Sublethal Effects of Imidacloprid on the Population Development of Western Flower Thrips Frankliniella occidentalis (Thysanoptera: Thripidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects, Plants, and Insecticides
2.2. Sublethal Effects of Imidacloprid on WFT
2.3. Statistical Analysis
3. Results
3.1. Sublethal Effects of Imidacloprid on WFT Development
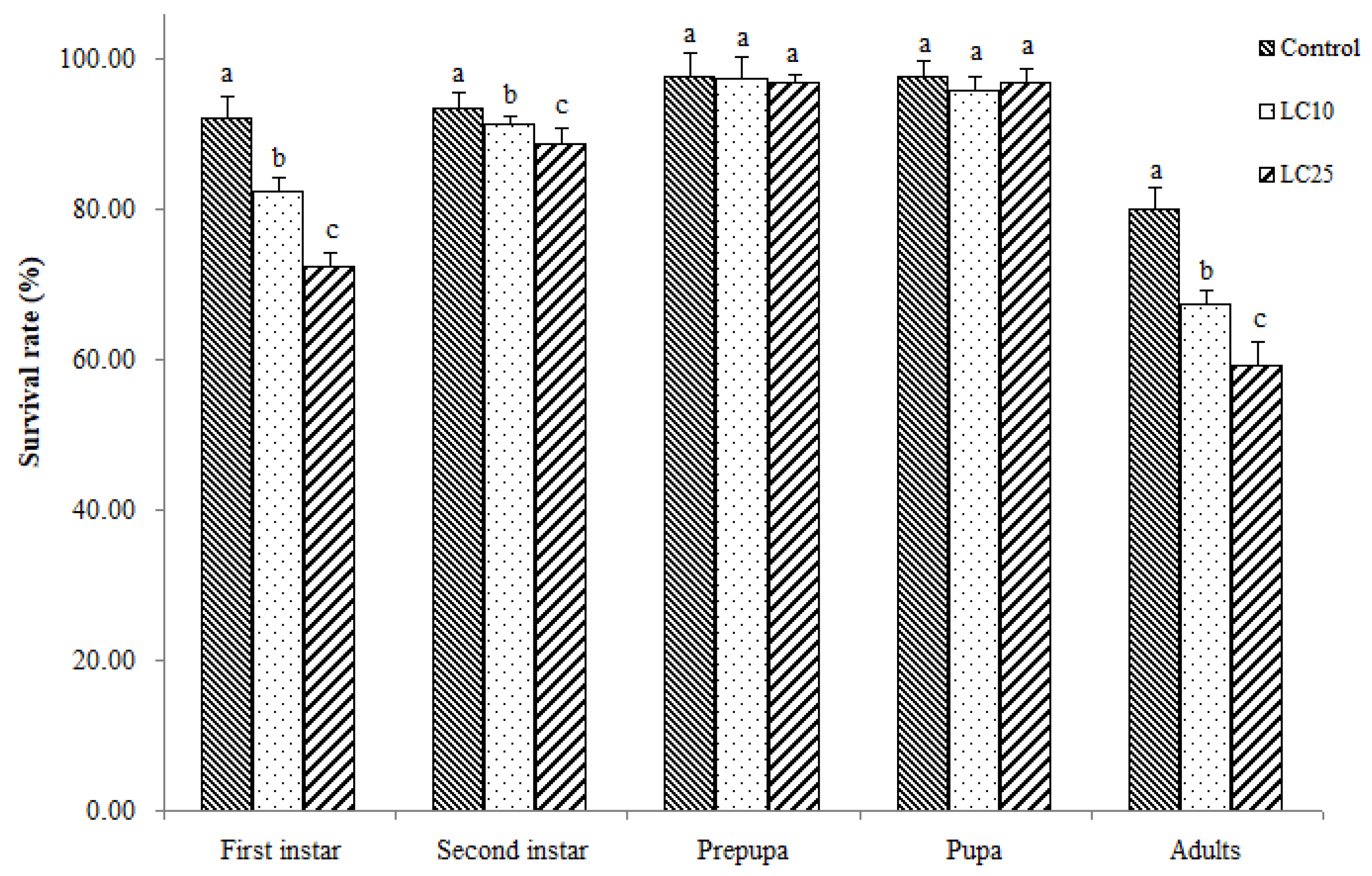
3.2. Sublethal Effects of Imidacloprid on WFT Survival
3.3. Sublethal Effects of Imidacloprid on WFT Longevity, Oviposition and Sex Ratios
3.4. Sublethal Effects of Imidacloprid on WFT Life Table Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reitz, S.R. Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): The making of a pest. Fla. Entomol. 2009, 1, 7–13. [Google Scholar] [CrossRef]
- Webster, C.; Reitz, S.R.; Perry, K.; Adkins, S. A natural M RNA reassortant arising from two species of plant- and insect infecting bunyaviruses and comparison of its sequence and biological properties to parental species. Virology 2011, 413, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, R.L.; Batuman, O.; Webster, G.; Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Reitz, S.R.; Gao, Y.L.; Lei, Z.R. Thrips: Pests of concern to China and the United States. Agric. Sci. China 2011, 10, 867–892. [Google Scholar]
- Zhang, Y.J.; Wu, Q.J.; Xu, B.Y.; Zhu, G.R. The occurrence and damage of Frankliniella occidentalis (Thysanoptera: Thripidae): A dangerous alien invasive pest in Beijing. Plant Prot. 2003, 4, 58–59. [Google Scholar]
- Lu, Y.B.; Zhang, Z.J.; Wu, Q.J.; Du, Y.Z.; Zhang, H.R.; Yu, Y.; Wang, E.D.; Wang, M.H.; Wang, M.Q.; Tong, X.L.; et al. Research progress of the monitoring, forecast and sustainable management of invasive alien pest Frankliniella occidentalis in China. Chin. J. Appl. Entomol. 2011, 48, 488–496. [Google Scholar]
- Gao, Y.L.; Lei, Z.R.; Reitz, S.R. Western flower thrips resistance to insecticides: Detection, mechanisms and management strategies. Pest Manag. Sci. 2012, 68, 1111–1121. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, L.; Wang, H.; Qiao, K.; Wang, D.; Wang, K. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest Manag. Sci. 2011, 67, 1528–1533. [Google Scholar] [CrossRef]
- Liang, P.; Tian, Y.A.; Biondi, A.; Desneux, N.; Gao, X.W. Short-term and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species. Ecotoxicology 2012, 21, 1889–1898. [Google Scholar] [CrossRef]
- Silva, I.M.A.; Martins, G.F.; Melo, C.R.; Santana, A.S.; Faro, R.R.N.; Blank, A.F.; Alves, P.B.; Picanço, M.C.; Cristaldo, P.F.; Araújo, A.P.A.; et al. Alternative control of Aedes aegypti resistant to pyrethroids: Lethal and sublethal effects of monoterpenes bioinsecticides. Pest Manag. Sci. 2017, 74, 1001–1012. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.F.; Zhang, H.; Zhang, R.J. Effect of fenvalerate on the reproduction and fitness costs of the brown planthopper, Nilaparvata lugens and its resistance mechanism. Pestic. Biochem. Physiol. 2011, 101, 148–153. [Google Scholar] [CrossRef]
- Mahmoudvand, M.; Moharramipour, S. Sublethal effects of fenoxycarb on the Plutella xylostella (Lepidoptera: Plutellidae). J. Insect Sci. 2015, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.H.; Xu, B.Y.; Zhang, Y.J.; Gao, X.W.; Wu, Q.J. Demonstration of an adaptive response to preconditioning Frankliniella occidentalis (pergande) to sublethal doses of spinosad: A hormetic-dose response. Ecotoxicology 2015, 24, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Elbert, A.; Becker, B.; Hartwig, J.; Erdelen, C. Imidacloprid-a new systemic insecticide. Pflanzenschutz-Nachrichten Bayer 1990, 44, 113–136. [Google Scholar]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.B.; Liu, S.H.; Gu, J.H.; Wang, X.Z.; Liang, X.L.; Liu, Z.W. Sublethal effects of four insecticides on the reproduction and wing formation of brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2009, 65, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, L.L.; Yang, H.; Wang, Z.; Long, G.Y.; Jin, D.C. Sublethal effects of imidacloprid on the development, reproduction, and susceptibility of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J. Asia-Pac. Entomol. 2017, 20, 996–1000. [Google Scholar] [CrossRef]
- Qu, C.; Zhang, W.; Li, F.; Tetreau, G.; Luo, C.; Wang, R. Lethal and sublethal effects of dinotefuran on two invasive whiteflies, Bemisia tabaci, (Hemiptera: Aleyrodidae). J. Asia-Pac. Entomol. 2017, 20, 325–330. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, W.; Che, W.; Qu, C.; Li, F.; Desneux, N.; Li, F.Q.; Desneux, N.; Luo, C. Lethal and sublethal effects of cyantraniliprole, a new anthranilic diamide insecticide, on Bemisia tabaci (Hemiptera: Aleyrodidae) med. Crop Prot. 2017, 91, 108–113. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Yu, Y.; Liu, Y.J.; Ma, J.Y. Resistance risk and resistance stability of Frankliniella occidentalis to imidacloprid, emamectin, benzoate, and phoxim. Chin. J. Appl. Ecol. 2012, 23, 3429–3434. [Google Scholar]
- Wang, S.Y.; Zhang, A.S.; Li, L.L.; Men, X.Y.; Zhou, X.H.; Zhai, Y.F.; Liu, Y.J.; Wei, S.J.; Yu, Y. Insecticide resistance status of field populations of Frankliniella occidentalis (Thysanoptera: Thripidae) in China and its control strategies. Acta Entomol. Sin. 2014, 57, 621–630. [Google Scholar]
- Cao, Y.; Li, C.; Ma, H.; Wang, C.; Zhi, J.R. Toxity measurements of five insecticides to Frankliniella occidentalis. Hubei Agric. Sci. 2015, 54, 3939–3944. [Google Scholar]
- Cao, Y.; Zhi, J.R.; Cong, C.L.; Margolies, D.C. Olfactory cues used in host selection by Frankliniella occidentalis (Thysanoptera: Thripidae) in relation to host suitability. J. Insect Behav. 2014, 27, 41–56. [Google Scholar] [CrossRef]
- Cao, Y.; Zhi, J.R.; Zhang, R.Z.; Li, C.; Liu, Y.; Lv, Z.Y.; Gao, Y.L. Different population performances of Frankliniella occidentalis and Thrips hawaiiensis on flowers of two horticultural plants. J. Pest Sci. 2017, 3, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wu, Q.J.; Li, X.F.; Zhang, Y.J.; Xu, B.Y.; Zhu, G.R. Life history of western flower thrips, Frankliniella occidentalis (Thysan., Thripidae), on five different vegetable leaves. J. Appl. Entomol. 2007, 131, 347–354. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Teulon, D.A.J.; Chapman, R.B.; Butler, R.C.; Drayton, G.M.; Philipsen, H. Comparison of life history parameters of two Frankliniella occidentalis (Thysanoptera: Thripidae) strains in New Zealand. Environ. Entomol. 2010, 2, 303–311. [Google Scholar] [CrossRef]
- Bielza, P. Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis. Pest Manag. Sci. 2008, 64, 1131. [Google Scholar] [CrossRef]
- Mouden, S.; Sarmiento, K.F.; Klinkhamer, P.G.; Leiss, K.A. Integrated pest management in western flower thrips: Past, present and future. Pest Manag. Sci. 2017, 73, 813–822. [Google Scholar] [CrossRef]
- Cutler, G.C. Insect, insecticides and hormesis: Evidence and considerations for study. Dose-Response 2013, 11, 154–177. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.G.; Ramanaidu, K.; Astatkie, T.; Isman, M.B. Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest Manag. Sci. 2009, 65, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Shen, G.Q.; Zhu, H.L.; Lu, Y.T. Imidacloprid-induced hormesis on the fecundity and juvenile hormone levels of the green peach aphid Myzus persicae (Sulzer). Pestic. Biochem. Physiol. 2010, 98, 238–242. [Google Scholar] [CrossRef]
- Azzam, S.; Wang, F.; Wu, J.C.; Shen, J.; Wang, L.P.; Yang, G.Q.; Guo, Y.R. Comparisons of stimulatory effects of a series of concentrations of four insecticides on reproduction in the rice brown planthopper Nilaparvata lugens Stål (Homoptera: Delphacidae). Int. J. Pest Manag. 2009, 55, 347–358. [Google Scholar] [CrossRef]
- Tan, Y.; Biondi, A.; Desneux, N.; Gao, X.W. Assessment of physiological sublethal effects of imidacloprid on the mirid bug Apolygus lucorum (Meyer-Dür). Ecotoxicology 2012, 21, 1989–1997. [Google Scholar] [CrossRef]
- Lu, Y.H.; Zheng, X.S.; Gao, X.W. Sublethal effects of imidacloprid on the fecundity, longevity, and enzyme activity of Sitobion avenae (Fabricius) and Rhopalosiphum padi (Linnaeus). Bull. Entomol. Res. 2016, 106, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Xiang, X.; Yuan, G.X.; Chen, Y.Q.; Wang, X.G. Spodoptera effects of sublethal doses of cyantraniliprole on the growth and development and the activities of detoxifying enzymes in Spodoptera exigua (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2015, 58, 634–641. [Google Scholar]
- Zhang, R.M.; Jang, E.B.; He, S.Y.; Chen, J.H. Lethal and sublethal effects of cyantraniliprole on Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Pest. Manag. Sci. 2015, 71, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Du, Z.B.; Wu, Y.Q.; Gong, Z.J.; Jiang, Y.L.; Duan, Y.; Li, T.; Lei, C.L. Sub-lethal effects of four neonicotinoid seed treatments on the demography and feeding behaviour of the wheat aphid Sitobion avenae. Pest Manag. Sci. 2014, 70, 55–59. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Cutler, G.C. Insecticide-induced hormesis and arthropod pest management. Pest Manag. Sci. 2014, 70, 690–697. [Google Scholar] [CrossRef]
- Qu, Y.Y.; Xiao, D.; Li, J.Y.; Zhou, C.; Biondi, A.; Desneux, N.; Gao, X.W.; Song, D.L. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology 2015, 24, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Zalizniak, L.; Nugegoda, D. Effect of sublethal concentrations of chlorpyrifos on three successive generations of Daphnia carinata. Ecotoxicol. Environ. Saf. 2006, 64, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Joost, P.H.; Riley, D.G. Imidacloprid effects on probing and settling behavior of Frankliniella fusca and Frankliniella occidentalis (Thysanoptera: Thripidae) in tomato. J. Econ. Entomol. 2005, 98, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.L.; Kennedy, G.G. Effect of cyantraniliprole on feeding behavior and virus transmission of Frankliniella fusca and Frankliniella occidentalis (Thysanoptera: Thripidae) on Capsicum annuum. Crop Prot. 2013, 54, 251–258. [Google Scholar] [CrossRef]
| Stage | Control | LC10 | LC25 |
|---|---|---|---|
| Egg | 2.59 ± 0.03 a | 2.55 ± 0.02 a | 2.51 ± 0.03 a |
| First instar | 2.20 ± 0.02 a | 1.99 ± 0.02 b | 1.96 ± 0.01 b |
| Second instar | 2.88 ± 0.03 a | 2.46 ± 0.02 b | 2.44 ± 0.02 b |
| Prepupa | 1.32 ± 0.00 a | 1.21 ± 0.00 ab | 1.15 ± 0.00 b |
| Pupa | 1.33 ± 0.00 a | 1.30 ± 0.00 a | 1.30 ± 0.00 a |
| Egg to adult | 10.31 ± 0.16 a | 9.57 ± 0.14 b | 9.38 ± 0.12 b |
| Parameters | Control | LC10 | LC25 |
|---|---|---|---|
| Longevity/female (day) | 26.15 ± 0.27 a | 25.20 ± 0.14 ab | 24.25 ± 0.51 b |
| Longevity/male (day) | 15.09 ± 0.13 a | 13.37 ± 0.83 b | 12.68 ± 0.75 b |
| Oviposition period (day) | 22.05 ± 0.48 a | 21.15 ± 0.32 a | 20.95 ± 0.95 b |
| Fecundity (first instars/female) | 72.65 ± 0.38 c | 79.02 ± 0.44 b | 82.20 ± 0.64 a |
| Oviposition rate (first instars/female/day) | 3.33 ± 0.04 b | 3.78 ± 0.05 a | 3.95 ± 0.03 a |
| Sex ratio of offspring (females/total) | 2.07 ± 0.03 c | 3.34 ± 0.05 b | 3.91 ± 0.02 a |
| Parameters | Control | LC10 | LC25 |
|---|---|---|---|
| Net reproductive rate (R0) | 38.80 ± 0.25 b | 42.26 ± 0.33 a | 40.90 ± 0.28 a |
| Intrinsic rate of increase (rm) | 0.154 ± 0.000 c | 0.171 ± 0.000 b | 0.181 ± 0.000 a |
| Mean generation time (T) | 23.76 ± 0.42 a | 21.88 ± 0.36 b | 20.50 ± 0.53 b |
| Finite rate of increase (λ) | 1.167 ± 0.000 b | 1.187 ± 0.000 a | 1.200 ± 0.000 a |
| Population doubling time (DT) | 4.501 ± 0.040 a | 4.052 ± 0.021 b | 3.829 ± 0.032 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Yang, H.; Li, J.; Wang, C.; Li, C.; Gao, Y. Sublethal Effects of Imidacloprid on the Population Development of Western Flower Thrips Frankliniella occidentalis (Thysanoptera: Thripidae). Insects 2019, 10, 3. https://doi.org/10.3390/insects10010003
Cao Y, Yang H, Li J, Wang C, Li C, Gao Y. Sublethal Effects of Imidacloprid on the Population Development of Western Flower Thrips Frankliniella occidentalis (Thysanoptera: Thripidae). Insects. 2019; 10(1):3. https://doi.org/10.3390/insects10010003
Chicago/Turabian StyleCao, Yu, Hong Yang, Jun Li, Chun Wang, Can Li, and Yulin Gao. 2019. "Sublethal Effects of Imidacloprid on the Population Development of Western Flower Thrips Frankliniella occidentalis (Thysanoptera: Thripidae)" Insects 10, no. 1: 3. https://doi.org/10.3390/insects10010003
APA StyleCao, Y., Yang, H., Li, J., Wang, C., Li, C., & Gao, Y. (2019). Sublethal Effects of Imidacloprid on the Population Development of Western Flower Thrips Frankliniella occidentalis (Thysanoptera: Thripidae). Insects, 10(1), 3. https://doi.org/10.3390/insects10010003






