Exposure of Larvae of the Solitary Bee Osmia bicornis to the Honey Bee Pathogen Nosema ceranae Affects Life History
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Ethical Statement
2.3. Preparation of Spore Suspension for Inoculation
2.4. Study Site and Experimental Set-Up
2.5. Treatment with N. ceranae
2.6. Sampling of Osmia Bee Larvae
Physiological States of Osmia Bee Larvae
2.7. Sampling of Pharates
Physiological States of the Pharate
2.8. Hatching Record of Imago
Physiological State of the Imago
2.9. Detection of N. ceranae in O. bicornis in Larvae and Pharate
2.10. Statistical Analysis
2.10.1. Statistical Analysis in Larvae and Pharates
2.10.2. Statistical Analysis of Imaginal Physiological State
3. Results
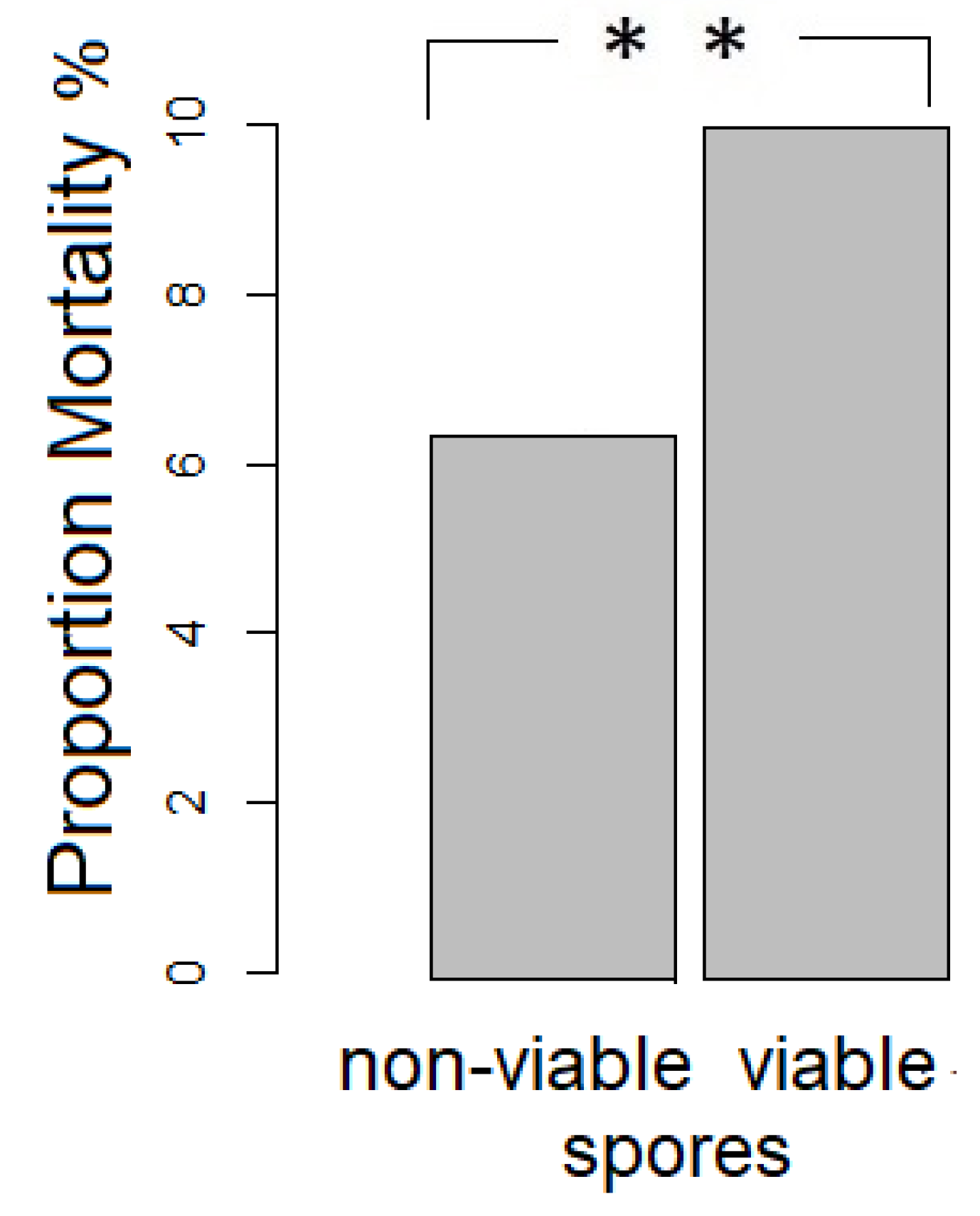
3.1. Mortality of Infected O. bicornis Larvae and Pharates
3.2. Detection of N. ceranae Spores
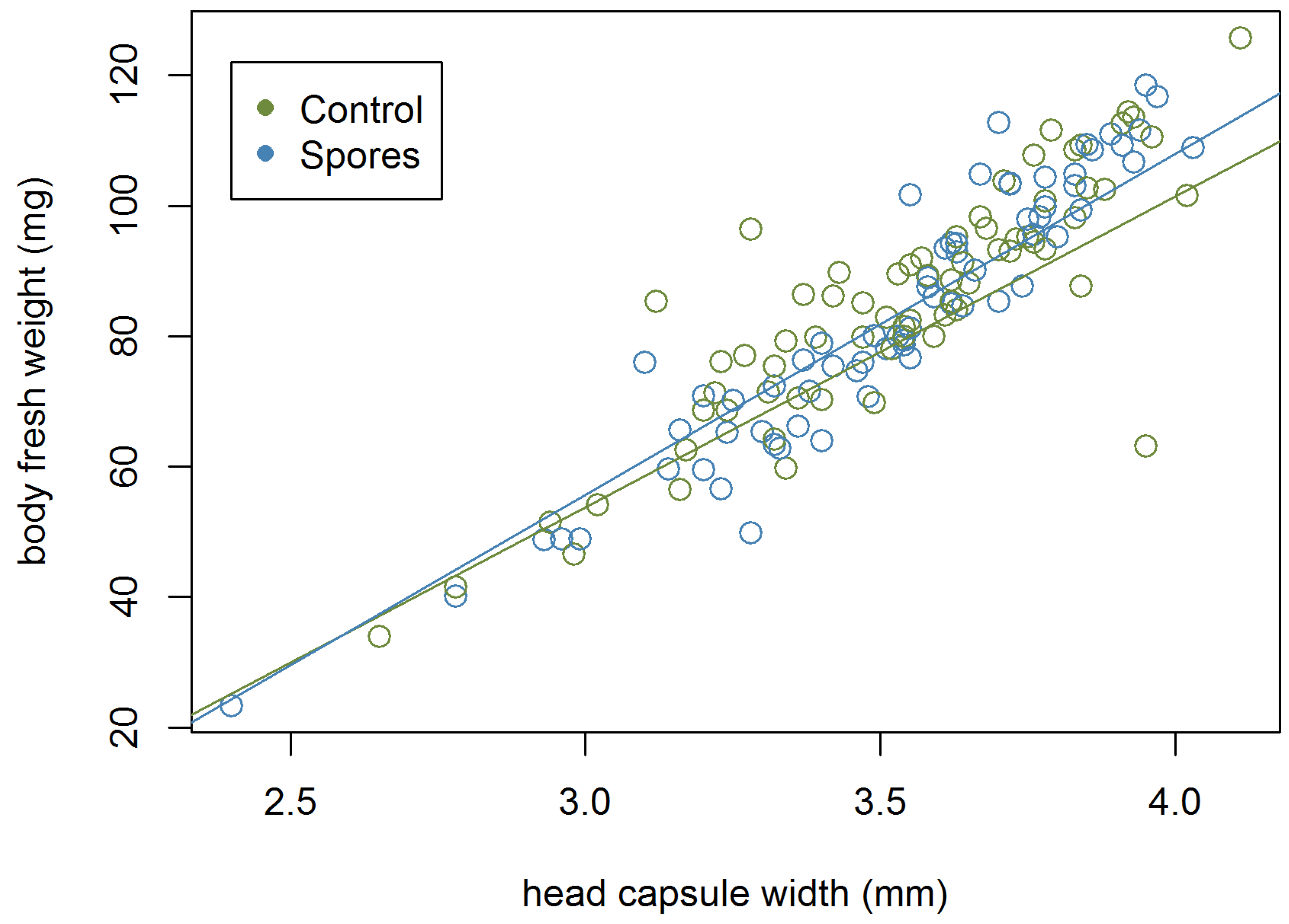
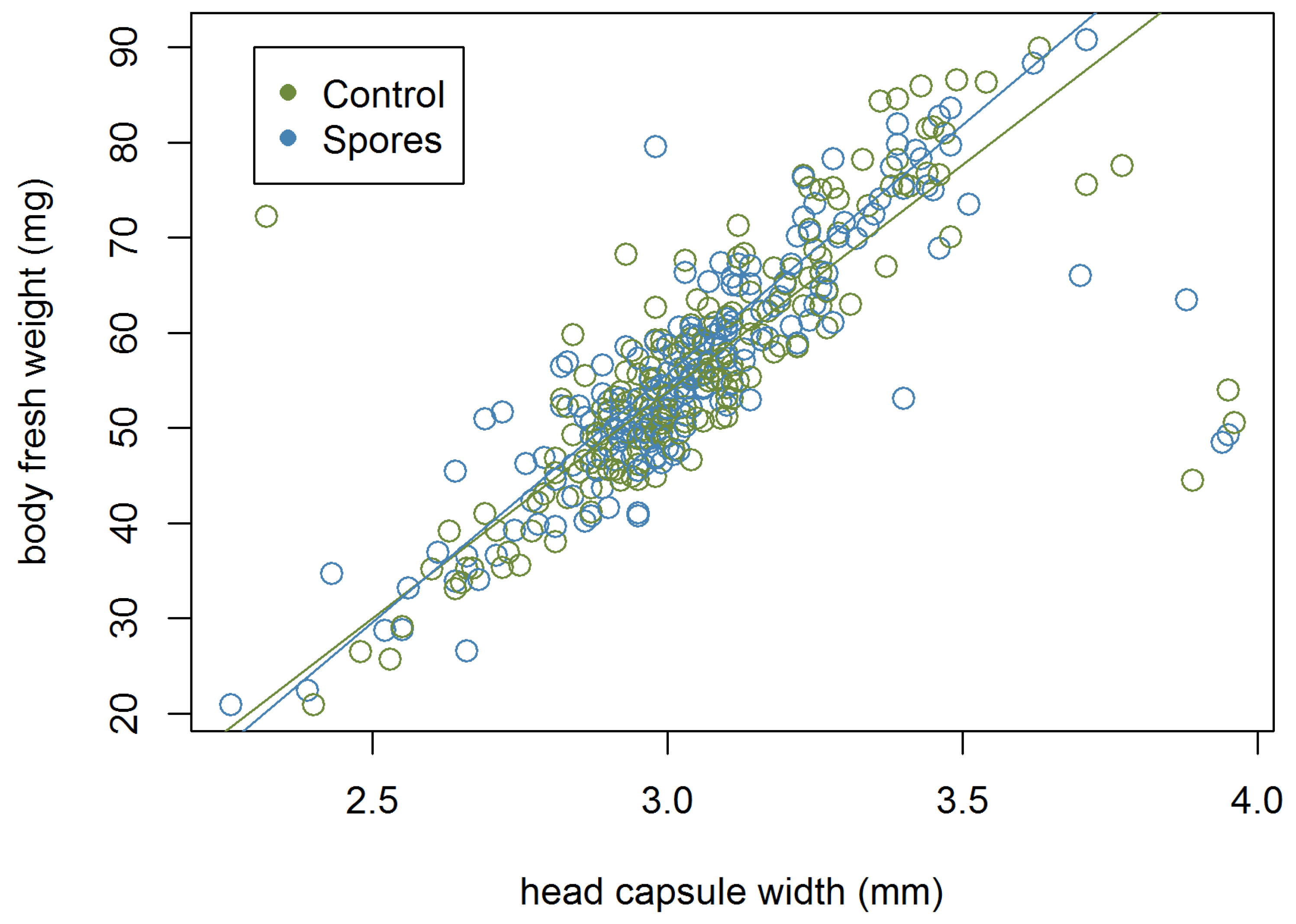
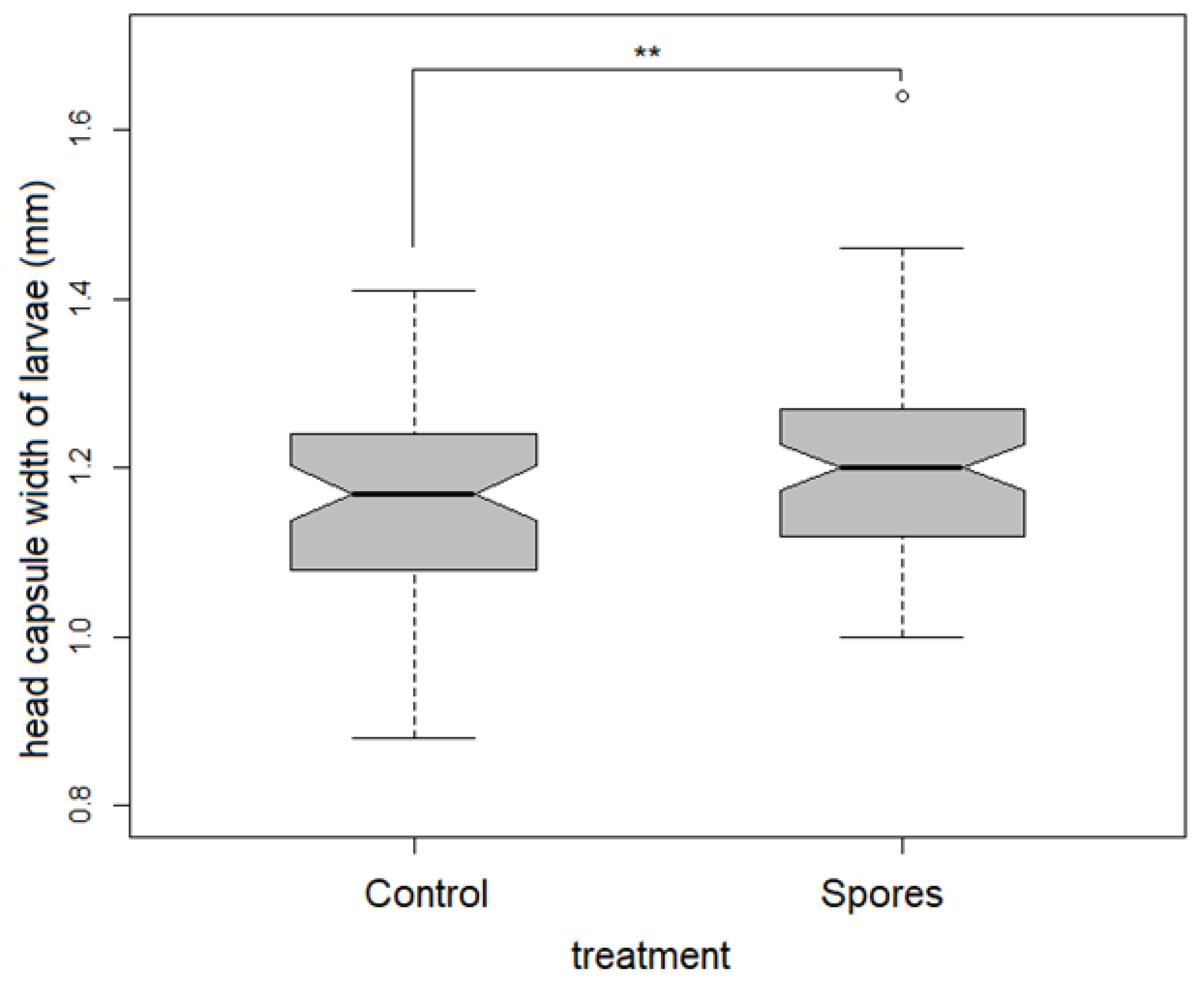
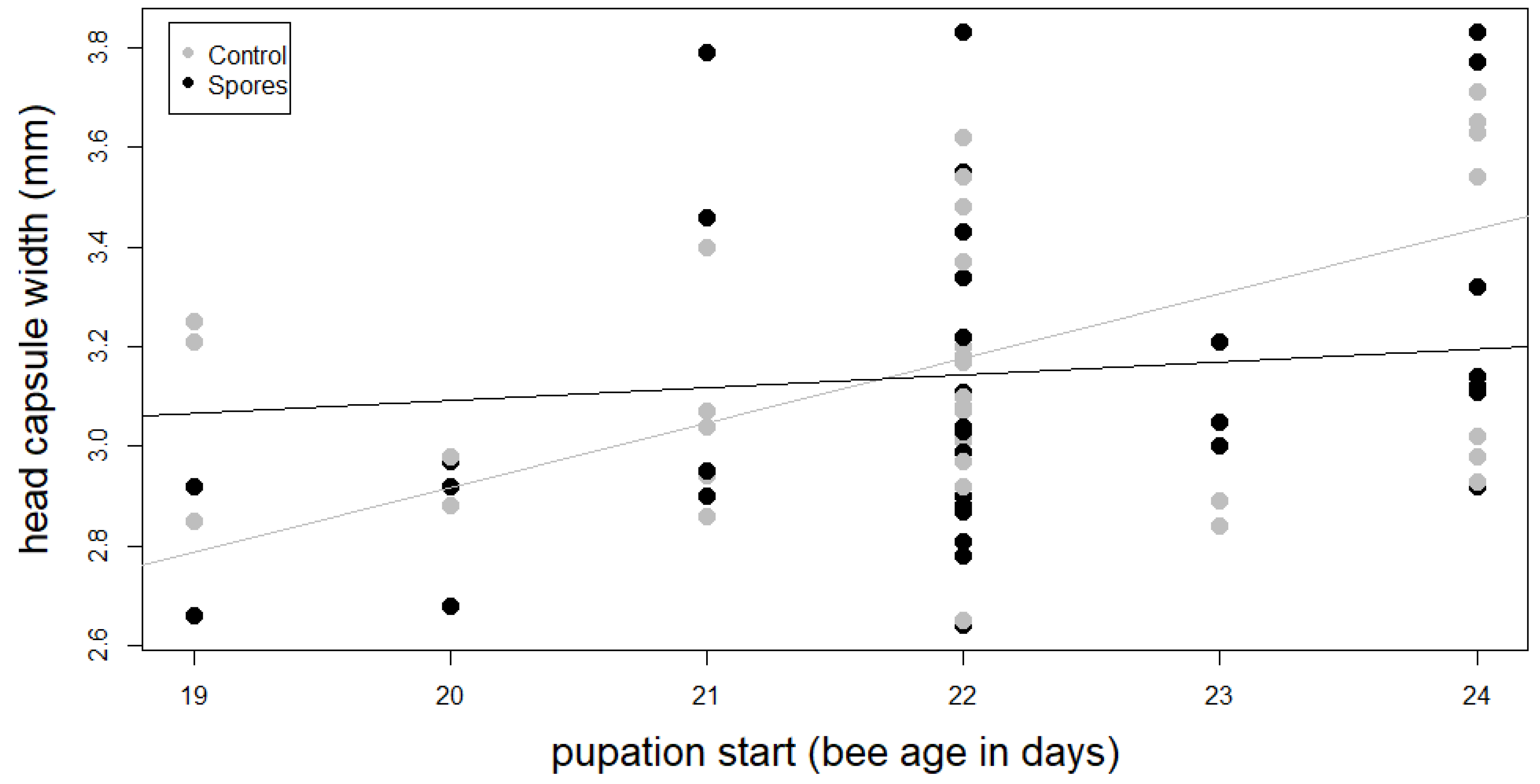
3.3. Development Time of the Larvae
3.4. Physiological State Imago
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sample size | Treatment | Count of Processed Part of the Larva for Spore Check | |
|---|---|---|---|
| Sum of larvae | whole larvae | separated larvae (body and gut) | |
| 61 | Control on food | 15 | 18 |
| Control on larva | 20 | 8 | |
| 77 | Spores on food | 35 | 2 |
| Spores on larva | 21 | 19 | |
| Sum of pharate | separated pharate (gut separated for spore check, spore check with n = 407) | ||
| 255 | Control on food | 127 | |
| Control on larva | 128 | ||
| 257 | Spores on food | 129 | |
| Spores on larva | 128 | ||
| Sex | Kind of Measurement | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| females | head capsule width | 139 | 3.52 | 0.30 | 2.40 | 4.11 |
| pupal weight (mg) | 97.33 | 23.16 | 25.98 | 148.10 | ||
| fresh body weight | 84.22 | 19.31 | 23.29 | 125.8 | ||
| males | head capsule width | 363 | 3.05 | 0.26 | 2.26 | 3.96 |
| pupal weight (mg) | 66.21 | 15.44 | 23.03 | 108.75 | ||
| fresh body weight | 56.28 | 21.155 | 20.89 | 90.83 |
| Sex | Kind of Measurement | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| females | head capsule width ‘Co’ | 59 | 3.54 | 0.28 | 2.40 | 4.03 |
| head capsule width ‘Sp’ | 80 | 3.51 | 0.32 | 2.65 | 4.11 | |
| pupal weight ‘Co’ | 59 | 97.59 | 21.91 | 25.98 | 131.12 | |
| pupal weight ‘Sp’ | 80 | 97.13 | 24.18 | 37.83 | 148.10 | |
| fresh body weight ‘Co’ | 59 | 84.23 | 18.38 | 23.29 | 112.81 | |
| fresh body weight ‘Sp’ | 80 | 84.22 | 20.08 | 34.01 | 125.80 | |
| males | head capsule width ‘Co’ | 193 | 3.06 | 0.27 | 2.26 | 3.96 |
| head capsule width ‘Sp’ | 170 | 3.05 | 0.24 | 2.32 | 3.89 | |
| pupal weight ‘Co’ | 193 | 64.78 | 15.10 | 23.03 | 108.75 | |
| pupal weight ‘Sp’ | 170 | 67.83 | 15.70 | 25.89 | 102.14 | |
| fresh body weight ‘Co’ | 193 | 55.18 | 12.32 | 20.89 | 90.83 | |
| fresh body weight ‘Sp’ | 170 | 57.53 | 12.78 | 22.43 | 85.92 |
| Treatment | Dataset/Treatment | N | Mean | SD | Min | Max |
| Complete dataset | 87 | 22.0 | 1.4 | 19 | 24 | |
| Control | Control on food | 24 | 22.1 | 1.4 | 19 | 24 |
| Control on larva | 23 | 21.5 | 1.3 | 19 | 24 | |
| Control sum | 47 | 21.8 | 1.4 | 19 | 24 | |
| Spores | Spores on food | 28 | 22.5 | 1.5 | 19 | 24 |
| Spores on larva | 12 | 21.8 | 0.5 | 21 | 22 | |
| Spores sum | 40 | 22.3 | 1.3 | 19 | 24 |
| Samples | Depen-Dent Variable | Independent Variable | Co-variate (C)/Interaction (I) | Num. d. f. | F-Value | p-Value | Estimate |
|---|---|---|---|---|---|---|---|
| pharate (history) | pupation start | treatment, inoculation type | C: head Capsule width | 73 | 4.304 | 0.0035 | Head 1.44769 |
| pharate (history) | pupation start | treatment | I: head Capsule width | 74 | 6.521 | 0.0005 | treatSp 6.8983 Head 2.5875 treatSp:head −2.0463 |
| larvae | head capsule width | treatment | 133 | 6.632 | 0.011 | treatSp 0.05150 | |
| pharate | head capsule width | fresh weight | C: sex | 499 | 950.1 | < 0.001 | Weight 0.0149293 Sexm −0.0552998 |
| pharate | head capsule width | cocoon weight | C: sex | 499 | 956.4 | < 0.001 | c.weight 0.0123092 sexm −0.0894422 |
| pharate | Fresh weight | cocoon weight | C: sex | 499 | 6.292e+04 | < 0.001 | c.weight 0.82060 sexm −2.40835 |
| pharate | weight difference | treatment | C: sex | 500 | 3.934 | 0.0479 | teatmentSp 0.6557 |
References
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services IPBES. Full Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Imperatriz Fonseca, V.L., Potts, G.S., Baste, I.A., Apau Oteng Yeboah, A., Eds.; IPBES: Bonn, Germany, 2016. [Google Scholar]
- Stokstad, E. The Case of the Empty Hives. Science 2007, 316, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, B.P. What’s Killing American Honey Bees? PLoS Biol. 2007, 5, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; Criado, M.G.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2015. [Google Scholar]
- Neumann, P.; Carreck, P. Honey Bee Colony Losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L.; Robinson, G.E. Patterns of Widespread Decline in North American Bumble Bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef]
- Fitzpatrick, Ú.; Murray, T.E.; Paxton, R.J.; Breen, J.; Cotton, D.; Santorum, V.; Brown, M.J.F. Rarity and Decline in Bumblebees—A Test of Causes and Correlates in the Irish Fauna. Biol. Conserv. 2007, 136, 185–194. [Google Scholar] [CrossRef]
- Bartomeus, I.; Ascher, J.S.; Gibbs, J.; Danforth, B.N.; Wagner, D.L.; Hedtke, S.M.; Winfree, R. Historical Changes in Northeastern US Bee Pollinators Related to Shared Ecological Traits. Proc. Natl. Acad. Sci. USA 2013, 110, 4656–4660. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Garratt, M.P. Threats to an Ecosystem Service: Pressures on Pollinators. Front. Ecol. Environ. 2013, 251–259. [Google Scholar] [CrossRef]
- Brown, M.J.F.F.; Paxton, R.J. The Conservation of Bees: A Global Perspective. Apidologie 2009, 40, 410–416. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schafers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherland. Science 2006, 313, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Winfree, R.; Kremen, C. Are Ecosystem Services Stabilized by Differences among Species? A Test Using Crop Pollination. Proc. R. Soc. B 2009, 276, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed Coating with a Neonicotinoid Insecticide Negatively Affects Wild Bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, B.A.; Isaac, N.J.B.; Bullock, J.M.; Roy, D.B.; Garthwaite, D.G.; Crowe, A.; Pywell, R.F. Impacts of Neonicotinoid Use on Long-Term Population Changes in Wild Bees in England. Nat. Commun. 2016, 7, 12459. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-Specific Effects of Neonicotinoid Pesticides on Honey Bees and Wild Bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding Pollinators and Their Values to Human Well-Being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Brown, M.J.F.; Dicks, L.V.; Paxton, R.J.; Baldock, K.C.R.; Barron, A.B.; Chauzat, M.-P.; Freitas, B.M.; Goulson, D.; Jepsen, S.; Kremen, C.; et al. A Horizon Scan of Future Threats and Opportunities for Pollinators and Pollination. PeerJ 2016, 4, e2249. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Sci. Express 2015, 347, 1–16. [Google Scholar] [CrossRef]
- Sánchez-bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are Bee Diseases Linked to Pesticides? A Brief Review. 2016, 90, 7–11. [Google Scholar]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, 1–13. [Google Scholar] [CrossRef]
- Brunner, F.S.; Schmid-Hempel, P.; Barribeau, S.M. Protein-Poor Diet Reduces Host-Specific Immune Gene Expression in Bombus Terrestris. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140128. [Google Scholar] [CrossRef] [PubMed]
- Bryden, J.; Gill, R.J.; Mitton, R.A.A.; Raine, N.E.; Jansen, V.A.A. Chronic Sublethal Stress Causes Bee Colony Failure. Ecol. Lett. 2013, 16, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid Clothianidin Adversely Affects Insect Immunity and Promotes Replication of a Viral Pathogen in Honey Bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [PubMed]
- Doublet, V.; Labarussias, M.; De Miranda, J.R.; Moritz, R.F.A.; Paxton, R.J. Bees under Stress: Sublethal Doses of a Neonicotinoid Pesticide and Pathogens Interact to Elevate Honey Bee Mortality across the Life Cycle. Environ. Microbiol. 2015, 17, 969–983. [Google Scholar] [CrossRef]
- Manley, R.; Boots, M.; Wilfert, L. Condition-dependent Virulence of Slow Bee Paralysis Virus in Bombus Terrestris: Are the Impacts of Honeybee Viruses in Wild Pollinators Underestimated? Oecologia 2017, 184, 305–315. [Google Scholar] [CrossRef]
- Garibaldi, L.; Steffen-Dewenter, I.; Winfree, R.; Al, E. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Bosch, J.; Bosch, J.; Kemp, W.P. Developing and Establishing Bee Species as Crop Pollinators: The Example of Osmia Spp. (Hymenoptera: Megachilidae) and Fruit Trees. Bull. Entomol. Res. 2002, 92, 3–16. [Google Scholar]
- McMenamin, A.J.; Genersch, E. Honey Bee Colony Losses and Associated Viruses. Curr. Opin. Insect Sci. 2015, 8, 121–129. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Wilfert, L.; Martin, S.J.; Jones, I.M.; Schroeder, D.C. Diversity in a Honey Bee Pathogen: First Report of a Third Master Variant of the Deformed Wing Virus Quasispecies. ISME J. 2016, 10, 1264–1273. [Google Scholar] [CrossRef]
- Remnant, E.J.; Shi, M.; Buchmann, G.; Blacquière, T.; Holmes, E.C.; Beekman, M.; Ashe, A. A Diverse Range of Novel RNA Viruses in Geographically Distinct Honey Bee Populations. J. Virol. 2017, 91, e00158-17. [Google Scholar] [CrossRef]
- Galbraith, D.A.; Fuller, Z.L.; Ray, A.M.; Brockmann, A.; Frazier, M.; Gikungu, M.W.; Martinez, J.F.I.; Kapheim, K.M.; Kerby, J.T.; Kocher, S.D.; et al. Investigating the Viral Ecology of Global Bee Communities with High-Throughput Metagenomics. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Budge, G.E.; Pietravalle, S.; Brown, M.; Laurenson, L.; Jones, B.; Tomkies, V.; Delaplane, K.S. Pathogens as Predictors of Honey Bee Colony Strength in England and Wales. PLoS ONE 2015, 10, e0133228. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive Markers of Honey Bee Colony Collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A Mutualistic Symbiosis between a Parasitic Mite and a Pathogenic Virus Undermines Honey Bee Immunity and Health. Proc. Natl. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Varroa-Virus Interaction in Collapsing Honey Bee Colonies. PLoS ONE 2013, 8, e57540. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E. Honey Bee Pathology: Current Threats to Honey Bees and Beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Highfield, A.C.; El Nagar, A.; Mackinder, L.C.M.; Noël, L.M.L.J.; Hall, M.J.; Martin, S.J.; Schroeder, D.C. Deformed Wing Virus Implicated in Overwintering Honeybee Colony Losses. Appl. Environ. Microbiol. 2009, 75, 7212–7220. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Vedova, G.D.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic Parasite-Pathogen Interactions Mediated by Host Immunity Can Drive the Collapse of Honeybee Colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef]
- Nguyen, B.K.; Ribière, M.; vanEngelsdorp, D.; Snoeck, C.; Saegerman, C.; Kalkstein, A.L.; Schurr, F.; Brostaux, Y.; Faucon, J.-P.; Haubruge, E. Effects of Honey Bee Virus Prevalence, Varroa Destructor Load and Queen Condition on Honey Bee Colony Survival over the Winter in Belgium. J. Apic. Res. 2011, 50, 195–202. [Google Scholar] [CrossRef]
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. Nosema Ceranae Has Infected Apis Mellifera in Europe since at Least 1998 and May Be More Virulent than Nosema Apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Natsopoulou, M.E.; Doublet, V.; Paxton, R.J. European Isolates of the Microsporidia Nosema Apis and Nosema Ceranae Have Similar Virulence in Laboratory Tests on European Worker Honey Bees. Apidologie 2016, 47, 57–65. [Google Scholar] [CrossRef][Green Version]
- Higes, M.; Martín-Hernández, R.; Garrido-Bailón, E.; García-Palencia, P.; Meana, A. Detection of Infective Nosema Ceranae (Microsporidia) Spores in Corbicular Pollen of Forager Honey bees. J. Invertebr. Pathol. 2008, 97, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Gisder, S.; Hedtke, K.; Mockel, N.; Frielitz, M.C.; Linde, A.; Genersch, E. Five-Year Cohort Study of Nosema Spp. in Germany: Does Climate Shape Virulence and Assertiveness of Nosema Ceranae? Appl. Environ. Microbiol. 2010, 76, 3032–3038. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.M.; Puerta, F.; Cousinou, M.; Dios-Palomares, R.; Campano, F.; Redondo, L. Asymptomatic Presence of Nosema Spp. in Spanish Commercial Apiaries. J. Invertebr. Pathol. 2012, 111, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.A.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J.F. Disease Associations between Hone ybees and Bumblebees as a Threat to Wild Pollinators. Nature 2014, 506, 364–366. [Google Scholar] [CrossRef]
- Graystock, P.; Yates, K.; Darvill, B.; Goulson, D.; Hughes, W.O.H. Emerging Dangers: Deadly Effects of an Emergent Parasite in a New Pollinator Host. J. Invertebr. Pathol. 2013, 114, 114–119. [Google Scholar] [CrossRef]
- Graystock, P.; Yates, K.; Evison, S.E.F.; Darvill, B.; Goulson, D.; Hughes, W.O.H. The Trojan Hives: Pollinator Pathogens, Imported and Distributed in Bumblebee Colonies. J. Appl. Ecol. 2013, 50, 1207–1215. [Google Scholar] [CrossRef]
- Ravoet, J.; De Smet, L.; Meeus, I.; Smagghe, G.; Wenseleers, T.; de Graaf, D.C. Widespread Occurrence of Honey Bee Pathogens in Solitary Bees. J. Invertebr. Pathol. 2014, 122, 55–58. [Google Scholar] [CrossRef]
- Shafer, A.B.A.; Williams, G.R.; Shutler, D.; Rogers, R.E.L.; Stewart, D.T.; Journal, T.; Shafer, B.A.; Williams, R.; Shutler, D.; Stewart, T. Cophylogeny of Nosema (Microsporidia: Nosematidae) and Bees (Hymenoptera: Apidae) Suggests Both Cospeciation and a Host-Switch Reviewed Work (s): Published by: The American Society of Parasitologists Content in a Trusted Digital Archive. We Use. J. Parasitol. 2009, 95, 198–203. [Google Scholar] [CrossRef]
- Sheldon, B.C.; Verhulst, S. Ecological Immunology: Costly Parasiet Defences and Trade-Offs in Evolutionary Ecology. Tree 1996, 5347, 317–321. [Google Scholar] [CrossRef]
- Armitage, S.A.O.; Thompson, J.J.W.; Rolff, J. Examining Costs of Induced and Constitutive Immune Investment in Tenebrio Molitor. J. Evol. Biol. 2003, 16, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Moret, Y.; Schmid-Hempel, P. Survival for Immunity: The Price of Immune System Activation for Bumblebee Workers. Science 2000, 290, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P. Variation in Immune Defence as a Question of Evolutionary Ecology. Proc. R. Soc. B Biol. Sci. 2003, 270, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Kirschman, L.J.; Crespi, E.J.; Warne, R.W. Critical Disease Windows Shaped by Stress Exposure Alter Allocation Trade-Offs between Development and Immunity. J. Anim. Ecol. 2018, 87, 235–246. [Google Scholar] [CrossRef]
- Stearns, S.C. Life History Evolution: Successes, Limitations, and Prospects. Naturwissenschaften 2000, 87, 476–486. [Google Scholar] [CrossRef]
- Strohm, E. How Can Cleptoparasitic Drosophilid Flies Emerge from the Closed Brood Cells of the Red Mason Bee? Physiol. Entomol. 2011, 36, 77–83. [Google Scholar] [CrossRef]
- Kornmilch, J.-C. Einsatz von Mauerbienen Zur Bestäubung von Obstkulturen—Handbuch Zur Nutzung Der Roten Mauerbiene in Obstplantagen Und Kleingärten. Available online: http://www.bund-lemgo.de/download/Handbuch_der_Mauerbienenzucht.pdf (accessed on 16 September 2019).
- Westrich, P. Die Wildbienen Baden-Würtembergs, 1. Teil: Lebensräume, Verhalten, Ökologie und Schutz, 2. Spezieller Teil: Die Gattungen und Arten; Eugen Ulmer Verlag: Stuttgart, Germany, 1989; p. 972. [Google Scholar]
- Radmacher, S.; Strohm, E. Effects of Constant and Fluctuating Temperatures on the Development of the Solitary Bee Osmia Bicornis (Hymenoptera: Megachilidae). Apidologie 2011, 42, 711–720. [Google Scholar] [CrossRef]
- Gisder, S.; Genersch, E. Molecular Differentiation of Nosema Apis and Nosema Ceranae Based on Species–Specific Sequence Differences in a Protein Coding Gene. J. Invertebr. Pathol. 2013, 113, 1–6. [Google Scholar] [CrossRef]
- Williams, G.R.; Alaux, C.; Costa, C.; Csáki, T.; Doublet, V.; Eisenhardt, D.; Fries, I.; Kuhn, R.; McMahon, D.P.; Medrzycki, P.; et al. Standard Methods for Maintaining Adult Apis Mellifera in Cages under in vitro Laboratory Conditions. J. Apic. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.-P.; Chen, Y.-P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard Methods for Nosema Research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Human, H.; Brodschneider, R.; Dietemann, V.; Dively, G.; Ellis, J.D.; Forsgren, E.; Fries, I.; Hatjina, F.; Hu, F.L.; Jaffé, R.; et al. Miscellaneous Standard Methods for Apis Mellifera Research. J. Apic. Res. 2013, 52, 1–56. [Google Scholar] [CrossRef]
- Cantwell, G.E. Standard Methods for Counting Nosema Spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- Fries, I. Nosema Ceranae in European Honey Bees (Apis Mellifera). J. Invertebr. Pathol. 2010, 103 (Suppl. 1), S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, E.; Fries, I. Comparative Virulence of Nosema Ceranae and Nosema Apis in Individual European Honey Bees. Vet. Parasitol. 2010, 170, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.J.; Uppala, S.S.; Lucas, H.M.; Sagili, R.R. Effects of Pollen Dilution on Infection of Nosema Ceranae in Honey Bees. J. Insect Physiol. 2016, 87, 12–19. [Google Scholar] [CrossRef]
- Eiri, D.M.; Suwannapong, G.; Endler, M.; Nieh, J.C. Nosema Ceranae Can Infect Honey Bee Larvae and Reduces Subsequent Adult Longevity. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Raw, A. The Biology of the Solitary Bee Osmia Rufa (L.) Megachilidae. Trans. R. Entemological Soc. Lond. 1972, 124, 213–229. [Google Scholar] [CrossRef]
- Vogelweith, F.; Thiery, D.; Moret, Y.; Moreau, J. Immunocompetence Increases with Larval Body Size in a Phytophagous Moth. Physiol. Entomol. 2013, 38, 219–225. [Google Scholar] [CrossRef]
- Bosch, J.; Vicens, N. Body Size as an Estimator of Production Costs in a Solitary Bee. Ecol. Entomol. 2002, 27, 129–137. [Google Scholar] [CrossRef]
- Amiet, F.; Krebs, A. Bienen Mitteleuropas. Gattungen, Lebensweise, Beobachtung. 2.korrigierte Auflage; Haupt Verlag: Bern, Switzerland, 2014; p. 424. [Google Scholar]
- Cane, J.H. Estimation of Bee Size Using Intertegular Span ( Apoidea ). J. Kansas Entomol. Soc. 1987, 60, 145–147. [Google Scholar]
- Amdam, G.V.; Omholt, S.W. The Regulatory Anatomy of Honeybee Lifespan. J. Theor. Biol. 2002, 216, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Mikolajewski, D.J.; De Block, M.; Stoks, R. The Interplay of Adult and Larval Time Constraints Shapes Species Differences in Larval Life History. Ecology 2015, 96, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- De Block, M.; Slos, S.; Johansson, F.; Stoks, R. Integrating Life History and Physiology to Understand Latitudinal Size Variation in a Damselfly. Ecography (Cop.) 2008, 31, 115–123. [Google Scholar] [CrossRef]
- Plaistow, S.; Siva-Jothy, M.T. Energetic Constraints and Male Mate-Securing Tactics in the Damselfly Calopteryx Splendens Xanthostoma (Charpentier). Proc. R. Soc. B Biol. Sci. 1996, 263, 1233–1239. [Google Scholar]
- Dussaubat, C.; Sagastume, S.; Gómez-Moracho, T.; Botías, C.; García-Palencia, P.; Martín-Hernández, R.; Le Conte, Y.; Higes, M. Comparative Study of Nosema Ceranae (Microsporidia) Isolates from Two Different Geographic Origins. Vet. Microbiol. 2013, 162, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Hacker, C.; Howell, M.; Bhella, D.; Lucocq, J. Strategies for Maximizing ATP Supply in the Microsporidian Encephalitozoon Cuniculi: Direct Binding of Mitochondria to the Parasitophorous Vacuole and Clustering of the Mitochondrial Porin VDAC. Cell. Microbiol. 2014, 16, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune Suppression in the Honey Bee (Apis Mellifera) Following Infection by Nosema Ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Huang, Q.; Kryger, P.; Le Conte, Y.; Moritz, R.F.A. Survival and Immune Response of Drones of a Nosemosis Tolerant Honey Bee Strain towards N. Ceranae Infections. J. Invertebr. Pathol. 2012, 109, 297–302. [Google Scholar] [CrossRef]
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome Analyses of the Honeybee Response to Nosema Ceranae and Insecticides. PLoS ONE 2014, 9, e91686. [Google Scholar] [CrossRef]
- Badaoui, B.; Fougeroux, A.; Petit, F.; Anselmo, A.; Gorni, C.; Cucurachi, M.; Cersini, A.; Granato, A.; Cardeti, G.; Formato, G.; et al. RNA-Sequence Analysis of Gene Expression from Honey bees (Apis Mellifera) Infected with Nosema Ceranae. PLoS ONE 2017, 12, e0173438. [Google Scholar] [CrossRef]
- Baer, B.; Armitage, S.A.O.; Boomsma, J.J. Sperm Storage Induces an Immunity Cost in Ants. Nature 2006, 441, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P.; Loosli, R. A Contribution to the Knowledge of Nosema Infections in Bumble Bees, Bombus Spp. Apidologie 1998, 29, 525–535. [Google Scholar] [CrossRef][Green Version]
- Higes, M.; Juarranz, Á.; Dias-Almeida, J.; Lucena, S.; Botías, C.; Meana, A.; García-Palencia, P.; Martín-Hernández, R. Apoptosis in the Pathogenesis of Nosema Ceranae (Microsporidia: Nosematidae) in Honey Bees (Apis Mellifera). Environ. Microbiol. Rep. 2013, 5, 530–536. [Google Scholar] [CrossRef]
- Fontbonne, R.; Garnery, L.; Vidau, C.; Aufauvre, J.; Texier, C.; Tchamitchian, S.; El Alaoui, H.; Brunet, J.L.; Delbac, F.; Biron, D.G. Comparative Susceptibility of Three Western Honeybee Taxa to the Microsporidian Parasite Nosema Ceranae. Infect. Genet. Evol. 2013, 17, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kurze, C.; Routtu, J.; Moritz, R.F.A. Parasite Resistance and Tolerance in Honey bees at the Individual and Social Level. Zoology 2016, 119, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Lochmiller, R.L.; Deerenberg, C. Trade-Offs in Evolutionary Immunology: Just what is the Cost of Immunity? Oikos 2000, 88, 87–98. [Google Scholar] [CrossRef]
- Moret, Y.; Sciences, P.; Sheffield, U.; Bank, W.; Tn, S.U.K. Explaining Ariable Costs of the Immune Response: Selection for Specific 7 Ersus Non—Specific Immunity and Facultati 7 e Life History Change. Oikos 2003, 102, 213–216. [Google Scholar] [CrossRef]
- Rowley, A.F.; Powell, A.; Rowley, A.F.; Powell, A. Invertebrate Immune Systems—Specific, Quasi-Specific, or Nonspecific? J. Immunol. 2007, 179, 7209–7214. [Google Scholar] [CrossRef]
- Zanchi, C.; Troussard, J.; Martinaud, G. Differential Expression and Costs between Maternally and Paternally Derived Immune Priming for Offspring in an Insect. J. Anim. Ecol. 2011, 2010, 1174–1183. [Google Scholar] [CrossRef]
- Laughton, A.M.; Boots, M.; Siva-Jothy, M.T. The Ontogeny of Immunity in the Honey Bee, Apis Mellifera L. Following an Immune Challenge. J. Insect Physiol. 2011, 57, 1023–1032. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic Stress in the Honeybee Apis Mellifera from Nosema Ceranae Infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Cabodevilla, O.; Villar, E.; Virto, C.; Murillo, R.; Williams, T.; Caballero, P. Intra- and Intergenerational Persistence of an Insect Nucleopolyhedrovirus: Adverse Effects of Sublethal Disease on Host Development, Reproduction, and Susceptibility to Superinfection. Appl. Environ. Microbiol. 2011, 77, 2954–2960. [Google Scholar] [CrossRef]
- Monobrullah, M.; Shankar, U. Sub-Lethal Effects of Splt MNPV Infection on Developmental Stages of Spodoptera Litura (Lepidoptera: Noctuidae). Biocontrol Sci. Technol. 2008, 18, 431–437. [Google Scholar] [CrossRef]
- Milks, M.L.; Burnstyn, I.; Myers, J.H. Influence of Larval Age on the Lethal and Sublethal Effects of the Nucleopolyhedrovirus of Trichoplusia Ni in the Cabbage Looper. Biol. Control 1998, 126, 119–126. [Google Scholar] [CrossRef]
- Mullen, L.; Goldsworthy, G. Birkbeck EPrints: An Open Access Repository of the Research Output of Birkbeck College Mullen, Lisa and Goldsworthy, Graham (2003). Changes in Lipophorins Are Related to the Activation of Phenoloxidase in the Haemolymph of Locusta Migratoria in Respo. Insect Biochem. Mol. Biol. 2003, 33, 661–670. [Google Scholar] [CrossRef]
- Cheon, H.; Shin, S.W.; Bian, G.; Park, J.; Raikhel, A.S. Regulation of Lipid Metabolism Genes, Lipid Carrier Protein Lipophorin, and Its Receptor during Immune Challenge in the Mosquito Aedes Aegypti. J. Biol. Chem. 2006, 281, 8426–8435. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, B. Thermoregulation in Bumblebees. J. Comp. Physiol. 1974, 88, 129–140. [Google Scholar] [CrossRef]
| Samples | Sample Size Registered Brood Cells (from N = 1592) | Sample Size for Mortality Rates (from N = 1085) | Data Set Size for Statistic (from N = 1085) | Head Capsule Width/Weight | Spore Check |
|---|---|---|---|---|---|
| All bees | 1085 | 915 | 783 | 645 | 545 |
| Larvae | 138 | 138 | 138 | ||
| Pharates | 512 | 507 | 407 | ||
| Imagines | 133 | - | - |
| Samples | Depen-dent Variable | Independent Variable | Co-Variate (C)/Interaction (I) | Num. d.f. | F-value | p-Value | Analysis |
|---|---|---|---|---|---|---|---|
| pharate (history) | pupation start | treatment, inoculation type | C: head capsule width | 73 | 4.304 | 0.0035 | lm: anova |
| pharate (history) | pupation start | treatment | I: head capsule width | 74 | 6.521 | 0.0005 | lm: anova |
| larva | head capsule width | treatment | 133 | 6.632 | 0.011 | lm: anova | |
| pharate | head capsule width | fresh weight | C: sex | 499 | 950.1 | <0.001 | regression |
| pharate | head capsule width | cocoon weight | C: sex | 499 | 956.4 | <0.001 | regression |
| pharate | Fresh weight | cocoon weight | C: sex | 499 | 6.292e+04 | <0.001 | regression |
| pharate | weight difference | treatment | C: sex | 500 | 3.934 | 0.0479 | lm: anova |
| Dependent Variable | Independent Variable | Shapiro-Test (p-Value) | Model | Residual Deviance | Num. d.f. | F-Value/ t-Value | p-Value |
|---|---|---|---|---|---|---|---|
| fatbody weight | males s/c | 0.03255 | glm | 2753.3 | 79 | 0.257 | 0.798 |
| fatbody weight | females s/c | 0.03404 | glm | 0.0025140 | 36 | (−)1.72 | 0.0941 |
| tegulae distance | males s/c | 0.3696 | lm | 79 | 0.4379 | 0.5101 | |
| tegulae distance | females s/c | 0.5313 | lm | 36 | (−)1558 | 0.1281 | |
| wing muscle weight | males s/c | 0.01899 | glm | 0.00036717 | 76 | (−)1360 | 0.178 |
| wing muscle weight | females s/c | 1.03 × 10−4 | glm | 0.00055552 | 37 | 0.994 | 0.32667 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bramke, K.; Müller, U.; McMahon, D.P.; Rolff, J. Exposure of Larvae of the Solitary Bee Osmia bicornis to the Honey Bee Pathogen Nosema ceranae Affects Life History. Insects 2019, 10, 380. https://doi.org/10.3390/insects10110380
Bramke K, Müller U, McMahon DP, Rolff J. Exposure of Larvae of the Solitary Bee Osmia bicornis to the Honey Bee Pathogen Nosema ceranae Affects Life History. Insects. 2019; 10(11):380. https://doi.org/10.3390/insects10110380
Chicago/Turabian StyleBramke, Kathrin, Uta Müller, Dino P. McMahon, and Jens Rolff. 2019. "Exposure of Larvae of the Solitary Bee Osmia bicornis to the Honey Bee Pathogen Nosema ceranae Affects Life History" Insects 10, no. 11: 380. https://doi.org/10.3390/insects10110380
APA StyleBramke, K., Müller, U., McMahon, D. P., & Rolff, J. (2019). Exposure of Larvae of the Solitary Bee Osmia bicornis to the Honey Bee Pathogen Nosema ceranae Affects Life History. Insects, 10(11), 380. https://doi.org/10.3390/insects10110380





