Compatibility and Efficacy of the Parasitoid Eretmocerus hayati and the Entomopathogenic Fungus Cordyceps javanica for Biological Control of Whitefly Bemisia tabaci
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and Insects for Testing
2.2. Entomopathogenic Fungus
2.3. Pathogenicity of C. javanica to E. hayati Pupae
2.4. Pathogenicity of C. javanica to E. hayati Adults
2.5. Effects of C. javanica on the Parasitic Potential of E. hayati
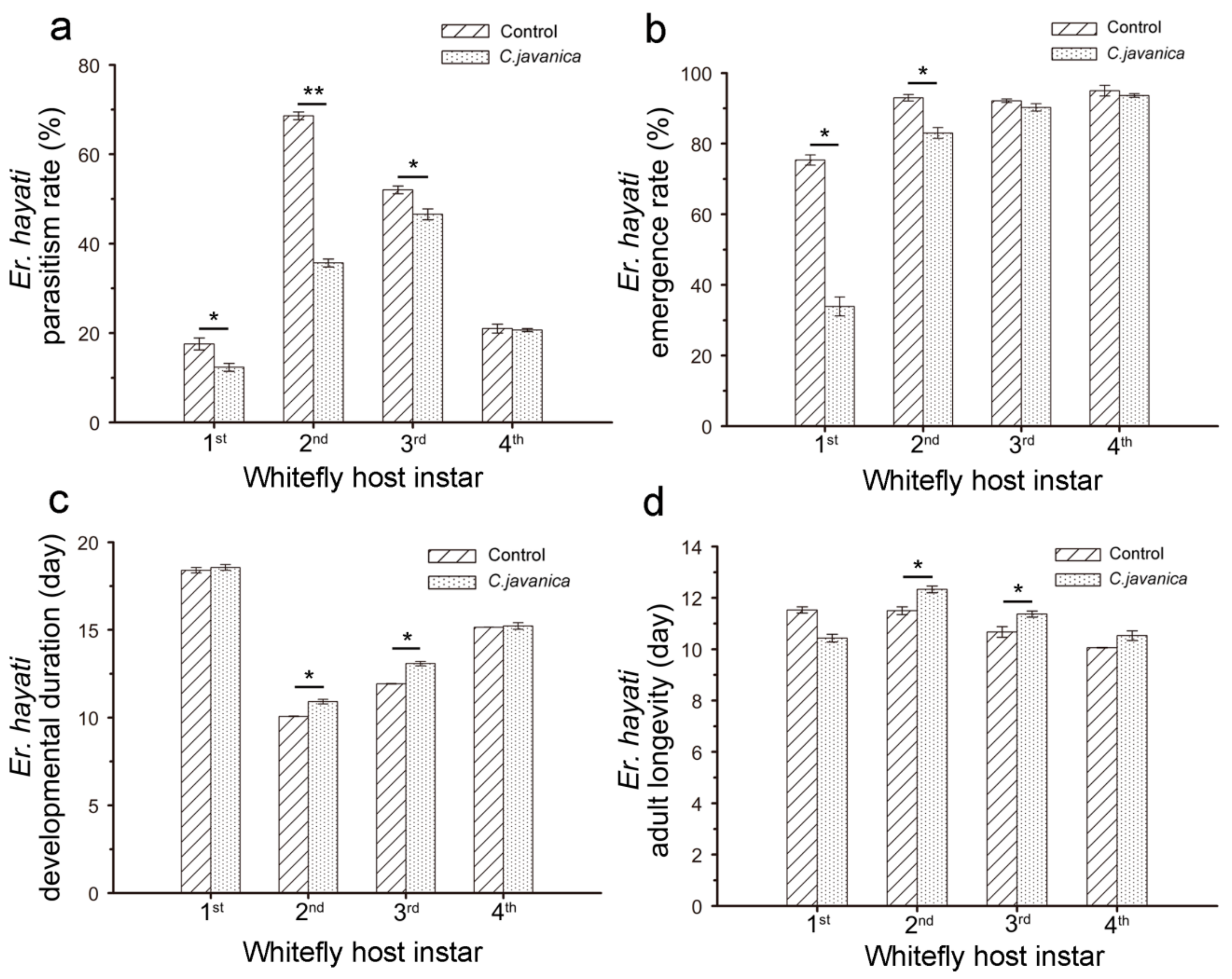
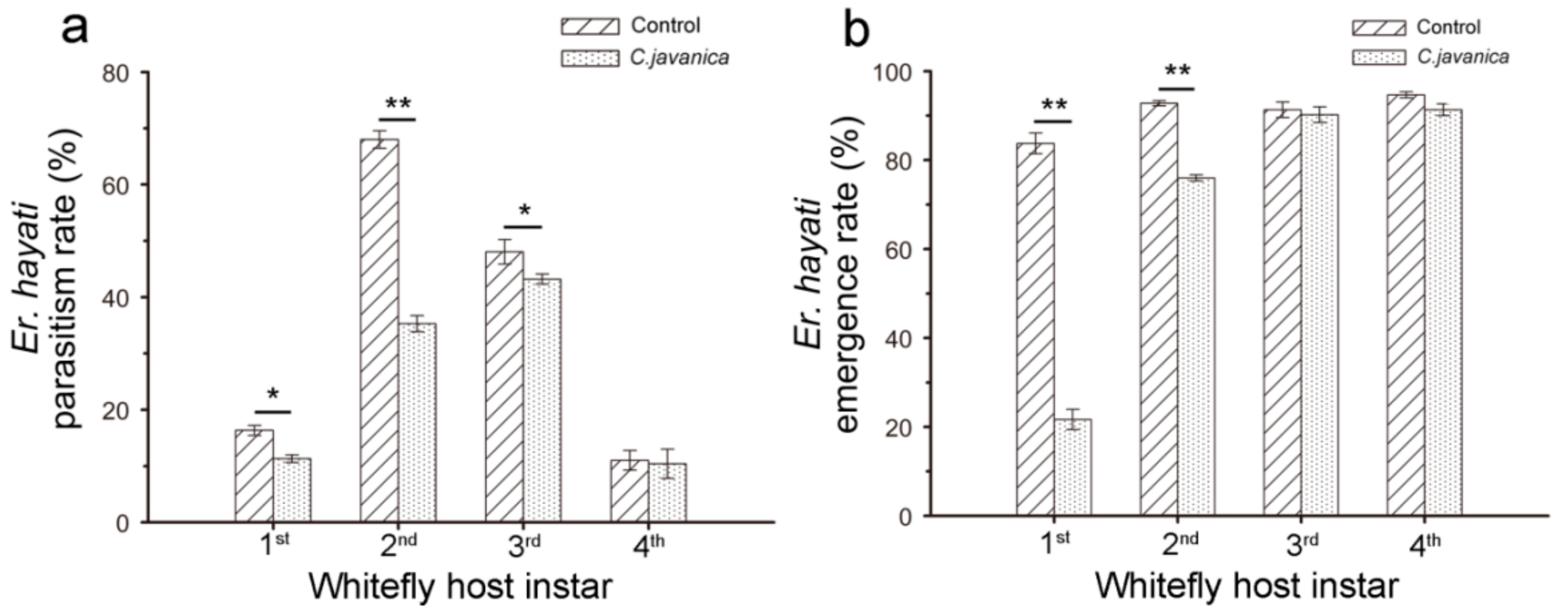
2.5.1. No-Choice Test (Single Age of Whitefly Nymphs)
2.5.2. Choice Test (Different Ages of Whitefly Nymphs)
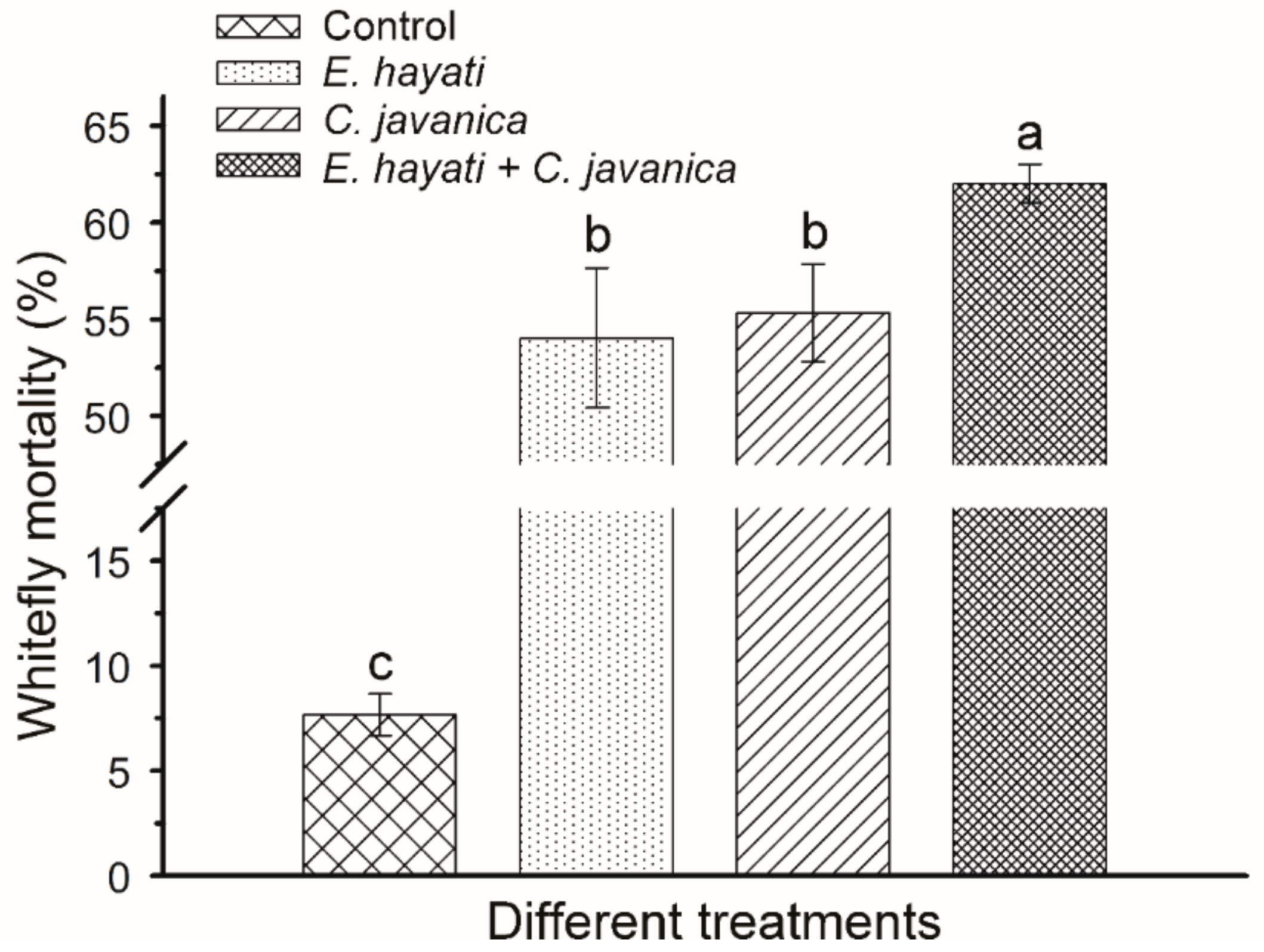
2.6. Compatibility of C. javanica and E. hayati against B. tabaci
2.7. Statistical Analysis
3. Results
3.1. Pathogenicity of C. javanica to E. hayati Pupae and Adults
3.2. Effects of C. javanica on E. hayati Wasp’s Parasitic Potential
3.3. Compatibility of C. javanica and E. hayati against B. tabaci
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fortes, I.M.; Sanchez-Campos, S.; Fiallo-Olive, E.; Diaz-Pendon, J.A.; Navas-Castillo, J.; Moriones, E. A novel strain of tomato leaf curl New Delhi virus has spread to the mediterranean basin. Viruses 2016, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Abrahamian, P.; Sobh, H.; Seblani, R.; Aboujawdah, Y. Co-infection of two criniviruses and a begomovirus enhances the disease severity in cucumber. Eur. J. Plant. Pathol. 2015, 142, 521–530. [Google Scholar] [CrossRef]
- Qiu, B.L.; Ren, S.X.; Sun, T.X.; Lin, L.; Kuang, Z.B. Preliminary report on the host plants of Bemisia tabaci in Guangzhou. J. South China Agric. Univ. 2001, 22, 43–47. [Google Scholar]
- Cahill, M.; Denholm, I.; Ross, G.; Gorman, K. Relationship between bioassay data and the simulated field performance of insecticides against susceptible and resistant adult Bemisia tabaci (Homoptera: Aleyrodidae). Bull. Entomol. Res. 1996, 86, 109–116. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Ishaaya, I. Dynamics of resistance to the neonicotinoids acetamiprid and thiamethoxam in Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 2004, 6, 2051–2056. [Google Scholar] [CrossRef]
- Silva, L.D.; Omoto, C.; Bleicher, E.; Dourado, P.M. Monitoring the susceptibility to insecticides in bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) populations from Brazil. Neotrop. Entomol. 2009, 38, 862–871. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Blackburn, L.F.; Eyre, D.P.; Cannon, R.J.C.; Miller, J.; Northing, P. Bemisia tabaci: The current situation in the UK and the prospect of developing strategies for eradication using entomopathogens. Insect Sci. 2011, 18, 1–10. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Ciomperlik, M.A.; Bcjr, L.; Legaspi, J.C.; Wendel, L.E. Laboratory and field evaluation of exotic parasitoids of Bemisia tabaci (gennadius) (biotype “B”) (Homoptera: Aleyrodidae) in the lower rio grande valley of Texas. Biol. Control 1998, 12, 127–135. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Khasdan, V.; Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. Physiol. 2005, 58, 216–225. [Google Scholar] [CrossRef]
- Wan, F.H.; Yang, N.W. Invasion and management of agricultural alien insects in China. Annu. Rev. Entomol. 2016, 61, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Frisvold, G.B. Economic value of biological control in integrated pest management of managed plant systems. Annu. Rev. Entomol. 2015, 60, 621–645. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.; Sridhar, J.; Murali-Baskaran, R.K.; Senthil-Nathan, S.; Kaushal, P.; Dara, S.K.; Arthurs, S. Microbial biopesticides for insect pest management in India: Current status and future prospects. J. Invertebr. Pathol. 2018, 165, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lahey, Z.; Stansly, P. An updated list of parasitoid hymenoptera reared from the Bemisia tabaci species complex (Hemiptera: Aleyrodidae). Fla. Entomol. 2015, 98, 456–463. [Google Scholar] [CrossRef]
- Yang, N.W.; Ji, L.L.; Lovei, G.L.; Wan, F.H. Shifting preference between oviposition vs. host-feeding under changing host densities in two aphelinid parasitoids. PLoS ONE 2012, 7, e41189. [Google Scholar] [CrossRef]
- Burger, W.; Reijnen, T.M.; Lenteren, J.C.; Vet, L.E.M. Host feeding in insect parasitoids: Why destructively feed upon a host that excretes an alternative? Entomol. Exp. Appl. 2010, 112, 207–215. [Google Scholar] [CrossRef]
- Yang, N.W.; Wan, F.H. Host suitability of different instars of Bemisia tabaci biotype B for the parasitoid Eretmocerus hayati. Biol. Control 2011, 59, 313–317. [Google Scholar] [CrossRef]
- Xu, H.Y.; Yang, N.W.; Chi, H.; Ren, G.D.; Wan, F.H. Comparison of demographic fitness and biocontrol effectiveness of two parasitoids, Encarsia sophia and Eretmocerus hayati (Hymenoptera: Aphelinidae), against Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2018, 74, 2116–2124. [Google Scholar] [CrossRef]
- De Barro, P.J.; Coombs, M.T. Post-release evaluation of Eretmocerus hayati Zolnerowich and Rose in Australia. Bull. Entomol. Res. 2009, 99, 193–206. [Google Scholar] [CrossRef]
- Asad, B.A. The Effects of Two Aphelinid Parasitoids at Low Bemisia Tabaci MEAM1 Densities and Optimizing Release Criteria in Greenhouse-Grown Tomatoes; Chinese Academy of Agricultural Sciences: Beijing, China, 2018; pp. 77–78. [Google Scholar]
- Kuang, W.; Yang, W.N.; Wan, F.H.; Yuan, Z.M. Effects of temperatures and Bemisia tabaci (gennadius) reared on different host plants on development and reproduction of parasitoid, Eretmocerus hayati (Zolnerowich and Rose). Chin. J. Biol. Control. 2011, 27, 152–156. [Google Scholar]
- Scholte, E.J.; Knols, B.G.J.; Samson, R.A.; Takken, W. Entomopathogenic fungi for mosquito control: A review. J. Insect Sci. 2004, 4, 19. [Google Scholar] [CrossRef]
- Hyweljones, N.L. Torrubiella luteorostrata: A pathogen of scale insects and its association with Paecilomyces cinnamomeus with a note on Torrubiella tenuis. Mycol. Res. 1993, 97, 1126–1130. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, Z.F.; Han, Y.Y.; Wang, X.M.; Wang, Z.Q.; Musa, P.D.; Qiu, B.L.; Ali, S. Effects of Aschersonia aleyrodis on the life table and demographic parameters of Bemisia tabaci. J. Integr. Agric. 2018, 17, 389–396. [Google Scholar] [CrossRef]
- Meng, X.; Hu, J.; Ouyang, G. The isolation and identification of pathogenic fungi from Tessaratoma papillosa Drury (Hemiptera: Tessaratomidae). PeerJ 2017, 5, e3888. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.E.; Steinkraus, D.C.; Eilenberg, J.; Hajek, A.E.; Pell, J.K. Bizarre interactions and endgames: Entomopathogenic fungi and their arthropod hosts. Annu. Rev. Entomol. 2006, 51, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, A.G.S.; Walters, K.F.A. Pathogenicity of the entomopathogenic fungus Lecanicillium muscarium against the sweetpotato whitefly Bemisia tabaci under laboratory and glasshouse conditions. Mycopathologia 2005, 160, 315–319. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Walters, K.F.A.; Northing, P. Susceptibility of Bemisia tabaci immature stages to the entomopathogenic fungus Lecanicillium muscarium on tomato and verbena foliage. Mycopathologia 2005, 159, 23–29. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Blackburn, L.F.; Northing, P.; Luo, W.; Cannon, R.J.C.; Walters, K.F.A. Further chemical compatibility testing of the entomopathogenic fungus Lecanicillium muscarium to control Bemisia tabaci in glasshouses. Int. J. Environ. Sci. Technol. 2010, 7, 405–409. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Alves Pereira-Junior, R.; Fernandes, E.K.K.; Quintela, E.D.; Dunlap, C.A.; Arthurs, S.P. Phenotype responses to abiotic stresses, asexual reproduction and virulence among isolates of the entomopathogenic fungus Cordyceps javanica (Hypocreales: Cordycipitaceae). Microbiol. Res. 2018, 216, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Ou, D.; Zhang, L.H.; Guo, C.F.; Chen, X.S.; Ali, S.; Qiu, B.L. Identification of a new Cordyceps javanica fungus isolate and its toxicity evaluation against Asian citrus psyllid. Microbiologyopen 2018, 8, e760. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, M.; Takatsuka, J. Isaria javanica (anamorphic Cordycipitaceae) isolated from gypsy moth larvae, Lymantria dispar (Lepidoptera: Lymantriidae), in Japan. Appl. Entomol. Zool. 2010, 45, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Gallou, A.; Serna-Dominguez, M.G.; Berlanga-Padilla, A.M.; Ayala-Zermeno, M.A.; Mellin-Rosas, M.A.; Montesinos-Matias, R.; Arredondo-Bernal, H.C. Species clarification of Isaria isolates used as biocontrol agents against Diaphorina citri (Hemiptera: Liviidae) in Mexico. Fungal Biol. 2016, 120, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento Silva, J.; Mascarin, G.M.; Dos Santos Gomes, I.C.; Tinoco, R.S.; Quintela, E.D.; Dos Reis Castilho, L.; Freire, D.M.G. New cost-effective bioconversion process of palm kernel cake into bioinsecticides based on Beauveria bassiana and Isaria javanica. Appl. Microbiol. Biotechnol. 2018, 102, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; Quintela, E.; Mascarin, G.; Santana, M. Enhanced mortality of Bemisia tabaci nymphs by Isaria javanica combined with sublethal doses of chemical insecticides. J. Appl. Entomol. 2018, 142, 598–609. [Google Scholar] [CrossRef]
- Xie, L.; Han, J.H.; Kim, J.J.; Sang, Y.L. Effects of culture conditions on conidial production of the sweet potato whitefly pathogenic fungus Isaria javanica. Mycoscience 2016, 57, 64–70. [Google Scholar] [CrossRef]
- Poprawski, T.J.; Legaspi, J.C.; Parker, P.E. Influence of entomopathogenic fungi on Serangium parcesetosum (Coleoptera: Coccinellidae), an important predator of whiteflies (homoptera: Aleyrodidae). Environ. Entomol. 1998, 27, 785–795. [Google Scholar] [CrossRef] [Green Version]
- Pell, J.K.; Vandenberg, J.D. Interactions among the aphid diuraphis noxia, the entomopathogenic fungus Paecilomyces fumosoroseus and the coccinellid Hippodamia convergens. Biocontrol Sci. Technol. 2002, 12, 217–224. [Google Scholar] [CrossRef]
- Di, X.; Ali, S.; Huang, Z.; Zhou, F.C.; Afzal, M.; Bashir, M.H. Influence of the entomopathogenic fungus, Verticillium lecanii on the whitefly predator, Axinoscymnus cardilobus (Coleoptera: Coccinellidae) under laboratory conditions. Pak. J. Zool. 2009, 41, 289–295. [Google Scholar]
- Barahona, C.F.S.; Threlkeld, B.S.; Avery, P.B.; Francis, A.W.; Cave, R.D. Compatibility and efficacy of the lady beetle Thalassa montezumae, and the entomopathogenic fungus Isaria fumosorosea, for biological control of the green croton scale: Laboratory and greenhouse investigations. Arthropod-Plant Interact. 2018, 12, 715–723. [Google Scholar] [CrossRef]
- Clara Scorsetti, A.; Pelizza, S.; Noelia Fogel, M.; Vianna, F.; Ines Schneider, M. Interactions between the entomopathogenic fungus Beauveria bassiana and the neotropical predator Eriopis connexa (Coleoptera: Coccinellidae): Implications in biological control of pest. J. Plant Prot. Res. 2017, 57, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Li, S.J.; Ahmed, M.Z.; Lv, N.; Shi, P.Q.; Wang, X.M.; Huang, J.L.; Qiu, B.L. Plant mediated horizontal transmission of Wolbachia between whiteflies. ISME J. 2017, 11, 1019–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.Z.; Ren, S.X.; Mandour, N.S.; Maruthi, M.N.; Naveed, M.; Qiu, B.-L. Phylogenetic analysis of Bemisia tabaci (Hemiptera: Aleyrodidae) populations from cotton plants in Pakistan, China, and Egypt. J. Pest Sci. 2009, 83, 135–141. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Blackburn, L.F.; Northing, P.; Luo, W.; Cannon, R.J.C.; Walters, K.F.A. Leaf dipping as an environmental screening measure to test chemical efficacy against Bemisia tabaci on poinsettia plants. Int. J. Environ. Sci. Technol. 2009, 6, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Qiu, B.L.; De Barro, P.J.; He, Y.R.; Ren, S.X. Suitability of Bemisia tabaci (Hemiptera: Aleyrodidae) instars for the parasitization by Encarsia bimaculata and Eretmocerus sp nr. furuhashii (Hymenoptera: Aphelinidae) on glabrous and hirsute host plants. Biocontrol Sci. Technol. 2007, 17, 823–839. [Google Scholar]
- IOBC (International Organization for Biological Control). IOBC Pesticide Side Effect Database. Available online: http://www.iobc-wprs.org/restricted_member/toolbox.cfm (accessed on 3 March 2016).
- Mesquita, D.A.L.M.; Lacey, L.A.; Leclant, F. Individual and combined effects of the fungus, Paecilomyces fumosoroseus and parasitoid, Aphelinus asychis Walker (hym. aphelinidae) on confined populations of russian wheat aphid, Diuraphis noxia (mordvilko) (hom. aphididae) under field conditions. J. Appl. Entomol. 2010, 121, 155–163. [Google Scholar] [CrossRef]
- Labbé, R.M.; Gillespie, D.R.; Cloutier, C.; Brodeur, J. Compatibility of an entomopathogenic fungus with a predator and a parasitoid in the biological control of greenhouse whitefly. Biocontrol Sci. Technol. 2009, 19, 429–446. [Google Scholar] [CrossRef]
- Ibarra-Cortes, K.H.; Guzman-Franco, A.W.; Gonzalez-Hernandez, H.; Ortega-Arenas, L.D.; Villanueva-Jimenez, J.A.; Robles-Bermudez, A. Susceptibility of Diaphorina citri (Hemiptera: Liviidae) and its parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) to entomopathogenic fungi under laboratory conditions. Neotrop. Entomol. 2018, 47, 131–138. [Google Scholar] [CrossRef]
- Goettel, M.S.; Hajek, A.E. Evaluation of non-target effects of pathogens used for management of arthropods. In Evaluating Indirect Ecological Effects of Biological Control; Wajnberg, E., Scott, J.K., Eds.; CABI Publishing: Cambridge, MA, USA, 2001; pp. 81–97. [Google Scholar]
- Ludwig, S.W.; Oetting, R.D. Susceptibility of natural enemies to infection by Beauveria bassiana and impact of insecticides on Ipheseius degenerans (Acari: Phytoseiidae). J. Agric. Urban Entomol. 2001, 18, 169–178. [Google Scholar]
- Seiedy, M.; Tork, M.; Deyhim, F. Effect of the entomopathogenic fungus Beauveria bassiana on the predatory mite Amblyseius swirskii (Acari: Phytoseiidae) as a non-target organism. Syst. Appl. Acarol. UK 2015, 20, 241–250. [Google Scholar]
- Huang, Z.; Sahar, F.; Ren, S.X.; Ali, S. Effect of Isaria fumosoroseus on Eretmocerus sp nr. furuhashii (Hymenoptera: Aphelinidae), a parasitoid of Bemisia tabaci (Hemiptera: Aleyrodidae). Pak. J. Zool. 2010, 42, 121–127. [Google Scholar]
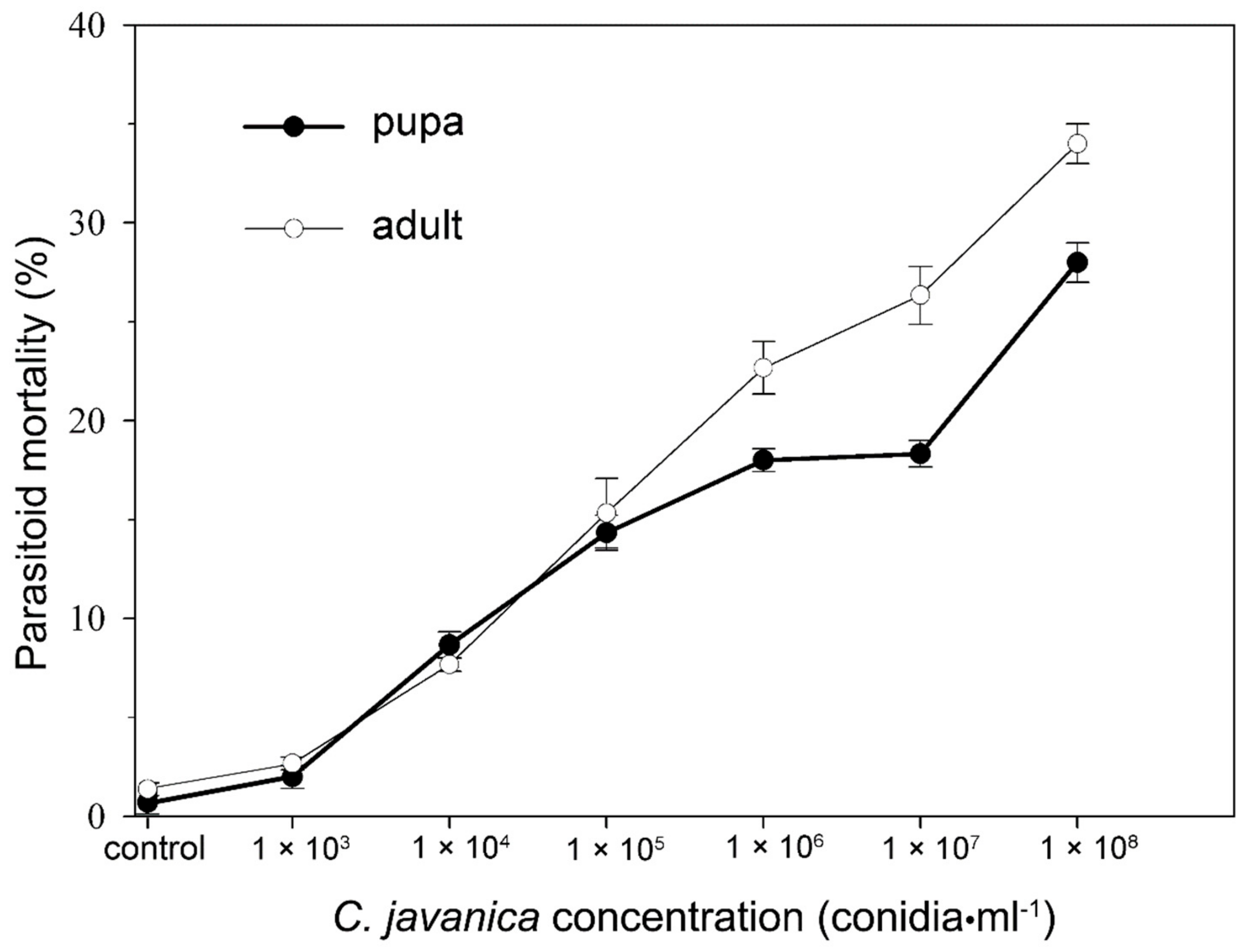
| Insect Stages | Regression Virulence Model | LC50 and 95% CI (conidia/mL) | R2 |
|---|---|---|---|
| Pupa | Y = 0.219X – 2.316 | 3.91 (0.55~72.2) × 1010 | 0.931 |
| Adult | Y = 0.241X – 2.350 | 5.56 (1.27~44.6) × 109 | 0.963 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, D.; Ren, L.-M.; -Liu, Y.; Ali, S.; Wang, X.-M.; Ahmed, M.Z.; Qiu, B.-L. Compatibility and Efficacy of the Parasitoid Eretmocerus hayati and the Entomopathogenic Fungus Cordyceps javanica for Biological Control of Whitefly Bemisia tabaci. Insects 2019, 10, 425. https://doi.org/10.3390/insects10120425
Ou D, Ren L-M, -Liu Y, Ali S, Wang X-M, Ahmed MZ, Qiu B-L. Compatibility and Efficacy of the Parasitoid Eretmocerus hayati and the Entomopathogenic Fungus Cordyceps javanica for Biological Control of Whitefly Bemisia tabaci. Insects. 2019; 10(12):425. https://doi.org/10.3390/insects10120425
Chicago/Turabian StyleOu, Da, Li-Mei Ren, Yuan -Liu, Shaukat Ali, Xing-Min Wang, Muhammad Z. Ahmed, and Bao-Li Qiu. 2019. "Compatibility and Efficacy of the Parasitoid Eretmocerus hayati and the Entomopathogenic Fungus Cordyceps javanica for Biological Control of Whitefly Bemisia tabaci" Insects 10, no. 12: 425. https://doi.org/10.3390/insects10120425
APA StyleOu, D., Ren, L.-M., -Liu, Y., Ali, S., Wang, X.-M., Ahmed, M. Z., & Qiu, B.-L. (2019). Compatibility and Efficacy of the Parasitoid Eretmocerus hayati and the Entomopathogenic Fungus Cordyceps javanica for Biological Control of Whitefly Bemisia tabaci. Insects, 10(12), 425. https://doi.org/10.3390/insects10120425






