Temperature and Sugar Feeding Effects on the Activity of a Laboratory Strain of Aedes aegypti
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
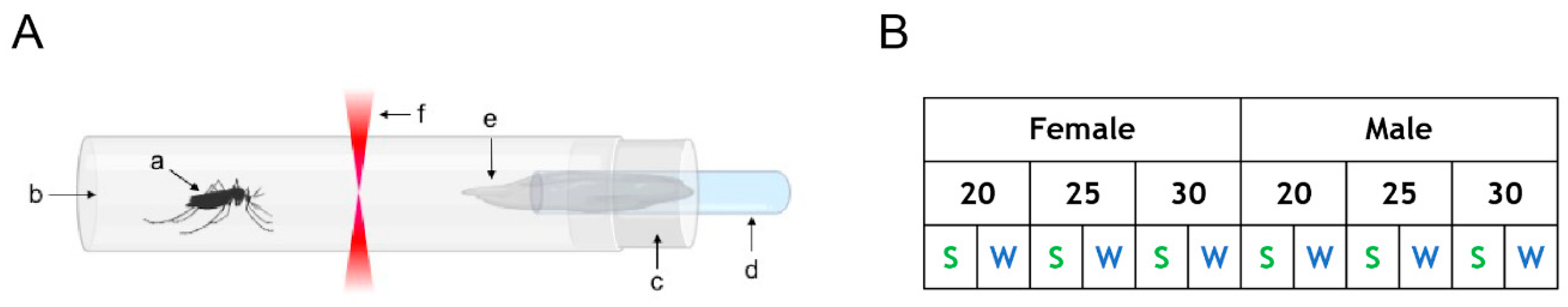
2.2. Actometer Experiments
3. Results
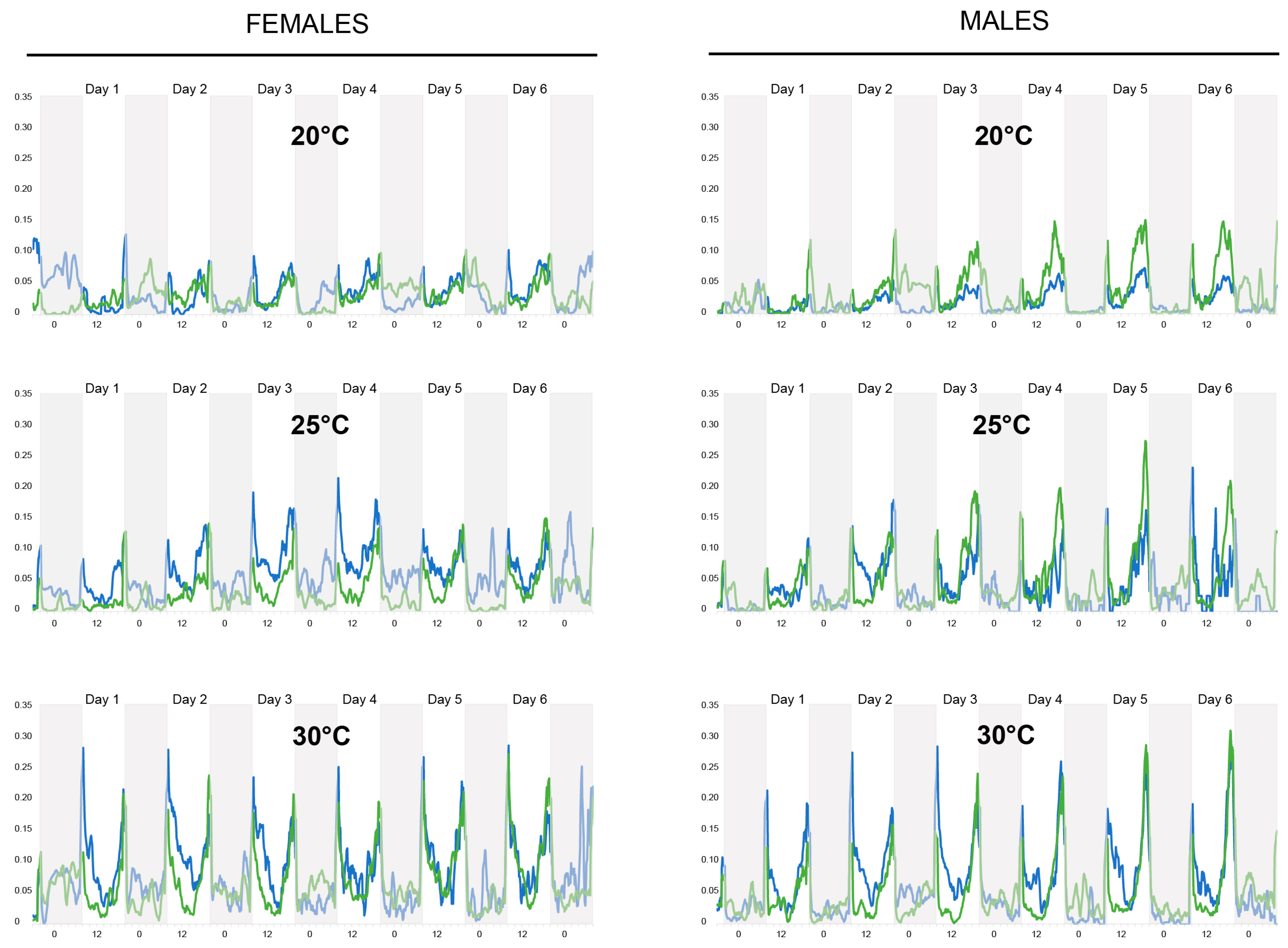
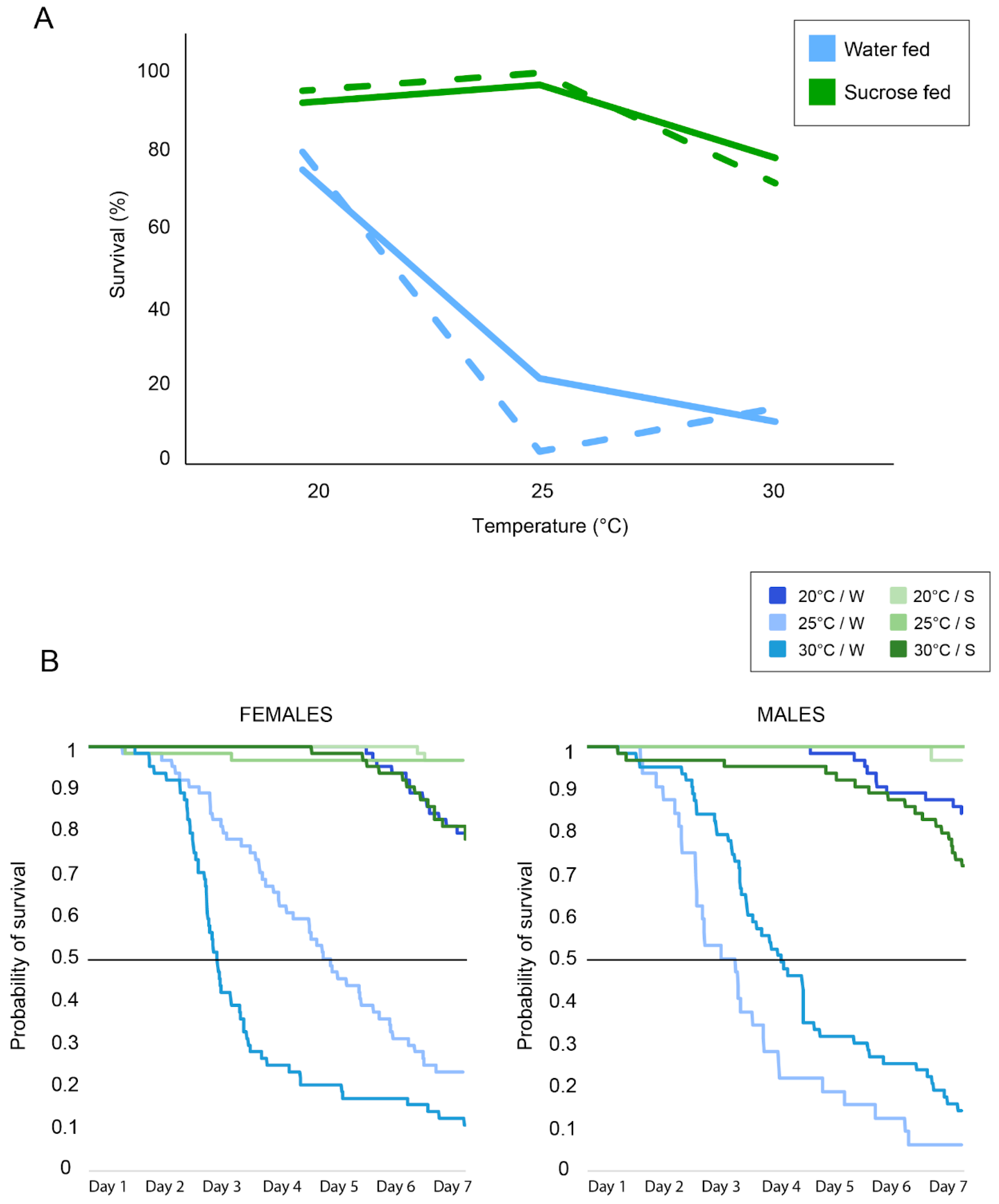
3.1. Actometer Experiments
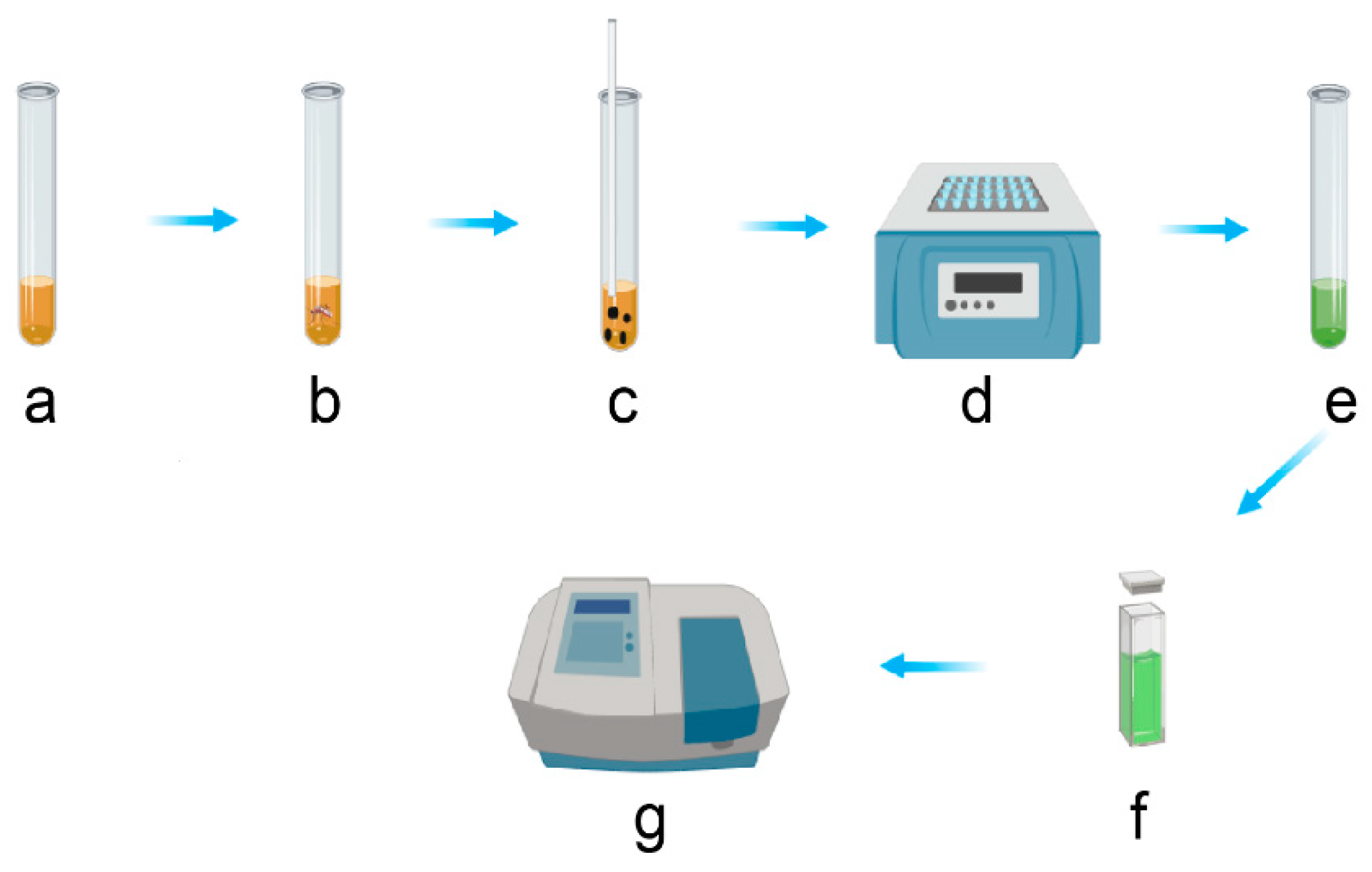
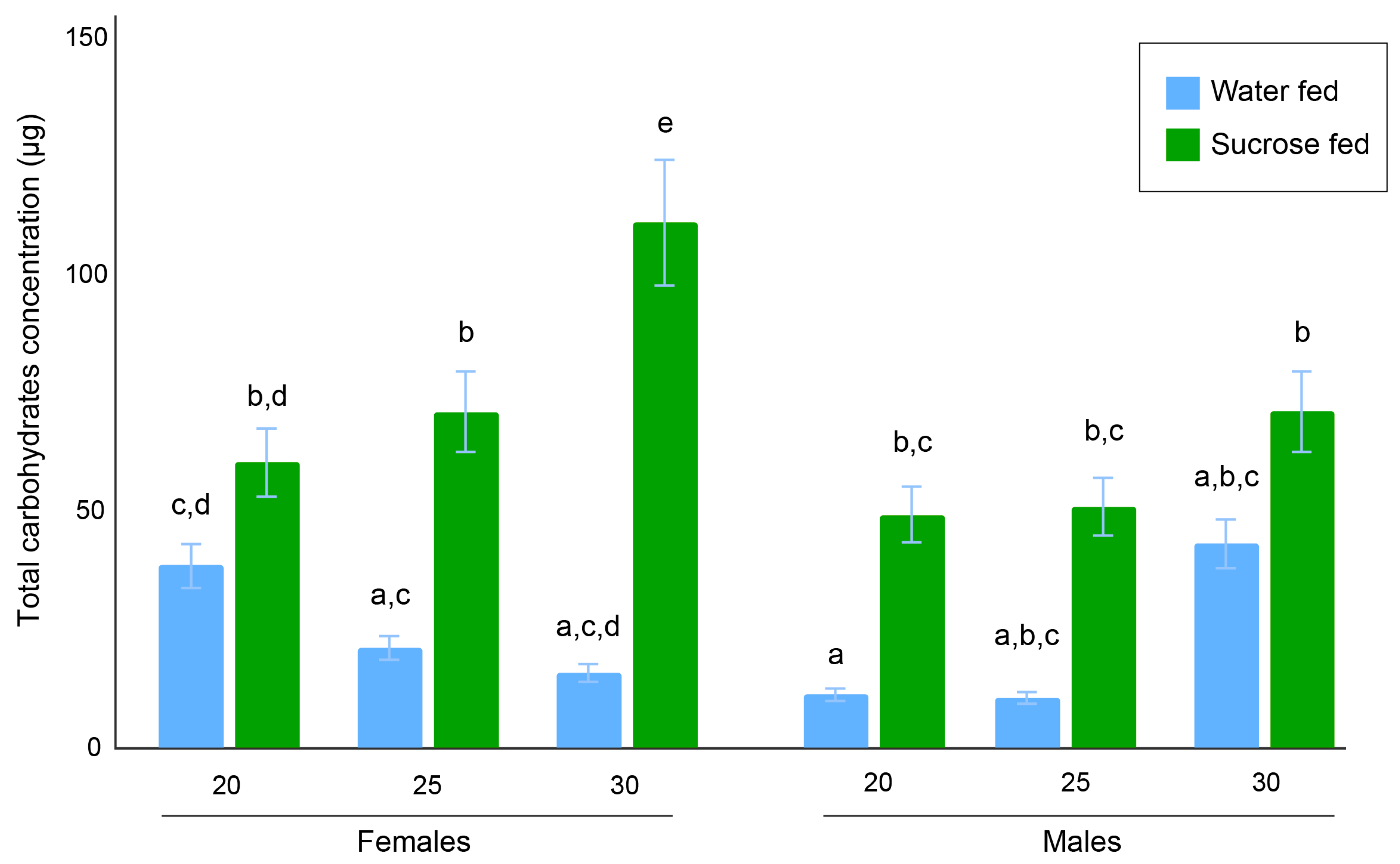
3.2. Total Carbohydrates Content Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coffel, E.D.; Horton, R.M.; Sherbinin, A. Temperature and humidity-based projections of a rapid rise in global heat stress exposure during the 21st century. Environ. Res. Lett. 2017, 13, 014001. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A. AR5 Synthesis Report: Climate Change 2014—IPCC (Synthesis Report No. 5); The Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014. [Google Scholar]
- Reidmiller, D.R.; Avery, C.W.; Easterling, D.R.; Kunkel, K.E.; Lewis, K.L.M.; Maycock, T.K.; Stewart, B.C.; Wuebbles, D.J.; Fahey, D.W.; Hibbard, K.A. Impacts, Risks and Adaptation in the United States: Fourth National Climate Assessment (Assessment No. 4), National Climate Assessment; US Global Change Research Program: Washington, DC, USA, 2018.
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef]
- Weaver, S.C. Arrival of Chikungunya Virus in the New World: Prospects for Spread and Impact on Public Health. PLOS Neglect. Trop. Dis. 2014, 8, e2921. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Hahn, M.B.; Eisen, L.; McAllister, J.; Savage, H.M.; Mutebi, J.-P.; Eisen, R.J. Updated Reported Distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in the United States, 1995–2016. J. Med. Entomol. 2017, 54, 1420–1424. [Google Scholar] [CrossRef]
- Capinha, C.; Rocha, J.; Sousa, C.A. Macroclimate determines the global range limit of Aedes aegypti. EcoHealth 2014, 11, 420–428. [Google Scholar] [CrossRef]
- Monaghan, A.J.; Sampson, K.M.; Steinhoff, D.F.; Ernst, K.C.; Ebi, K.L.; Jones, B.; Hayden, M.H. The potential impacts of 21st century climatic and population changes on human exposure to the virus vector mosquito Aedes aegypti. Clim. Chang. 2018, 146, 487–500. [Google Scholar] [CrossRef]
- Khormi, H.M.; Kumar, L. Climate change and the potential global distribution of Aedes aegypti: Spatial modelling using GIS and CLIMEX. Geospat. Health 2014, 8, 405–415. [Google Scholar] [CrossRef]
- Reinhold, J.; Lazzari, C.R.; Lahondère, C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: A review. Insects 2018, 9, 158. [Google Scholar] [CrossRef]
- de Almeida Costa, E.A.P.; de Mendonça, E.M.; Correia, J.C.; de Albuquerque, C.M.R. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae). Rev. Bras. Entomol. 2010, 54, 488–493. [Google Scholar] [CrossRef]
- Foster, W.A. Mosquito Sugar Feeding and Reproductive Energetics. Annu. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Denlinger, D.L. Suppression of water loss during adult diapause in the northern house mosquito, Culex pipiens. J. Exp. Biol. 2007, 210, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Lopez-Martinez, G.; Phillips, Z.P.; Patrick, K.R.; Denlinger, D.L. Heat shock proteins contribute to mosquito dehydration tolerance. J. Insect Physiol. 2010, 56, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Friend, W.G. Physical Factors Affecting the Feeding Responses of Culiseta inornata to Atp, Sucrose, and Blood. Ann. Entomol. Soc. Am. 1978, 71, 935–940. [Google Scholar] [CrossRef]
- Friend, W.G.; Schmidt, J.M.; Smith, J.J.B.; Tanner, R.J. The effect of sugars on ingestion and diet destination in Culiseta inornata. J. Insect Physiol. 1988, 34, 955–961. [Google Scholar] [CrossRef]
- Friend, W.G.; Smith, J.J.B.; Schmidt, J.M.; Tanner, R.J. Ingestion and diet destination in Culiseta inornata: Responses to water, sucrose and cellobiose. Physiol. Entomol. 1989, 14, 137–146. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Friend, W.G. Ingestion and diet destination in the mosquito Culiseta inornata: Effects of carbohydrate configuration. J. Insect Physiol. 1991, 37, 817–828. [Google Scholar] [CrossRef]
- House, H.L. Insect Nutrition. Annu. Rev. Entomol. 1961, 6, 13–26. [Google Scholar] [CrossRef]
- Nayar, J.K.; Van Handel, E. The fuel for sustained mosquito flight. J. Insect Physiol. 1971, 17, 471–481. [Google Scholar] [CrossRef]
- Briegel, H.; Knüsel, I.; Timmermann, S.E. Aedes aegypti: Size, reserves, survival, and flight potential. J. Vector Ecol. 2001, 26, 21–31. [Google Scholar]
- Clements, A.N. The Biology of Mosquitoes. Volume 2: Sensory Reception and Behavior; CABI Publishing: Oxfordshire, UK, 1999. [Google Scholar]
- Nielsen, E.T.; Greve, H. Studies on the swarming habits of mosquitos and other Nematocera. Bull. Entomol. Res. 1950, 41, 227–258. [Google Scholar] [CrossRef]
- Haeger, J.S. The non-blood feeding habits of Aedes taeniorhynchus (Diptera, Culicidae) on Sanibel Island, Florida. Mosq. News. 1955, 15, 21–26. [Google Scholar]
- Lahondère, C.; Vinauger, C.; Okubo, R.P.; Wolff, G.; Akbari, O.S.; Riffell, J.A. The olfactory basis of orchid pollination by mosquitoes. bioRxiv 2019, 643510. [Google Scholar] [CrossRef]
- Farquharson, C.O. Harpagomyia and other Diptera fed by Crematogaster ants in S. Nigeria. Trans. Entomol. Soc. Lond. 1918, 5, 66: xxix-xxxviii. [Google Scholar]
- Farquharson, C.O.; Poulton, E.B.; Bagnall, R.S.; Bethune-Baker, G.T.; Chappman, T.A.; Collin, J.E.; Durrant, J.H.; Edwards, F.W.; Eltringham, H.; Gatenby, J.B.; et al. Five Years’ Observations (1914–1918) on the Bionomics of Southern Nigerian Insects, chiefly directed to the Investigation of Lycaenid Life-histories and to the Relation of Lycaenidae, Diptera, and other Insects to Ants. Trans. R. Entomol. Soc. Lond. 1922, 69, 319–324. [Google Scholar] [CrossRef]
- James, S.P. Summary of a year’s mosquito work in Colombo. Indian J. Med. Res. 1914, 2, 227–267. [Google Scholar]
- Dittmer, J.; Alafndi, A.; Gabrieli, P. Fat body–specific vitellogenin expression regulates host-seeking behaviour in the mosquito Aedes albopictus. PLoS Biol. 2019, 17, e3000238. [Google Scholar] [CrossRef]
- Christophers, S.R. Aedes aegypti (L.), the Yellow Fever Mosquito: Its Life History, Bionomics and Structure; Cambridge University Press: London, UK, 1960. [Google Scholar]
- Airs, P.M.; Kudrna, K.E.; Bartholomay, L.C. Impact of sugar composition on meal distribution, longevity, and insecticide toxicity in Aedes aegypti. Acta Trop. 2019, 191, 221–227. [Google Scholar] [CrossRef]
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Ann. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Qualls, W.A.; Müller, G.C.; Traore, S.F.; Traore, M.M.; Arheart, K.L.; Doumbia, S.; Schlein, Y.; Kravchenko, V.D.; Xue, R.-D.; Beier, J.C. Indoor use of attractive toxic sugar bait (ATSB) to effectively control malaria vectors in Mali, West Africa. Malar. J. 2015, 14, 301. [Google Scholar] [CrossRef]
- Russell, T.L.; Beebe, N.W.; Cooper, R.D.; Lobo, N.F.; Burkot, T.R. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar. J. 2013, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Fiorenzano, J.M.; Koehler, P.G.; Xue, R.D. Attractive Toxic Sugar Bait (ATSB) For Control of Mosquitoes and Its Impact on Non-Target Organisms: A Review. Int. J. Environ. Res. Public Health 2017, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Global plan for insecticide resistance management in malaria vectors, WHO. Global Plan for Insecticide Resistance Management in Malaria Vectors; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Khallaayoune, K.; Qualls, W.A.; Revay, E.E.; Allan, S.A.; Arheart, K.L.; Kravchenko, V.D.; Xue, R.-D.; Schlein, Y.; Beier, J.C.; Müller, G.C. Attractive toxic sugar baits: Control of mosquitoes with the low-risk active ingredient dinotefuran and potential impacts on nontarget organisms in Morocco. Environ. Entomol. 2013, 42, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Revay, E.E.; Müller, G.C.; Qualls, W.A.; Kline, D.; Naranjo, D.P.; Arheart, K.L.; Kravchenko, V.D.; Yfremova, Z.; Hausmann, A.; Beier, J.C.; et al. Control of Aedes albopictus with attractive toxic sugar baits (ATSB) and potential impact on non-target organisms in St. Augustine, Florida. Parasitol. Res. 2014, 113, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Junnila, A.; Revay, E.E.; Müller, G.C.; Kravchenko, V.; Qualls, W.A.; Xue, R.; Allen, S.A.; Beier, J.C.; Schlein, Y. Efficacy of attractive toxic sugar baits (ATSB) against Aedes albopictus with garlic oil encapsulated in beta-cyclodextrin as the active ingredient. Acta Trop. 2015, 152, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Qualls, W.A.; Xue, R.; Revay, E.E.; Allan, S.A.; Müller, G.C. Implications for operational control of adult mosquito production in cisterns and wells in St. Augustine, FL using attractive sugar baits. Acta Trop. 2012, 124, 158–161. [Google Scholar] [CrossRef]
- Naranjo, D.P.; Qualls, W.A.; Müller, G.C.; Samson, D.M.; Roque, D.; Alimi, T.; Arheart, K.; Beier, J.C.; Xue, R.-D. Evaluation of boric acid sugar baits against Aedes albopictus (Diptera: Culicidae) in tropical environments. Parasitol. Res. 2013, 112, 1583–1587. [Google Scholar] [CrossRef]
- Müller, G.C.; Beier, J.C.; Traore, S.F.; Toure, M.B.; Traore, M.M.; Bah, S.; Doumbia, S.; Schlein, Y. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar. J. 2010, 9, 210. [Google Scholar] [CrossRef]
- Stewart, Z.P.; Oxborough, R.M.; Tungu, P.K.; Kirby, M.J.; Rowland, M.W.; Irish, S.R. Indoor Application of Attractive Toxic Sugar Bait (ATSB) in Combination with Mosquito Nets for Control of Pyrethroid-Resistant Mosquitoes. PLoS ONE 2013, 8, e84168. [Google Scholar] [CrossRef]
- Müller, G.C.; Junnila, A.; Qualls, W.; Revay, E.E.; Kline, D.L.; Allan, S.; Schlein, Y.; Xue, R.D. Control of Culex quinquefasciatus in a storm drain system in Florida using attractive toxic sugar baits. Med. Vet. Entomol. 2010, 24, 346–351. [Google Scholar] [CrossRef]
- Qualls, W.A.; Naranjo, D.P.; Subía, M.A.; Ramon, G.; Cevallos, V.; Grijalva, I.; Gómez, E.; Arheart, K.L.; Fuller, D.O.; Beier, J.C. Movement of Aedes aegypti following a sugar meal and its implication in the development of control strategies in Durán, Ecuador. J. Vector Ecol. 2016, 41, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.J. Studies on the Biting Habits and Medical Importance of East African Mosquitos in the Genus Aëdes. I.—Subgenera Aëdimorphus, Banksinella and Dunnius. Bull. Entomol. Res. 1960, 50, 759–779. [Google Scholar] [CrossRef]
- Eilerts, D.; VanderGiessen, M.; Bose, E.; Broxton, K.; Vinauger, C. Odor-specific daily rhythms in the olfactory sensitivity and behavior of Aedes aegypti mosquitoes. Insects 2018, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Meireles-Filho, A.C.; Britto, C.; Lima, J.B.P.; Valle, D.; Peixoto, A.A. Cloning and daily expression of the timeless gene in Aedes aegypti (Diptera:Culicidae). Insect Biochem. Mol. Biol. 2006, 36, 878–884. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 31 July 2019).
- Van Handel, E. Rapid determination of glycogen and sugars in mosquitoes. J. Am. Mosq. Control Assoc. 1985, 1, 299–301. [Google Scholar]
- Harrington, D.P.; Fleming, T.R. A class of rank test procedures for censored survival data. Biometrika 1982, 69, 553–566. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Van Handel, E. The obese mosquito. J. Insect Physiol. 1965, 181, 478–486. [Google Scholar] [CrossRef]
- Gary, R.E.; Foster, W.A. Diel timing and frequency of sugar feeding in the mosquito Anopheles gambiae, depending on sex, gonotrophic state and resource availability. Med. Vet. Entomol. 2006, 20, 308–316. [Google Scholar] [CrossRef]
- McCrae, A.W.R.; Boreham, P.F.L.; Ssenkubuge, Y. The behavioural ecology of host selection in Anopheles implexus (Theobald) (Diptera, Culicidae). Bull. Entomol. Res. 1976, 66, 587–631. [Google Scholar] [CrossRef]
- Yuval, B.; Holliday-hanson, M.L.; Washino, R.K. Energy budget of swarming male mosquitoes. Ecol. Entomol. 1994, 19, 74–78. [Google Scholar] [CrossRef]
- Smith, S.M.; Gadawski, R.M. Nectar feeding by the early-spring mosquito Aedes provocans. Med. Vet. Entomol. 1994, 8, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, P.R.; DeFoliart, G.R. Nectar sources of Wisconsin mosquitoes. J. Med. Entomol. 1974, 11, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, N.P.; Burkepile, D.E.; Parker, J.D. Variable effects of temperature on insect herbivory. PeerJ 2014, 2, e376. [Google Scholar] [CrossRef] [Green Version]
- Berrigan, D.; Partridge, L. Influence of temperature and activity on the metabolic rate of adult Drosophila melanogaster. Comp. Biochem. Physiol. A Physiol. 1997, 118, 1301–1307. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef]
- Klepsatel, P.; Wildridge, D.; Gáliková, M. Temperature induces changes in Drosophila energy stores. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Vorhees, A.S.; Gray, E.M.; Bradley, T.J. Thermal resistance and performance correlate with climate in populations of a widespread mosquito. Physiol. Biochem. Zool. 2012, 86, 73–81. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Comeau, G.; Monaghan, A.J.; Williamson, D.J.; Ernst, K.C. Effects of desiccation stress on adult female longevity in Aedes aegypti and Ae. albopictus (Diptera: Culicidae): Results of a systematic review and pooled survival analysis. Parasites Vectors 2018, 11, 267. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Hagan, R.W.; Didion, E.M.; Rosselot, A.E.; Holmes, C.J.; Siler, S.C.; Rosendale, A.J.; Hendershot, J.M.; Elliot, K.S.; Jennings, E.C.; Nine, G.A.; et al. Dehydration prompts increased activity and blood feeding by mosquitoes. Sci. Rep. 2018, 8, 6804. [Google Scholar] [CrossRef] [PubMed]
| Factors | Df | Sum Sq | Mean Sq | F Value | Pr(>F) | Significance |
|---|---|---|---|---|---|---|
| Temperature | 2 | 121,442 | 60,721 | 37.876 | 6.09 × 10−16 | *** |
| Food source | 1 | 110,499 | 110,499 | 68.926 | 1.18 × 10−15 | *** |
| Sex | 1 | 49,724 | 49,724 | 31.016 | 4.38 × 10−8 | *** |
| Temperature:Food source | 2 | 10,465 | 5232 | 3.264 | 0.03914 | * |
| Temperature:Sex | 2 | 1628 | 814 | 0.508 | 0.6022 | |
| Food source:Sex | 1 | 6 | 6 | 0.004 | 0.9522 | |
| Temperature:Food source:Sex | 2 | 17,728 | 8864 | 5.529 | 0.00424 | ** |
| Residuals | 455 | 729,438 | 1603 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upshur, I.F.; Bose, E.A.; Hart, C.; Lahondère, C. Temperature and Sugar Feeding Effects on the Activity of a Laboratory Strain of Aedes aegypti. Insects 2019, 10, 347. https://doi.org/10.3390/insects10100347
Upshur IF, Bose EA, Hart C, Lahondère C. Temperature and Sugar Feeding Effects on the Activity of a Laboratory Strain of Aedes aegypti. Insects. 2019; 10(10):347. https://doi.org/10.3390/insects10100347
Chicago/Turabian StyleUpshur, Irvin Forde, Elizabeth Annadel Bose, Cameron Hart, and Chloé Lahondère. 2019. "Temperature and Sugar Feeding Effects on the Activity of a Laboratory Strain of Aedes aegypti" Insects 10, no. 10: 347. https://doi.org/10.3390/insects10100347
APA StyleUpshur, I. F., Bose, E. A., Hart, C., & Lahondère, C. (2019). Temperature and Sugar Feeding Effects on the Activity of a Laboratory Strain of Aedes aegypti. Insects, 10(10), 347. https://doi.org/10.3390/insects10100347





