,676.3 |
| gamma-Cadinene/beta Cubebene | 13.489 | 49,388,138.3 | 13.488 | 68,758,807 | 13.487 | 79,022,234.3 |
| gamma-Muurolene/Eremophilene | 13.660 | 18,646,364.5 | 13.656 | 14,572,749.3 | 13.652 | 17,154,062 |
| Humulene * | 13.812 | 17,520,372 | 13.815 | 21,593,178.7 | 13.813 | 25,674,739 |
| Germacrene D | 14.227 | 252,723,612 | 14.224 | 251,971,967 | 14.220 | 253,423,376 |
| gamma-Cadinene | 14.7 | 17,871,571 | 14.697 | 19,851,101.7 | 14.693 | 14,956,966.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aluja, M.; Guillén, L.; Castro, Á.; Cárdenas, M.L.; Hurtado, M.; Durán, Ó.; Arévalo-Peñaranda, E. Physalis peruviana L. (Solanaceae) Is Not a Host of Ceratitis capitata (Diptera: Tephritidae): Evidence from Multi-Year Field and Laboratory Studies in Colombia. Insects 2019, 10, 434. https://doi.org/10.3390/insects10120434
Aluja M, Guillén L, Castro Á, Cárdenas ML, Hurtado M, Durán Ó, Arévalo-Peñaranda E. Physalis peruviana L. (Solanaceae) Is Not a Host of Ceratitis capitata (Diptera: Tephritidae): Evidence from Multi-Year Field and Laboratory Studies in Colombia. Insects. 2019; 10(12):434. https://doi.org/10.3390/insects10120434
Chicago/Turabian StyleAluja, Martín, Larissa Guillén, Ángela Castro, Martha Liliana Cárdenas, Maribel Hurtado, Óscar Durán, and Emilio Arévalo-Peñaranda. 2019. "Physalis peruviana L. (Solanaceae) Is Not a Host of Ceratitis capitata (Diptera: Tephritidae): Evidence from Multi-Year Field and Laboratory Studies in Colombia" Insects 10, no. 12: 434. https://doi.org/10.3390/insects10120434
APA StyleAluja, M., Guillén, L., Castro, Á., Cárdenas, M. L., Hurtado, M., Durán, Ó., & Arévalo-Peñaranda, E. (2019). Physalis peruviana L. (Solanaceae) Is Not a Host of Ceratitis capitata (Diptera: Tephritidae): Evidence from Multi-Year Field and Laboratory Studies in Colombia. Insects, 10(12), 434. https://doi.org/10.3390/insects10120434





